ABSTRACT
Rhabdomyosarcoma (RMS) is a rare mesenchymal tumor. The aim of the present study was to develop a patient-derived orthotopic xenograft (PDOX) mouse model of RMS and compare the PDOX model to a subcutaneous (s.c.)-transplant model. A patient RMS from a striated muscle was grown orthotopically in the right biceps femoris muscle and right quadriceps muscle of nude mice to establish a PDOX model, as well as under the skin to establish an s.c. model. PDOX tumors grew at a statistically-significant faster rate compared to the s.c. tumors. Recurrence after surgical resection occurred only in PDOX tumors, not in the s.c. model. Histologically, only the PDOX model was shown to be invasive. In conclusion, these results indicate that the PDOX model of adult RMS is malignant and the subcutaneous model is benign. These results emphasize that a proper tumor microenvironment is necessary for patient-like behavior of a tumor in a mouse model.
Introduction
Rhabdomyosarcoma (RMS) is a recalcitrant mesenchymal tumor that occurs rarely,Citation1,2 and usually in the first 2 decades of life.Citation3,4 A clinically-relevant mouse model of RMS could make possible important advances in the understanding and therapy of RMS.
Discrepancies have been repeatedly described between the malignant behavior of tumors in the patient compared to their benign tumor behavior in the subcutaneous (s.c.)-transplanted xenografts in nude mice.Citation5
Our laboratory pioneered the patient-derived orthotopic xenograft (PDOX) nude mouse model with the technique of surgical orthotopic implantation (SOI) of intact cancer tissue.Citation5,6 PDOX models from patients with colon,Citation6 pancreatic,Citation7-11 breast,Citation12 ovarian,Citation13 lung,Citation14 cervical,Citation15 stomach cancer,Citation16 and mesotheliomaCitation17 were established by our laboratory resulting in primary and metastatic tumor growth very similar to that of the patient.
We have recently established PDOX models of soft tissue sarcoma including follicular dendritic-cell sarcoma, leiomyosarcoma, and Ewing's sarcomaCitation18-21 and melanoma.Citation22 In the present report, we describe the very different behavior of orthotopic and s.c. models of adult pleomorphic RMS in nude mouse models.
Results and discussion
Growth rate of the RMS PDOX is greater than RMS subcutaneous tumor in nude mice
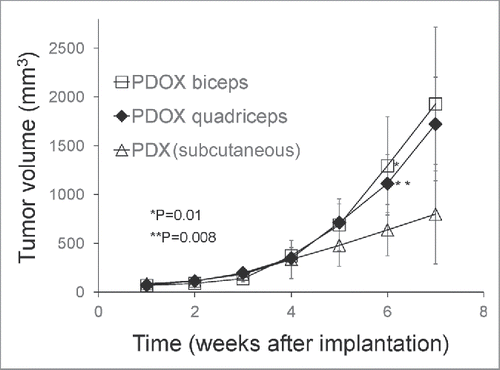
Two weeks after tumor implantation, the tumor take ratio for the RMS subcutaneous (s.c.) mouse models was 90% and for the PDOX models was 100%. Six weeks after tumor implantation, tumor volume in the PDOX models growing either into the biceps femoris muscle (1294.3 ± 5 mm3, p = 0.01); or quadriceps femoris muscle (1111.5 ± 3 mm3, p = 0.008) was larger compared to the s.c. tumors (636.7 ± 3 mm3) (). There were no significant difference in volume between tumors growing in the biceps femoris muscle or the quadriceps femoris muscle.
Figure 1. Growth of rhabdomyosarcoma (RMS) in the PDOX and subcutaneous-transplant models. Line graph shows tumor volume at each point. Six weeks after tumor implantation, the tumor volume in both PDOX models was significantly larger, both in the biceps-femoris muscle (G2) (1294.3 ± 504.8 mm3) (p = 0.01); and quadriceps-femoris muscle (G3) (1111.5 ± 296.6 mm3) (p = 0.008) compared to mice with subcutaneous tumors (G1) (636.7 ± 263.2 mm3). There were no significant differences in growth rate between tumors growing in either the biceps-femoris muscle or quadriceps-femoris muscle.
