Microcephaly is a disorder characterized by severe impairment in brain development and reduced brain and head size. Many cases result from genetic causes, which have provided insight into the mechanisms responsible for normal and abnormal neocortical development.Citation1 The neocortex, at the outer surface of the brain, is responsible for many higher order brain functions. Its development relies on extensive stem cell proliferation within a limited brain region, the ventricular zone. Radial glial progenitors (RGPs) represent the majority of stem cells in this region and generate most neurons and glial cells in the developing neocortex. The RGPs are highly elongated, with an apical process in contact with the ventricular surface, and a basal process in contact with the outer surface of the brain throughout development ().
Figure 1. We have identified 3 distinct non-mitotic stages of the cell cycle where NDE1 is required in radial glia progenitor cells (RPGs): (1) regulation of primary cilium dynamics at the G1-to-S transition; (2) apical nuclear migration during G2; and (3) entry into mitosis at the G2-to-M transition. Cell cycle duration is not drawn to scale.
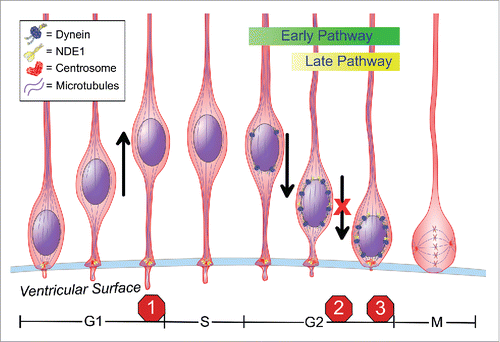
The RGPs exhibit highly unusual cell cycle behavior. Mitosis occurs only when the cell body is at the ventricular surface.Citation2 Once in G1, the nucleus migrates away from the ventricular surface through the narrow basal process of the cell, progresses through S-phase, and then returns to the ventricular surface during G2 for the next mitotic division (). How the nuclear oscillatory behavior is entrained with cell cycle progression, and the biological purpose of this behavior have remained mysterious until recently.
The cell cycle-dependent nuclear behavior, termed interkinetic nuclear migration (INM), is now known to be powered by microtubule motor proteins. Our lab found that the kinesin Kif1A transports the nucleus basally during G1 along a cytoskeleton of near-uniformly oriented microtubules, which emanates from the centrosome located at the apical terminus of the RGP cell.Citation3 How Kif1a is activated in a G1-specific manner for nuclear transport is unknown.
G2-specific apical nuclear migration is carried out by cytoplasmic dynein, which has been found to be recruited to the nuclear envelope through two distinct and successive mechanisms.Citation2,4 Early G2 nuclear envelope recruitment is activated by Cdk1 phosphorylation of the nucleoporin RanBP2, which, in turn, recruits BicD2, dynein, dynactin, and Lis1. Late G2 nuclear envelope recruitment involves another nucleoporin, Nup133, which recruits CENP-F once it has left the late G2 nucleus through an incompletely understood Cdk1-mediated mechanism. CENP-F, in turn, recruits NDE1 and, through it, additional cytoplasmic dynein ().
Human NDE1 mutations cause severe forms of microcephaly,Citation5 but how was uncertain. We found that acute depletion of NDE1 by in utero electroporation of shRNAs into embryonic day 16 rat brain severely inhibits apical INM.Citation6 Depletion of NDEL1, a NDE1 paralog, had no such effect. However, NDE1 and NDEL1 double knock down arrested RGP nuclei far from the ventricular surface, and in G1 rather than G2.Citation6 This result suggested an unexpected additional cell cycle role for these proteins, perhaps in the G1-S transition.
Recent work has revealed a role for NDE1 in non-neuronal cells during the G1-to-S transition, mediated by the behavior of primary cilia, which elongate during G1 but must begin resorption for S-phase entry.Citation7 Similar observations have been reported by inhibiting Tctex-1, LC8, trichoplein, or CPAP in other model systems.Citation7
We found, in fact, that NDE1/NDEL1 knockdown causes a doubling in RGP primary cilium length.Citation6 Additional knockdown of the IFT172 gene, which inhibits cilium assembly, overrode the G1-S block. These results support an important role for primary cilium dynamics in RGP cell cycle progression.
When present, cilia protrude from the RGP cells into the ventricle, where signaling factors could potentially act on them to regulate the cell cycle. However, the role of NDE1 and NDEL1, as well as cytoplasmic dynein, in this mechanism remains to be more fully elucidated, as does a potential contribution of primary cilium-mediated cell signaling.
Restriction of RGP cell division to the ventricular surface may be the most important biological purpose of INM, but how this behavior is controlled is still poorly understood. Although the effects of NDE1 knockdown on INM could be rescued by expression of RNAi-insensitive NDE1, expression of NDEL1 rescued apical INM. However, once at the ventricular surface, the nucleus failed to enter mitosis.Citation6 Artificially targeting additional dynein to the nuclear envelope by overexpressing the early recruitment factor BicD2 had the same result.Citation6 These observations strongly suggest an additional role for NDE1 in the unique spatial control of mitotic entry observed in this system. The particular contribution of NDE1 in this mechanism emerges as an important new question.
The multiple contributions of NDE1 to cell cycle progression, in addition to its known role in mitosis, present multiple opportunities for NDE1 mutations to impair RGP cell proliferation. In further analysis we found an additional, later requirement for NDE1 in postmitotic neuronal migration,Citation6 which helps explain why patients with NDE1 mutations may also exhibit neuronal lamination defects, such as lissencephaly.Citation5 Nonetheless, the multiple roles of NDE1 in cell cycle progression should encourage a reexamination of cell cycle effects associated with other microcephaly genes.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB. A developmental and genetic classification for malformations of cortical development: update 2012. Brain 2012; 135:1348-69; PMID:22427329; http://dx.doi.org/10.1093/brain/aws019
- Hu DJ-K, Baffet AD, Nayak T, Akhmanova A, Doye V, Vallee RB. Dynein recruitment to nuclear pores activates apical nuclear migration and mitotic entry in brain progenitor cells. Cell 2013; 154:1300-13; PMID:24034252; http://dx.doi.org/10.1016/j.cell.2013.08.024
- Carabalona A, Hu DJ-K, Vallee RB. KIF1A inhibition immortalizes brain stem cells but blocks BDNF-mediated neuronal migration. Nat Neurosci 2016; 19:253-62; PMID:26752160; http://dx.doi.org/10.1038/nn.4213
- Baffet AD, Hu DJ, Vallee RB. Cdk1 activates pre-mitotic nuclear envelope dynein recruitment and apical nuclear migration in neural stem cells. Developmental Cell 2015; 33:703-16; PMID:26051540; http://dx.doi.org/10.1016/j.devcel.2015.04.022
- Alkuraya FS, Vai X, Emery C, Mochida GH, Al-Dosari MS, Felie JM, Hill RS, Barry BJ, Partlow JN, Gascon GG, et al. Human mutations in NDE1 cause extreme microcephaly with lissencephaly. Am J Hum Genet 2011; 88:536-47; PMID:21529751; http://dx.doi.org/10.1016/j.ajhg.2011.04.003
- Doobin DJ, Kemal S, Dantas TJ, Vallee RB. Severe NDE1-mediated microcephaly results from neural progenitor cell cycle arrests at multiple specific stages. Nat Commun 2016; 7:12551; PMID:27553190; http://dx.doi.org/10.1038/ncomms12551
- Izawa I, Goto H, Kasahara K, Inagaki M. Current topics of functional links between primary cilia and cell cycle. Cilia 2015; 4:12; PMID:26719793; http://dx.doi.org/10.1186/s13630-015-0021-1
