The cell surface of virtually all cell types harbors a great variety of glycosylphosphatidylinositol (GPI)-anchored proteins with diverse biological functions. GPI-anchoring is a complex post-translational modification that anchors proteins in the outer leaflet of the plasma membrane.Citation1 GPI-anchoring may allow for lateral movement and some structural flexibility of membrane proteins, but otherwise the biological function of GPI anchors has long remained a mystery. Certain GPI-anchored proteins are involved in signal transduction but, by their very nature, they lack intrinsic signaling capacity. Instead, they must rely on direct communication with nearby partners. For example, by binding growth factors or/and through lateral interactions with transmembrane receptors, GPI-anchored proteins can function as co-receptors to modulate signaling pathways. Some GPI-anchored proteins are released from their anchor and detected in body fluids, implying involvement of putative GPI-specific phospholipases. But the mechanism and physiological significance, if any, of GPI-anchor cleavage has long remained unexplored. A unique GPI-specific phospholipase D (GPI-PLD) has often been assumed to release GPI-anchored proteins, but this enzyme does not function on native membranes.
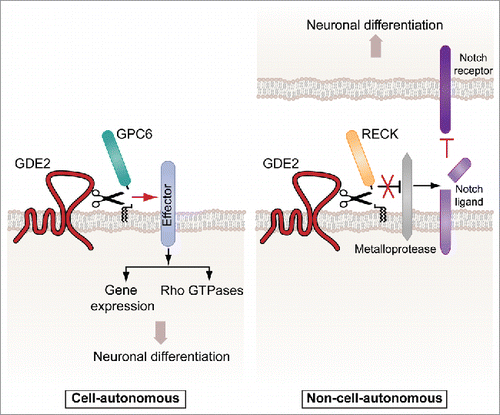
Recent studies have advanced the field by showing that selective cleavage of GPI-anchored proteins by an ecto-phosphodiesterase, termed GDE2, leads to neuronal differentiation.Citation2,3 GDE2 (encoded by GDPD5) is a member of the glycerophosphodiesterase familyCitation4 and characterized by 6 transmembrane domains and a large catalytic ectodomain. GDE2 was originally identified as a retinoic acid-inducible gene that promotes spinal motor neuron differentiation by inhibiting Notch signaling in adjacent neuronal progenitors.Citation5 Mechanistically, the Notch transmembrane receptor is activated by binding membrane-bound ligands on contacting cells. GDE2 was reported to cleave and release a GPI-anchored Notch regulator, termed RECK, that normally inhibits protease-dependent shedding of a Notch ligand.Citation2 By releasing RECK, GDE2 inactivates the ligand-Notch signaling axis and thereby promotes differentiation in contacting neuronal cells (). Thus, in the developing spinal cord, GDE2 promotes neurogenesis in a non-cell-autonomous manner, requiring cell-cell contact.Citation2
Figure 1. GDE2 cleaves GPI-anchored substrates to promote neuronal differentiation in distinct manners. Left panel, GDE2-induced cleavage of glypican-6 (GPC6) signals differentiation of neuroblastoma cells, involving both Rho GTPase-driven cytoskeletal remodeling and new gene transcription.3 Right panel, RECK cleavage is a trigger to downregulate Notch signaling in adjacent neural progenitors.2 It is unclear if GDE2 acts as a PLC or PLD.
In our laboratory, GDE2 was identified and tested as a candidate ecto-phospholipase D that might produce the lipid mediator lysophosphatidic acid (LPA) (unpublished work). LPA has long been known to induce growth cone collapse and neurite retraction in immature neuronal cells through activation of RhoA,Citation6 one of the master small-GTPases that regulate the actin cytoskeleton and cellular phenotype.Citation7 Unexpectedly, however, GDE2 was found to oppose rather than promote LPA-induced neurite retraction and to induce phenotypic differentiation of neuroblastoma cells. In this case, GDE2 acted in a cell-autonomous manner, not requiring cell-cell contact.Citation3 Through overexpression and knockdown studies, GDE2 was found to initiate a neuronal differentiation program that involves a change in the Rac-RhoA activity balance leading to cell spreading, neurite outgrowth, inhibition of RhoA-driven neurite retraction and reduced cell motility in both mouse and human neuroblastoma cells. In addition, GDE2 was found to regulate multiple differentiation-associated genes, including the transcription factor NEUROD1, a key player in nervous system development.Citation3
We discovered that GDE2 promotes neuronal differentiation through GPI-anchor cleavage of the heparan sulfate proteoglycan glypican-6 (GPC6) (). While little is known about the normal biological function of GPC6, heparan sulfate proteoglycans such as the glypicans play key roles in neurodevelopment, being capable of recruiting and distributing growth factors at the cell surface, dictated by the structure of their glycosaminoglycan chains. Glypican release may thus result in growth factor redistribution with either stimulatory or inhibitory effects on receptor signaling pathways. As we discussed (ref. Citation3), glypicans may also serve as membrane-tethered ligands in their own right by directly interacting with transmembrane receptors, such as the type-II receptor tyrosine phosphatases. In the latter scenario, GDE2-induced GPC6 release could trigger phosphotyrosine-based signaling events to impact neuronal differentiation, an attractive hypothesis that warrants further study.