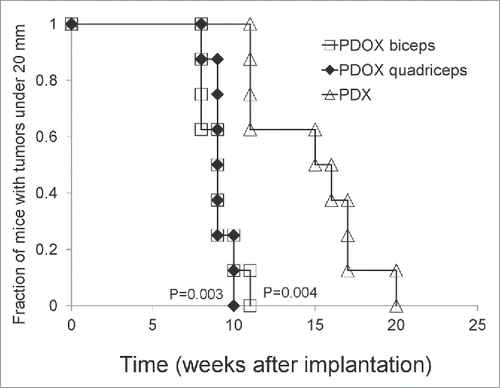
PDOX tumors reached 20 mm earlier in the biceps femoris muscle group (9 ± 1.1 weeks, p = 0.004); and quadriceps femoris group (9.13 ± 0.6 weeks, p = 0.003) compared to the s.c. group (14.75 ± 3.4 weeks), demonstrated with Kaplan-Mayer analysis (). These results indicate that the RMS in the PDOX model grew faster than in the s.c. model.
Figure 2. Time of PDOX and subcutaneous tumors to reach 20 mm diameter. Tumors in the PDOX group growing either in the biceps-femoris (G2) (9 ± 1.07 weeks, p = 0.004) or quadriceps-femoris muscle (G3) (9.13 ± 0.64 weeks, p = 0.003) reached 20 mm at an earlier time compared to the subcutaneous group (G1) (14.75 ± 3.41 weeks), shown with Kaplan-Mayer analysis.
Tumor recurrence after resection only in the PDOX model
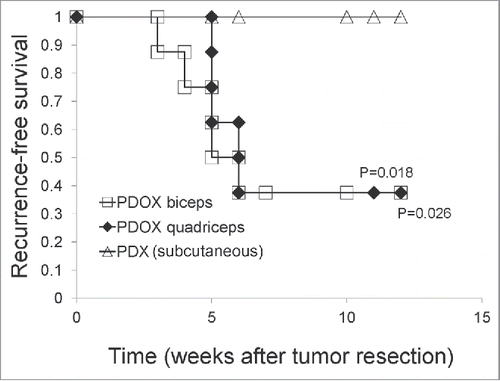
When the s.c. or PDOX tumors reached 20 mm (), they were resected. After tumor resection, recurrence was observed only in the PDOX mice. Kaplan–Meier analysis with the log-rank test demonstrated that recurrence-free survival was significantly shorter in the mice with orthotopically-implanted tumors in the biceps-femoris muscle (p = 0.018) and quadriceps-femoris muscle (p=0.026) compared to the mice with subcutaneous tumors ().
Figure 3. Recurrence-free survival of rhabdomyosarcoma PDOX and subcutaneous tumors after surgical resection. Recurrence-free survival was significantly shorter in the PDOX biceps-femoris muscle (G2) (p = 0.018); and quadriceps-femoris muscle (G3) (p = 0.026) compared to mice with subcutaneous tumors (G1).
Tumor invasiveness only in the PDOX model
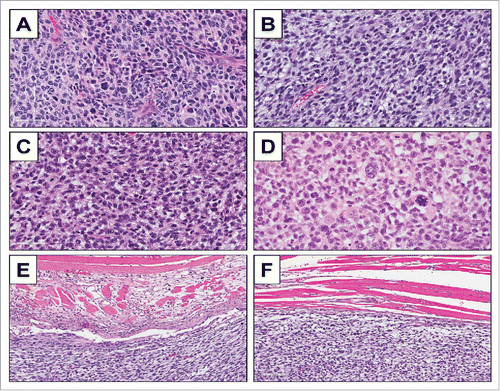
High power microscopy of the original patient tumor displayed solid sheets of tumor cells characterized by pleomorphic, hyperchromatic, enlarged nuclei with coarse chromatin and moderate amounts of lightly-eosinophilic cytoplasm. Numerous mitotic figures, including atypical forms are present (). High power microscopy of subcutaneously-implanted tumors and orthotopically-implanted tumors displayed identical features including pleomorphic, hyperchromatic, enlarged nuclei with coarse chromatin and moderate amounts of lightly-eosinophilic cytoplasm. Numerous mitotic figures, including atypical forms were also present (). Recurrent tumors appearing after excision of orthotopic tumors displayed almost identical features as the original patient tumor and primary-implanted tumor (). High power microscopy of the tumor muscle margin of orthotopically-implanted tumors showed infiltration of cancer cells in surrounding muscle tissues ().
Figure 4. Tumor histology. H & E-stained sections of the original patient tumor (A); subcutaneously-implanted tumor (B); orthotopically-implanted tumor (C); and recurrence of orthotopically-implanted tumor after marginal excision (D); H & E-stained sections of tumor muscle margin of the orthotopically-implanted tumor (E, F).
