Neurogenin 3 (Neurog3) is a pro-endocrine transcription factor required for endocrine-lineage specification during mouse pancreas development. It was a long-standing notion that Neurog3 activation in Sox9+ epithelial cells, a subset of early multipotent progenitors that produce ductal and endocrine lineages, triggers a rapid progression to a post-mitotic, Neurog3HI endocrine-committed precursor state. We challenged this model by demonstrating that a mitotic progenitor state, in which Neurog3 is transcriptionally active but at a low level (Neurog3TA.LO), pre-empts endocrine-commitment.Citation1 We postulated a new model in which this Neurog3TA.LO progenitor state is a mitotic, metastable condition in which low Neurog3 levels regulate decisions regarding progenitor maintenance and endocrine-lineage specification.Citation1 Our work showed that Neurog3TA.LO progenitors resemble other progenitor populations, such as intestinal and haematopoietic progenitors, in their ability to undergo multiple rounds of division that seem to be symmetric and produce either more Neurog3TA.LO progenitors, or two endocrine-committed daughters.Citation1 This modification of the endocrine-lineage specification model focuses attention on the Neurog3TA.LO progenitor state as being the stage wherein the critical decision is made as to which endocrine cell fate will be established within committed cells emerging from Neurog3TA.LO progenitors. Below, we discuss potential connections between the cell cycle and Neurog3 protein levels in Neurog3TA.LO progenitors, and the implications of bringing this model together with a systems-level understanding of the 3-dimensional and dynamic nature of an epithelial plexus state that represents the endocrine-birth niche. Understanding how cycling Neurog3TA.LO progenitors integrate their behavior within the surrounding dynamics of a developing epithelial endocrine birth niche will be important. For example, what determines progenitor-maintaining or symmetric endocrine-birth divisions, and the end-fate endocrine potential of Neurog3TA.LO progenitors, could inform on how to generate multilineage islets rather than simply populations of β cells.
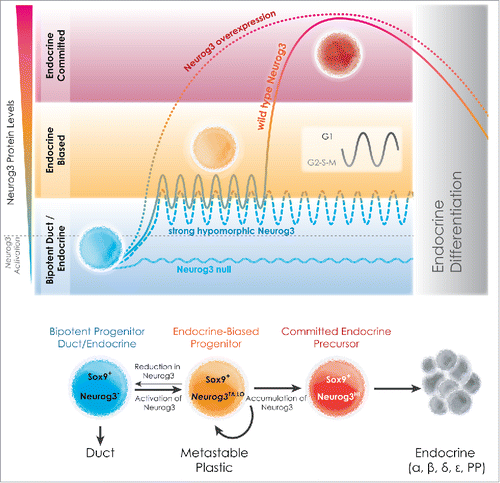
Gain and loss of function studies show the power of Neurog3 in instructing endocrine differentiation, but other studies had already begun to suggest correlations between a more finely adjusted Neurog3 expression level and the potential of the Neurog3TA.LO population to produce endocrine cells (). Wang et al. (2010) reported that a strong Neurog3 hypomorphic condition significantly decreased the degree of endocrine biasing, diverting many cells to non-endocrine acinar or ductal fates ().Citation2 Our analysis of the same hypomorphic condition showed a doubling of the mitotic index of Neurog3TA.LO progenitors, but not of the surrounding non-endocrine-biased cells, and expansion of the Neurog3TA.LO pool at the expense of endocrine-committed cells.Citation1 In addition to low-level Neurog3 expression within the endocrine-biased mitotic population, a number of Neurog3 downstream targets are also expressed at relatively low levels, suggesting that endocrine-biased cells undertake a concerted effort to build the framework of a gene regulatory network that primes intraepithelial Neurog3TA.LO progenitors toward the endocrine lineage, before their committment. Such a concept has been referred to as lineage-priming and is a familiar concept regarding haematopoietic and intestinal stem cells. We propose that the Neurog3TA.LO state represents a mitotic metastable progenitor state in which low Neurog3 levels, under normal conditions, begin to drive cells toward the endocrine lineage (). However, small reductions of the Neurog3 level within Neurog3TA.LO progenitors allow movement toward other lineages (). The notion that Neurog3 can maintain a metastable progenitor state at low levels, but at higher levels trigger cell-cycle exit and endocrine-lineage commitment is a concept not without precedent. In neural progenitors, the Neurog3 sister protein Neurog2 is actively phosphorylated by Cdks during S-G2-M phases, resulting in a low-level unstable form that activates progenitor-associated target genes.Citation3 During G1, however, the increased activity of Cdk-inhibitors results in accumulation of underphosphorylated Neurog2 which is stabilized and targets different suites of genes that promote neural differentiation. Similarly, in the pancreas, we found that Neurog3 protein levels are low-to-undetectable in cycling Neurog3TA.LO progenitors and transition to an endocrine-committed Neurog3HI state ∼2–3 hours after the symmetric mitotic division, during G1.Citation3 Previous work using Xenopus egg extract showed stabilization of Neurog3 in the presence of the Cdk-inhibitor p27Kip1, and that high Neurog3 levels promote expression of the Cdk-inhibitor Cdkn1a - suggesting a threshold-dependent feedforward mechanism coupling Neurog3 protein accumulation with cell-cycle exit.Citation1,3 We hypothesize that Neurog3 protein levels, as suggested for Neurog2, oscillate over the various phases of the cell cycle, with an extension of G1 providing the context for promoting Neurog3 stabilization, accumulation and thus endocrine commitment (). Of particular interest would be if cell-cycle phase-length variations were connected to the ability to produce α vs. β vs. δ cells. We propose that the Neurog3TA.LO progenitor state is where we need to understand how cell-intrinsic mechanisms such as cell-cycle length variation regulate Neurog3 levels and balance endocrine-lineage commitment with maintenance of the lineage-primed endocrine-biased mitotic pool.
