Cellular identity is established and maintained by a coordinated series of signals that result in the activation and repression of defined set of genes. In general, this is regulated by the activity of transcription factors (TFs) that act directly at promoters and or at regulatory elements to stimulate or inhibit transcription, for instance, by modulating DNA accessibility via modifications of the chromatin environment. At the same time, cells requires to maintain a certain degree of plasticity that allows them to react promptly to a multitude of stimuli or environmental signals in order to preserve tissue homeostasis. The major mechanism by which cells can “lock” defined repressed states and at the same time maintain transcriptional plasticity comes from the Polycomb machinery.Citation1-2
Discovered almost 30 years ago in Drosophila melanogaster, the Polycomb group of proteins (PcG) maintains transcriptional repression by modifying histone tails to promote chromatin compaction. Polycomb proteins assemble in 2 large multi-protein complexes: the Polycomb repressive complex 1 (PRC1) and 2 (PRC2). While PRC1 mediates the mono-ubiquitination of lysine 119 on histone H2A through the activity of the E3 ligases RING1A and RING1B; the SET domain proteins EZH2 or EZH1 – in association with the scaffold proteins EED and SUZ12 to form the core of PRC2 – mediates the methylation of lysine 27 on histone H3. These activities regulate common transcriptional pathways, maintaining transcriptional repression of a large set of common target genes.Citation3
The essential role of both activities in early embryogenesis has limited our knowledge regarding the role of these activities in the homeostasis of adult tissues.Citation4 Using conditional models for tissue-specific inactivation of critical PRC1 or PRC2 components, we have recently uncovered the role of both activities in supporting intestinal homeostasis.Citation5-6 The intestinal epithelium is one of the tissues of the body endowed with the greater regenerative potentials and a level of cell plasticity rarely seen in other adult epithelia. A pool of fast cycling stem cells (ISC) located at the bottom of the crypts of Lieberkühn and marked by the expression of the RSPO1 receptor LGR5, continuously provide new cells to regenerate the entire epithelium that undergoes complete renewal every 5–6 days. This is crucial to face the harsh conditions present in the intestinal lumen. LGR5+ cells generate all the differentiated cell types of the intestinal epithelium belonging to both secretory and absorptive lineage and can be regenerated by different pools of progenitor cells upon extensive damages that compromise their viability.Citation7
Our recent work has shown that loss of function of global PRC1 activity (Ring1a/b double knock-out) severely affects intestinal homeostasis resulting in weight loss and morbidity.Citation5 In particular, we demonstrated that PRC1 is essential to maintain the pool of LGR5+ ISCs. Transcriptomic analysis demonstrated that the absence of PRC1 force stem cells to exit the niche without acquiring any defined differentiation program. This correlates with the transcriptional activation of a number of genes that are strongly enriched for DNA binding TFs specific for other tissues or lineages. Among these, we identified potential negative regulators of WNT-signaling that were sufficient to impair intestinal homeostasis in 3D organoids cultures. This set of TFs can bind directly the TCF7L2-CTNNB1 complex displacing the complex from chromatin in the stem cells affecting WNT transcriptional response. Consistently, WNT constitutive activation in vivo was not sufficient to revert this phenotype preventing the development of intestinal adenomas. Together our findings demonstrate that PRC1 activity is required to maintain ISC identity by repressing non lineage-specific genes, whose ectopic activation can directly interfere with the TCF7L2-CTNNB1 transcriptional complex.
Figure 1. An Eph-induced signaling cascade prevents cytokinesis completion: possible physiological and pathological consequences. Activation of Eph signaling in dividing cells interrupts or delays cytokinesis which increases the fraction of polyploid cells. In the neocortex polyploid neurons could participate in generating neuronal diversity while in other tissues, cytokinesis defects could increase genomic instability and participate in tumor initiation.