The present report demonstrates fundamental differences between the PDOX and s.c. mouse models of RMS. This is consistent with our previous observations of direct comparison of orthotopic and s.c. models of other tumor types such as stomach,Citation16 breast,Citation12 prostate,Citation23 kidneyCitation24 and others.Citation5 The orthotopic and s.c. and other ectopic sites have different tumor microenvironments which accounts for the different behavior of the tumors at each site.Citation25,26
In vivo imaging at the cellular level of GFP-labeled cancer cells showed that invasion could start within 1 or 2 days, with metastasis occurring by 4 days after orthotopic implantation, but never occurred at the s.c. site.Citation23 Although simplistic hyphotheses suggest a single growth factor such as TGF-β may be responsible for a “pro-metastatic” effect,Citation27,28 the fundamental difference between the s.c. and orthotopic tumor microenvironment's ability to enable malignant behavior, such as observed in the present report, indicates a more complex mechanism.
Materials and methods
Animal care
Athymic nu/nu nude mice (AntiCancer Inc., San Diego, CA), 4–6 weeks old, were used in this study. All animal studies were conducted with an AntiCancer Institutional Animal Care and Use Committee (IACUC)-protocol specifically approved for this study and in accordance with the principles and procedures outlined in the National Institute of Health Guide for the Care and Use of Animals under Assurance Number A3873–1. In order to minimize any suffering of the animals, anesthesia and analgesics were used for all surgical experiments. Animals were anesthetized by subcutaneous injection of a 0.02 ml solution of 20 mg/kg ketamine, 15.2 mg/kg xylazine, and 0.48 mg/kg acepromazine maleate. The response of animals during surgery was monitored to ensure adequate depth of anesthesia. The animals were observed on a daily basis and humanely sacrificed by CO2 inhalation if they met the following humane endpoint criteria: severe tumor burden (more than 20 mm in diameter), prostration, significant body-weight loss, difficulty breathing, rotational motion and body temperature drop. Animals were housed in a barrier facility on a high-efficiency particulate-arrestance (HEPA)-filtered rack under standard conditions of 12-hour light/dark cycles. The animals were fed an autoclaved laboratory rodent diet.
Patient-derived tumor
A 68-years-old male diagnosed with a pleomorphic rhabdomyosarcoma (RMS) in the right high thigh underwent surgical resection at Department of Surgery, University of California, Los Angeles (UCLA). The patient did not receive any chemotherapy or radiotherapy prior to surgery. Written informed consent was obtained from the patient as part of a UCLA Institutional Review Board-approved protocol (IRB #10-001857).
Surgical orthotopic implantation (SOI) for establishment of the RMS PDOX model
A fresh sample of the tumor from the patient was obtained and transported immediately to the laboratory at AntiCancer, Inc., on wet ice. The sample was cut into 5 mm fragments and implanted subcutaneously in nude mice. The grown tumors were harvested and cut into small fragments (3–4 mm). After the nude mice were anesthetized, a 5 mm skin incision was made on the right high thigh, then the biceps femoris or quadriceps femoris was split to make space for tumor implantation. A single tumor fragment was implanted orthotopically into the space to establish a PDOX model. A single fragment was implanted under the skin to establish the s.c. model. The wounds were closed with 6–0 nylon suture (Ethilon, Ethicon, Inc., NJ, USA).
Study design for the PDOX and subcutaneous models
The mice were divided into 3 groups, G1: subcutaneous-transplant model (n = 10); G2: PDOX model with tumors transplanted in the biceps-femoris muscle (n = 10), G3: PDOX model with tumors transplanted in the quadriceps-femoris muscle (n = 10). Tumor length and width were measured once a week with calipers. Tumor volume was calculated with the following formula: Tumor volume (mm3) = length (mm) × width (mm) × width (mm) × 1/2. Data are presented as mean ± SD. When the tumors reached 20 mm, they were resected under anesthesia induced by the ketamine-solution anesthesia described above. After tumor resection, the mice were further evaluated for incidence of local recurrence.
Histological examination
Fresh tumor samples were fixed in 10% formalin and embedded in paraffin before sectioning and staining. Tissue sections (5 μm) were deparaffinized in xylene and rehydrated in an ethanol series. Hematoxylin and eosin (H&E) staining was performed according to standard protocols. Histological examination was performed with a BHS System Microscope (Olympus Corporation, Tokyo, Japan). Images were acquired with INFINITY ANALYZE software (Lumenera Corporation, Ottawa, Canada).
Disclosure of potential conflicts of interest
The authors have no conflicts of interest to disclose.