The new findings are of clinical relevance, since elevated GDPD5 expression was strongly associated with improved survival in neuroblastoma,Citation3 a childhood cancer of the developing sympathetic nervous system characterized by impaired neuronal differentiation. Our study thus reveals GDE2 as a cell-autonomous inducer of neuronal differentiation through GPC6 release, and defines GDE2 as a marker of clinical outcome. A better mechanistic understanding of the GDE2-GPC6 signaling axis in neuroblastoma may hopefully suggest new therapeutic strategies for this often fatal malignancy.
Since GDE2 hydrolyzes distinct substrates to promote neural differentiation, it will be important to explore the substrate selectivity of GDE2 in further detail and also that of its close relative GDE3 (ref.Citation4). In addition to the above substrates, multiple GPI-anchored proteins are known to participate in the development and proper functioning of the nervous system. These include GPI-anchored neural cell adhesion molecules (N-CAM, Contactin), neurotrophic receptors (CNTFR, GDNFR) as well as the neurodegeneration-associated prion protein (PrP). Future studies should address whether (some of) these proteins can serve as GDE substrates, and how their cleavage and release may impact neuronal functions in health and disease. It will also be important to understand how GDE2 expression and activity are regulated by either cell-intrinsic or cell-extrinsic cues.
While many questions remain to be addressed in the emerging GDE field of research, the new findings highlight GPI-anchor hydrolysis by GDE2 as a cell-intrinsic signaling mechanism to promote neuronal differentiation and, in a broader context, help to clarify the raison d'être of the once mysterious GPI anchors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Paulick MG, Bertozzi CR. The glycosylphosphatidylinositol anchor: a complex membrane-anchoring structure for proteins. Biochemistry 2008; 47:6991-7000; PMID:18557633; http://dx.doi.org/10.1021/bi8006324
- Park S, Lee C, Sabharwal P, Zhang M, Meyers CL, Sockanathan S. GDE2 promotes neurogenesis by glycosylphosphatidylinositol-anchor cleavage of RECK. Science 2013; 339:324-8; PMID:23329048; http://dx.doi.org/10.1126/science.1231921
- Matas-Rico E, van Veen M, Leyton-Puig D, van den Berg J, Koster J, Kedziora KM, Molenaar B, Weerts MJ, de Rink I, Medema RH, et al. Glycerophosphodiesterase GDE2 Promotes Neuroblastoma Differentiation through Glypican Release and Is a Marker of Clinical Outcome. Cancer Cell 2016; 30:548-62; PMID:27693046; http://dx.doi.org/10.1016/j.ccell.2016.11.008
- Corda D, Mosca MG, Ohshima N, Grauso L, Yanaka N, Mariggio S. The emerging physiological roles of the glycerophosphodiesterase family. FEBS J 2014; 281:998-1016; PMID:24373430; http://dx.doi.org/10.1111/febs.12699
- Sabharwal P, Lee C, Park S, Rao M, Sockanathan S. GDE2 regulates subtype-specific motor neuron generation through inhibition of Notch signaling. Neuron 2011; 71:1058-70; PMID:21943603; http://dx.doi.org/10.1016/j.neuron.2011.07.028
- Jalink K, van Corven EJ, Hengeveld T, Morii N, Narumiya S, Moolenaar WH. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol 1994; 126:801-10; PMID:8045941
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 2002; 420:629-35; PMID:12478284; http://dx.doi.org/10.1038/nature01148
