ABSTRACT
The tumor microenvironment is critical for metastasis to occur. Subcutaneous xenografts of tumors in immunodeficient mice are usually encapsulated and rarely metastasize as opposed to orthotopic tumors which metastasize if the original tumor was metastatic. In the present report, we were able to reconstitute a metastatic tumor microenvironment by subcutaneously co-transplanting a human cervical cancer cell line and human cervical cancer-associated fibroblasts (CAFs), in athymic mice, which resulted in lymph node metastasis in 40% of the animals. In contrast, no metastasis occurred from the cervical cancer without CAFs. These results suggest that CAFs can overcome an anti-metastatic tumor environment and are a potential target to prevent metastasis.
Introduction
The tumor microenvironment (TME) is essential for cancer progression.Citation1,2 For example, the majority of human solid tumors do not metastasize when grown subcutaneously in immunocompromised mice because they are in a heterotopic TME. There are numerous discrepancies between the invasive and metastatic behavior of tumors in the patient compared with their benign behavior as s.c.-transplanted xenografts in nude mice.Citation3 In contrast, orthotopic (literally: correct surface) implantation of intact tumor tissue can lead to metastasis that mimics that is seen in patients, since the orthotopic tumor is in the proper TME.Citation4
The first use of athymic nude mice for human tumor growth, was Rygaard and PovlsenCitation5 in 1969. A metastatic colon cancer from a 74-year-old patient was transplanted subcutaneously (s.c.), in nude mice which grew as a well-differentiated adenocarcinoma similar to that from the donor patient. The tumors grew as local nodules and did not metastasize, over 7 y and 76 passages.Citation5
Wang and Sordat et al.Citation6 in 1982 were among the first to implant human tumors orthotopically in nude mice. Metastases as well as local tumor growth occurred but not when the cells were planted s.c. This seminal study indicated that tumor implantation at the orthotopic site allows metastasis to occur.Citation6
Orthotopically-implanted intact tumor tissue resulted in greater metastasis compared with orthotopically-implanted cell suspensions.Citation7 We also demonstrated that cells from the TME were necessary for metastasis.Citation8
In the present report, we reconstituted a metastatic-resistant tumor microenvironment at the subcutaneous site of nude mice by co-transplanting a human cervical cancer cell line and human cancer-associated fibroblasts (CAFs), derived from a patient cervical cancer.
Results and discussion
CAFs were isolated from patient cervical cancer tissue and co-transplanted subcutaneously with the human cervical cancer cell line ME180 expressing GFP, (ME180/GFP) in 10 nude mice. After 8 to 10 weeks, the mice were killed and metastasis was imaged by GFP fluorescence. In the nude mice, which were transplanted with ME180/GFP only, no animal developed metastasis. In contrast, in 4 of 10 nude mice which were co-transplanted with ME180/GFP and CAFs, lymph node metastasis were observed (). The metastatic sites included inguinal and subcutaneous lymph nodes (, 2, ).
Figure 1. (A) Inguinal lymph node metastasis. The white arrow indicates an inguinal lymph node metastasis (left). Green fluorescent protein (GFP) expression visualized the metastasis (right). ME180-GFP cells (1.5 × 106), co-transplanted s.c. with CAFs (0.6 × 106), formed inguinal lymph node metastasis after 8 weeks. GFP-labeled ME180 facilitated localization of the metastasis. White bar = 10 mm. (B) H&E and fluorescence images of lymph node metastasis. H&E staining (left). Fluorescence immunostaining of the inguinal-lymph-node metastasis (right). Normal lymph-node structure can be seen in the deep purple area that is darkly-stained in the H&E section (left). ME180/GFP cells were labeled with GFP and lymphocytes were immunoreacted with an anti-LCA antibody labeled with Alexa594 (red) (right). Metastatic ME180 cells occupied a large area of the lymph node. DAPI, with blue fluorescence, labeled the cell nuclei (right). Bars = 1 mm. (C) White arrows indicate the lymph node metastasis.