References
- Stout AP. Rhabdomyosarcoma of the skeletal muscles. Ann Surg 1946; 123:447-72; http://dx.doi.org/10.1097/00000658-194603000-00011
- Dagher R, Helman L. Rhabdomyosarcoma: an overview. Oncologist 1999; 4:34-44; PMID:10337369
- Ferrari A, Dileo P, Casanova M, Bertulli R, Meazza C, Gandola L, Navarria P, Collini P, Gronchi A, Olmi P, et al. Rhabdomyosarcoma in adults. A retrospective analysis of 171 patients treated at a single institution. Cancer 2003; 98:571-80; PMID:12879475; http://dx.doi.org/10.1002/cncr.11550
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal S, Thun MJ. Cancer statistics. CA Cancer J Clin 2006; 56:106-30; PMID:16514137; http://dx.doi.org/10.3322/canjclin.56.2.106
- Hoffman RM. Patient-derived orthotopic xenografts: better mimic of metastasis than subcutaneous xenografts. Nature Rev Cancer 2015; 15:451-2; http://dx.doi.org/10.1038/nrc3972
- Fu X, Besterman JM, Monosov A, Hoffman RM. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc Natl Acad Sci USA 1991; 88:9345-9; PMID:1924398; http://dx.doi.org/10.1073/pnas.88.20.9345
- Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically from histologically intact patient specimens. Proc Natl Acad Sci USA 1992; 89:5645-9; PMID:1608975; http://dx.doi.org/10.1073/pnas.89.12.5645
- Hiroshima Y, Zhao M, Maawy A, Zhang Y, Katz MH, Fleming JB, Uehara F, Miwa S, Yano S, Momiyama M, et al. Efficacy of Salmonella typhimurium A1-R versus chemotherapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX). J Cell Biochem 2014; 115:1254-61; PMID:24435915; http://dx.doi.org/10.1002/jcb.24769
- Hiroshima Y, Maawy A, Zhang Y, Murakami T, Momiyama M, Mori R, Matsuyama R, Katz MH, Fleming JB, Chishima T, et al. Metastatic recurrence in a pancreatic cancer patient derived orthotopic xenograft (PDOX) nude mouse model is inhibited by neoadjuvant chemotherapy in combination with fluorescence-guided surgery with an anti-CA 19-9-conjugated fluorophore. PLOS ONE 2014; 9:e114310; PMID:25463150; http://dx.doi.org/10.1371/journal.pone.0114310
- Hiroshima Y, Zhang Y, Murakami T, Maawy AA, Miwa S, Yamamoto M, Yano S, Sato S, Momiyama M, Mori R, et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R in combination with anti-angiogenesis therapy on a pancreatic cancer patient-derived orthotopic xenograph (PDOX) and cell line mouse models. Oncotarget 2014; 5:12346-57; PMID:25402324; http://dx.doi.org/10.18632/oncotarget.2641
- Hiroshima Y, Maawy A, Zhan Y, Murakami T, Momiyama M, Mori R, Matsuyama R, Chishima T, Tanaka K, Ichikawa Y, et al. Fluorescence-guided surgery, but not bright-light surgery, prevents local recurrence in a pancreatic cancer patient-derived orthotopic xenograft (PDOX) model resistant to neoadjuvant chemotherapy (NAC). Pancreatology 2015; 15:295-301; PMID:25800176; http://dx.doi.org/10.1016/j.pan.2015.02.008
- Fu X, Le P, Hoffman RM. A metastatic-orthotopic transplant nude mouse model of human patient breast cancer. Anticancer Res 1993; 13:901-4; PMID:8352558
- Fu X, Hoffman RM. Human ovarian carcinoma metastatic models constructed in nude mice by orthotopic transplantation of histologically-intact patient specimens. Anticancer Res 1993; 13:283-6; PMID:8517640
- Wang X, Fu X, Hoffman RM. A new patient-like metastatic model of human lung cancer constructed orthotopically with intact tissue via thoracotomy in immunodeficient mice. Int J Cancer 1992; 51:992-5; PMID:1639545; http://dx.doi.org/10.1002/ijc.2910510621
- Hiroshima Y, Zhang Y, Zhang M, Maawy A, Mii S, Yamamoto M, Uehara F, Miwa S, Yano S, Murakami T, et al. Establishment of a patient-derived orthotopic xenograph (PDOX) model of HER-2-positive cervical cancer expressing the clinical metastatic pattern. Plos One 2015; 10:e0117417; PMID:25689852; http://dx.doi.org/10.1371/journal.pone.0117417
