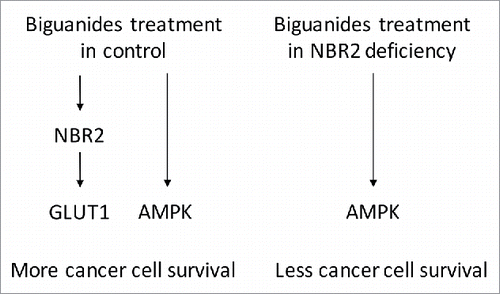
Biguanides, including metformin and phenformin, are inhibitors of mitochondrial respiratory chain complex I, which are shown to be involved in cancer prevention and treatment.Citation1 Biguanides induced energy stress in cancer cells may restrict tumor growth through multiple mechanisms. Notably, AMP activated protein kinase (AMPK), a crucial sensor of cellular energy status, is activated upon biguanides treatment.Citation2 Activation of AMPK is reported to inhibit cancer development in many cancers.Citation3 Paradoxically, the energy stress induced AMPK activation allows for the survival of cancer cells in response to metabolic stressed conditions.Citation4 Similarly, the AMPK activation in mediating the sensitivity of cancer cells to biguanides is also controversial. While some studies showed the important role of AMPK activation in mediating cancer cell killing by biguanides, it is also reported that cancer cells with defective AMPK pathway, like LKB1 deficiency, sensitizes tumors to biguanides treatment.Citation5 The discrepancy may come from distinct cell models or different approaches used in these studies to manipulate AMPK through either direct AMPK targeting or indirectly targeting its related pathways. Thus, the exact role of AMPK activation in regulating the response to biguanides treatment remains unclear and complicated in various scenarios. In this regard, the status of AMPK activation may not be an ideal marker for stratifying patients for the treatment of biguanides. The study by Liu et al. in the 15(24) issue suggested that non-protein coding RNA NBR2 (neighbor of BRCA1 gene 2) regulates the sensitivity of cancer cells to biguanides ().Citation6 The authors previously showed that NBR2 is induced under glucose deprivation and interacts with AMPK to promote AMPK kinase activity.Citation7 Although phenformin treatment also induced NBR2 expression, NBR2 deficiency does not affect phenformin induced AMPK activation. This suggests that metabolic stress and phenformin act through distinct mechanisms to promote AMPK activation. Notably, NBR2 is required for phenformin induced expression of glucose transporter GLUT1, and GLUT1 restoration rescues the cell death in NBR2 deficient cancer cells. This study not only highlights the importance of the NBR2-GLUT1 axis in the cellular homeostasis under the stress of phenformin treatment, but also suggests that NBR2 and GLUT1 could be potential markers for phenformin sensitivity in the clinical applications.
How GLUT1 is regulated by the non-coding RNA NBR2 remains to be answered in the future studies. Since mRNA level of GLUT1 is reduced upon NBR2 knockdown, it is possible that the transcription of GLUT1 is regulated by NBR2. More importantly, it is also puzzling why glucose deprivation triggered AMPK activation is NBR2 dependent, but phenformin treatment induced AMPK activation is NBR2 independent. As glucose starvation is known to affect many pathways other than energy restriction, it is possible that other functions of glucose are required for the interaction between NBR2 and AMPK. Conversely, phenformin may also execute a unique mechanism toward AMPK activation, which differs from that by glucose deprivation. This work by Liu et al also suggests a possible AMPK independent effect of phenformin, and future studies comparing AMPK dependent and independent effects of phenformin would be important to dissect the mechanisms of biguanides in cancer cell killing. As NBR2 is induced by glucose deprivation, it would be also interesting to assess NBR2-GLUT1 axis in the cancer cell survival upon glucose deprivation. Together, the study by Liu et al. revealing that NBR2 mediated expression of GLUT1 protects cancer cells from phenformin treatment offers novel markers to predict the response to biguanides.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Pernicova I, Korbonits M. Metformin–mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol 2014; 10:143-56; PMID:24393785; http://dx.doi.org/10.1038/nrendo.2013.256
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001; 108:1167-74; PMID:11602624; http://dx.doi.org/10.1172/JCI13505
- Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer 2009; 9:563-75; PMID:19629071; http://dx.doi.org/10.1038/nrc2676
- Li W, Saud SM, Young MR, Chen G, Hua B. Targeting AMPK for cancer prevention and treatment. Oncotarget 2015; 6:7365-78; PMID:25812084; http://dx.doi.org/10.18632/oncotarget.3629
- Shackelford DB, Abt E, Gerken L, Vasquez DS, Seki A, Leblanc M, Wei L, Fishbein MC, Czernin J, Mischel PS, et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell 2013; 23:143-58; PMID:23352126; http://dx.doi.org/10.1016/j.ccr.2012.12.008
- Liu X, Gan B. lncRNA NBR2 modulates cancer cell sensitivity to phenformin through GLUT1. Cell Cycle 2016; 15(24):3471-3481; PMID:27792451; http://dx.doi.org/10.1080/15384101.2016.1249545
- Liu X, Xiao ZD, Han L, Zhang J, Lee SW, Wang W, Lee H, Zhuang L, Chen J, Lin HK, et al. LncRNA NBR2 engages a metabolic checkpoint by regulating AMPK under energy stress. Nat Cell Biol 2016; 18:431-42; PMID:26999735; http://dx.doi.org/10.1038/ncb3328