Figure 1. Endocrine-biasing and cell-cycle regulation - a model for lineage-priming in pancreas organogenesis. Normally, low-level Neurog3 activation in Sox9+ progenitors defines an actively cycling endocrine-biased progenitor state (orange). Neurog3 levels are postulated to oscillate over the cell cycle (inset), with G1 lengthening promoting Neurog3 accumulation, and its targeting of genes that cause endocrine commitment. While increasing (by Neurog3 overexpression) or eliminating Neurog3 expression (Neurog3 null) enforces or blocks endocrine-lineage allocation, respectively, greatly reducing Neurog3 (strong hypomorph) perpetuates the mitotic state, and allows drift from this metastable state to other lineages. Figure courtesy of Jean-Philippe Cartailler.
Current efforts generating relatively high-functioning β cells from human embryonic stem cells (hESC) use complex multifactorial protocols in 2-dimensional culture systems. Going beyond producing pure β cells, aiming at functional multilineage islet organoids, however, will likely depend on creating in vitro environments that better reflect the in vivo endocrine-birthing niche, and thus also respond more appropriately to the intrinsic and extrinsic factors driving endocrine-lineage specification. Previous studies of mouse pancreas development suggest a strong reciprocal connection between the number of Neurog3TA.LO progenitors and the architecture and integrity of the epithelial “plexus state” defined as the endocrine-birthing niche.Citation1,4,5 To date, production of multi-lineage islet-like clusters from hESC-derived pancreas progenitors has only been reported following implantation and maturation under the kidney capsule in vivo, allowing the transplant to adopt a complex 3-D structure.Citation6 Recent studies indicate that Neurog3 is just as critical to human pancreatic endocrine development as in mice, with true Neurog3 null mutations leading to neonatal diabetes and a block in β-cell differentiation from hESC.Citation7 Therefore, it seems plausible that knowledge of how the cell cycle connects to the behavior and lineage potential of endocrine-biased Neurog3TA.LO progenitors, during both mouse and human endocrine-lineage specification, will be key to understanding the engineering principles that guide the formation of, and endocrine specification from, a 3-D epithelial plexus state endocrine-birth niche. Focusing on generating the correct 3-D endocrine-birthing niche via hESC directed differentiation could activate the appropriate morphogenic programs to generate nascent islet clusters that resemble their in vivo counterparts.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Bechard ME, Bankaitis ED, Hipkens SB, Ustione A, Piston DW, Yang Y-P, Magnuson MA, Wright CVE. Precommitment low-level Neurog3 expression defines a long-lived mitotic endocrine-biased progenitor pool that drives production of endocrine-committed cells. Genes Dev 2016; 30:1852-65; PMID:27585590; http://dx.doi.org/10.1101/gad.284729.116
- Wang S, Yan J, Anderson DA, Xu Y, Kanal MC, Cao Z, Wright CVE, Gu G. Neurog3 gene dosage regulates allocation of endocrine and exocrine cell fates in the developing mouse pancreas. Dev Biol 2010; 339:26-37; PMID:20025861; http://dx.doi.org/10.1016/j.ydbio.2009.12.009
- Hardwick LJA, Philpott A. Nervous decision-making: to divide or differentiate. Trends Genet 2014; 30:254-61; PMID:24791612; http://dx.doi.org/10.1016/j.tig.2014.04.001
- Bankaitis ED, Bechard ME, Wright CVE. Feedback control of growth, differentiation, and morphogenesis of pancreatic endocrine progenitors in an epithelial plexus niche. Genes Dev 2015; 29:2203-16; PMID:26494792; http://dx.doi.org/10.1101/gad.267914.115
- Kesavan G, Sand FW, Greiner TU, Johansson JK, Kobberup S, Wu X, Brakebusch C, Semb H. Cdc42-Mediated tubulogenesis controls cell specification. Cell 2009; 139:791-801; PMID:19914171; http://dx.doi.org/10.1016/j.cell.2009.08.049
- Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, Gauvin R, Narayan K, Karanu F, O'Neil JJ, et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes 2012; 61:2016-29; PMID:22740171; http://dx.doi.org/10.2337/db11-1711
- McGrath PS, Watson CL, Ingram C, Helmrath MA, Wells JM. The Basic Helix-Loop-Helix transcription factor NEUROG3 is required for development of the human endocrine pancreas. Diabetes 2015; 64:2497-505; PMID:25650326; http://dx.doi.org/10.2337/db14-1412