- Furukawa T, Kubota T, Watanabe M, Kitajima M, Fu X, Hoffman RM. Orthotopic transplantation of histologically intact clinical specimens of stomach cancer to nude mice: correlation of metastatic sites in mouse and individual patient donors. Int J Cancer 1993; 53:608-12; PMID:8436434; http://dx.doi.org/10.1002/ijc.2910530414
- Astoul P, Wang X, Colt HG, Boutin C, Hoffman RM. A patient-like human malignant pleural mesothelioma nude-mouse model. Oncol Rep 1996; 3:483-7; PMID:21594397
- Murakami T, DeLong J, Eilber FC, Zhao M, Zhang Y, Zhang N, Singh A, Russell T, Deng S, Reynoso J, et al. Tumor-targeting Salmonella typhimurium A1-R in combination with doxorubicin eradicate soft tissue sarcoma in a patient-derived orthotopic xenograft PDOX model. Oncotarget 2016; 7:12783-90; PMID:26859573
- Kiyuna T, Murakami T, Tome Y, Kawaguchi K, Igarashi K, Zhang Y, Zhao M, Li Y, Bouvet M, Kanaya F, et al. High efficacy of tumor-targeting Salmonella typhimurium A1-R on a doxorubicin- and dactolisib-resistant follicular dendritic-cell sarcoma in a patient-derived orthotopic xenograft PDOX nude mouse model. Oncotarget 2016; 7:33046-54; PMID:27105519
- Murakami T, Singh AS, Kiyuna T, Dry SM, Li Y, James AW, Igarashi K, Kawaguchi K, DeLong JC, Zhang Y, et al. Effective molecular targeting of CDK4/6 and IGF-1R in a rare FUS-ERG fusion CDKN2A-deletion doxorubicin-resistant Ewing's sarcoma in a patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget; Epub ahead of print; http://dx.doi.org/10.18632/Oncotarget.9879
- Kiyuna T, Murakami T, Tome Y, Igarashi K, Kawaguchi K, Russell T, Eckhardt MA, Crompton J, Singh A, Bernthal N, et al. Labeling the stroma of a patient-derived orthotopic xenograft (PDOX) mouse models of undifferentiated pleomorphic soft-tissue sarcoma with red fluorescent protein for rpaid non-invasive drug screening. J Cell Biochem, Epub ahead of print; PMID:27357060; http://dx.doi.org/10.1002/jcb25643
- Yamamoto M, Zhao M, Hiroshima Y, Zhang Y, Shurell E, Eilber FC, Bouvet M, Noda M, Hoffman RM. Efficacy of tumor-targeting Salmonella typhimurium A1-R on a melanoma patient-derived orthotopic xenograft (PDOX) nude-mouse model. Plos One 2016; 11:e0160882; PMID:27500926; http://dx.doi.org/10.1371/journal.pone.0160882
- Zhang Y, Toneri M, Ma H, Yang Z, Bouvet M, Goto Y, Seki N, Hoffman RM. Real-time GFP intravital imaging of the difference in cellular and angiogenic behavior of subcutaneous and orthotopic nude-mouse models of human PC-3 prostate cancer. J Cell Biochem 2016; 117:2546-51; PMID:27012365; http://dx.doi.org/10.1002/jcb.25547
- An Z, Jiang P, Wang X, Moossa AR, Hoffman RM. Development of a high metastatic orthotopic model of human renal cell carcinoma in nude mice: Benefits of fragment implantation compared to cell-suspension injection. Clin Exp Metastasis 1999; 17:265-70; PMID:10432012; http://dx.doi.org/10.1023/A:1006654600095
- Togo S, Wang X, Shimada H, Moossa AR, Hoffman RM. Cancer seed and soil can be highly selective: human-patient colon tumor lung metastasis grows in nude mouse lung but not colon or subcutis. Anticancer Res 1995; 15:795-8; PMID:7645960
- Fidler IJ. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res 1990; 50:6130-8; PMID:1698118
- Padua D, Massagué J. Roles of TGFbeta in metastasis. Cell Res 2009; 19:89-102; PMID:19050696; http://dx.doi.org/10.1038/cr.2008.316
- Stankic M, Pavlovic S, Chin Y, Brogi E, Padua D, Norton L, Massagué J, Benezra R. TGF-β-Id1 signaling opposes Twist1 and promotes metastatic colonization via a mesenchymal-to-epithelial transition. Cell Rep 2013; 5:1228-42; PMID:24332369; http://dx.doi.org/10.1016/j.celrep.2013.11.014