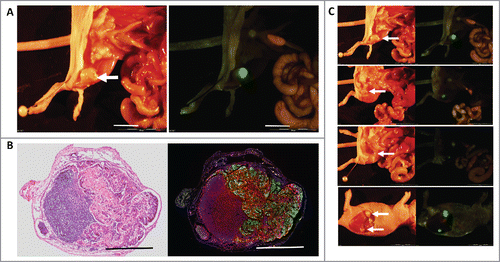
Table 1A. CAFs can enable metastasis in an anti-metastatic subcutaneous tumor microenvironment.
Table 1B.
GFP expression by ME180 cells enabled facile visualization of the lymph node metastasis (). Hematoxylin and eosin (H&E) staining and anti-LCA (Rabbit polyclonal, Abcam) immunostaining confirmed the lymph-node metastasis. The lymph-node metastasis comprised GFP fluorescent ME180/GFP cells and Alexa594-labeled red-fluorescent lymphocytes ().
These results suggest that CAFs can overcome an anti-metastatic tumor environment and also that they can be used as a target to prevent metastasis. Cairns and HillCitation9 showed that ME180 transplanted to the uterine cervix of immunodeficient mice metastasized initially to local lymph nodes and later to lung, a pattern consistent with the clinical course of uterine cervical cancer.
We previously showed that heparin-binding epidermal growth factor (HB-EGF) is produced by uterine cervical-cancer CAFs. Co-culturing the ME180 cell with cervical-cancer CAFs in vitro indicated that CAFs enhanced the proliferation of ME180 cells. Platelet–derived growth factor (PDGF) produced by ME180 cells enhanced CAF HB-EGF expression, suggesting that these growth factors contribute to the reciprocal interaction of cancer cells and CAFs.Citation10 Wilson et al.Citation17 reported tumor formation from only 10 melanoma cells, if they were injected together with cultured human fibroblasts in nude mice. Kojima et al.Citation18 showed that subperitoneal fibroblasts stimulated colon-cancer cells to metastasize when they were co-transplanted subcutaneously in SCID mice, further demonstrating the role of different types of fibroblasts in promoting metastasis.
Previously-developed concepts and strategies of highly selective tumor targeting can take advantage of molecular and cellular targeting of tumors, including stroma such as described in the present report.Citation11-16
Materials and methods
Cell lines
ME180 cells were was stably transfected with GFP as previously-described.Citation10 ME180/GFP (1.5 × 106 cells) and CAFs (0.6 × 106 cells) were co-transplanted s.c. in 10 nude mice. ME180/GFP cells (1.5 × 106 cells) alone were transplanted in 10 nude mice, as a control. After 8 to 10 weeks, mice were killed and tumor size and metastasis status were analyzed by imaging of GFP green fluorescence.
Animal experiments
Athymic (nu/nu) nude mice (CLEA, Kawasaki, Japan) were maintained in a barrier facility under HEPA filtration and fed with autoclaved laboratory rodent diet. All animal studies were conducted in accordance with the principles and procedures outlined in the National Institute of Health Guide for the Care and Use of Laboratory Animal.