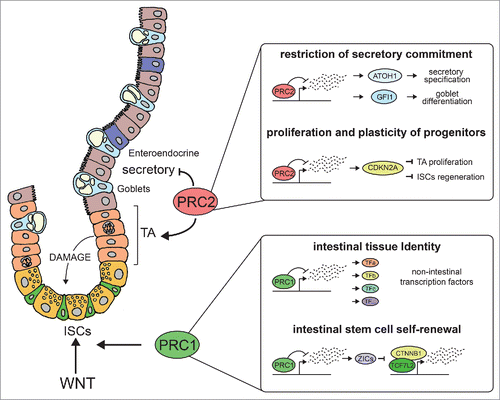
Considering that PRC1 and PRC2 activities “sit” together on a large set of common target promoters, we extended this analysis to PRC2 to uncover common or specific feature of this activity.Citation6 By knocking out PRC2 activity in the entire intestinal epithelium (Eed knockout), we found that while intestinal homeostasis and ISC maintenance was not affected, its regenerative capabilities upon extensive damage were strongly compromised. Loss of Eed reduces cell proliferation of transit amplifying cells and increases the number of secretory lineage progenitors leading to a non-physiological accumulation of goblet and enteroendocrine cells. While the proliferation of progenitors and their plasticity to regenerate the ISC pool were dependent on PRC2-mediated repression of the Cdkn2a locus, the accumulation of secretory cells was not an indirect consequence of these proliferation defects. Indeed, the accumulation of secretory cells was a result of an unscheduled activation of master transcription factors that are required and sufficient to instruct secretory cell fate. These transcription factors are maintained repressed by PRC2 and needs to be displaced from these loci when cells are undergoing secretory commitment.
Together, these works provide important understanding about how distinct layer of repressive control are required to maintain intestinal homeostasis. Our data highlight the distinct biological functions of PRC1 and PRC2 demonstrating that PRC1 acts redundantly over PRC2 in regulating stem cells self-renewal and suggest that the extent of overlap and redundancy between these activities is likely to be context dependent. This implies that different layers of repression are imposed by PcG complexes at different promoters, which likely involve the biochemical complexity behind PRC1 composition and the mechanisms that mediate the recruitment of distinct PcG activities at specific genomic loci.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Laugesen A, Helin K. Chromatin repressive complexes in stem cells, development, and cancer. Cell Stem Cell 2014; 14:735-51; PMID:24905164; http://dx.doi.org/10.1016/j.stem.2014.05.006
- Orkin SH, Hochedlinger K. Chromatin connections to pluripotency and cellular reprogramming. Cell 2011; 145:835-50; PMID:21663790; http://dx.doi.org/10.1016/j.cell.2011.05.019
- Pasini D, Di L. Croce Emerging roles for Polycomb proteins in cancer. Curr Opin Genet Dev 2016; 36:50-8; PMID:27151431; http://dx.doi.org/10.1016/j.gde.2016.03.013
- Avgustinova A, Benitah SA. Epigenetic control of adult stem cell function. Nat Rev Mol Cell Biol 2016; 17:643-58; PMID:27405257; http://dx.doi.org/10.1038/nrm.2016.76
- Chiacchiera F, Rossi A, Jammula S, Piunti A, Scelfo A, Ordonez-Moran P, Huelsken J, Koseki H, Pasini D. Polycomb complex PRC1 preserves intestinal stem cell identity by sustaining Wnt/beta-catenin transcriptional activity. Cell Stem Cell 2016; 18:91-103; PMID:26526724; http://dx.doi.org/10.1016/j.stem.2015.09.019
- Chiacchiera F, Rossi A, Jammula S, Zanotti M, Pasini D. PRC2 pre-serves intestinal progenitors and restricts secretory lineage commitment. EMBO J 2016; 35:2301-2314; PMID:27585866; http://dx.doi.org/10.15252/130embj.201694550
- Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 2014; 15:19-33; PMID:24326621; http://dx.doi.org/10.1038/nrm3721