Immunohistochemistry
Frozen sections of the specimens were dissected and fixation was performed with 4% paraformaldehyde for 30 min. Section was stained with hematoxylin and eosin. Consecutive fixed section was used for immunohistochemistry. Anti-LCA antibody (Rabbit polyclonal, Abcam: ab10558, Tokyo, Japan) was reacted for 1 hour at 4°C and washed with Tris-buffered saline for 5 min 3 times. Then Alexa594 Goat anti-rabbit IgG(H+L) (Thermo Fisher Scientific, Kanagawa, Japan) was reacted for 30 min at 4°C and washed with Tris-buffered saline for 5 min 3 times. SlowFade Gold Antibody Mountant with DAPI (Thermo Fisher Scientific, Kanagawa, Japan) was used for mounting.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012; 21:309-22; PMID: 22439926; http://dx.doi.org/10.1016/j.ccr.2012.02.022
- Hoffman RM, Bouvet M. Imaging the microenvironment of pancreatic cancer patient-derived orthotopic xenografts (PDOX) growing in transgenic nude mice expressing GFP, RFP, or CFP. Cancer Lett 2016; 380:349-55; PMID:26742463; http://dx.doi.org/10.1016/j.canlet.2015.12.021
- Garralda E, Paz K, López-Casas PP, Jones S, Katz A, Kann LM, López-Rios F, Sarno F, Al-Shahrour F, Vasquez D, et al. Integrated next-generation sequencing and avatar mouse models for personalized cancer treatment. Clin Cancer Res 2014; 20:2476-84; PMID: 24634382; http://dx.doi.org/10.1158/1078-0432.CCR-13-3047
- Hoffman RM. Patient-derived orthotopic xenografts: better mimic of metastasis than subcutaneous xenografts. Nat Rev Cancer 2015; 15:451-2; PMID: 26422835; http://dx.doi.org/10.1038/nrc3972
- Rygaard J, Povlsen CO. Heterotransplantation of a human malignant tumour to “Nude” mice. Acta Pathol Microbiol Scand 1969; 77:758-60; PMID: 5383844; http://dx.doi.org/10.1111/j.1699-0463.1969.tb04520.x
- Wang WR, Sordat B, Piguet D, Sordat M. Human Colon Tumors in Nude Mice: Implantation Site and Expression of the Invasive Phenotype 1. In: Immune-Deficient Animals-4th International Workshop on Immune-Deficient Animals in Experimental Research (ed. Sordat, B.) (Karger) 239-245 (1982).
- Furukawa T, Fu X, Kubota T, Watanabe M, Kitajima M, Hoffman RM. Nude mouse metastatic models of human stomach cancer constructed using orthotopic implantation of histologically intact tissue. Cancer Res 1993; 53:1204-8. PMID: 8439965
- Bouvet M, Tsuji K, Yang M, Jiang P, Moossa AR, Hoffman RM. In vivo color-coded imaging of the interaction of colon cancer cells and splenocytes in the formation of liver metastases. Cancer Res 2006; 66:11293-7; PMID: 17145875; http://dx.doi.org/10.1158/0008-5472.CAN-06-2662
- Cairns RA, Hill RP. A fluorescent orthotopic model of metastatic cervical carcinoma. Clin Exp Metastasis 2004; 21:275-81; PMID: 15387378; http://dx.doi.org/10.1023/B:CLIN.0000037729.75981.9e
- Murata T, Mizushima H, Chinen I, Moribe H, Yagi S, Hoffman RM, Kimura T, Yoshino K, Ueda Y, Enomoto T, Mekada E. HB-EGF and PDGF mediate reciprocal interactions of carcinoma cells with cancer-associated fibroblasts to support progression of uterine cervical cancers. Cancer Res 2011; 71:6633-42; PMID: 22009535; http://dx.doi.org/10.1158/0008-5472.CAN-11-0034
- Blagosklonny MV. Matching targets for selective cancer therapy. Drug Discov Today 2003; 8:1104-7; PMID: 14678733; http://dx.doi.org/10.1016/S1359-6446(03)02806-X
- Blagosklonny MV. Teratogens as anti-cancer drugs. Cell Cycle 2005;4:1518-21; PMID: 16258270; http://dx.doi.org/10.4161/cc.4.11.2208
- Blagosklonny MV. Treatment with inhibitors of caspases, that are substrates of drug ransporters, selectively permits chemotherapy-induced apoptosis in multidrug-resistant cells but protects normal cells. Leukemia 2001; 15:936-41; PMID: 11417480; http://dx.doi.org/10.1038/sj.leu.2402127
- Blagosklonny MV. Target for cancer therapy: proliferating cells or stem cells. Leukemia 2006;20:385-91; PMID: 16357832; http://dx.doi.org/10.1038/sj.leu.2404075
- Apontes P, Leontieva OV, Demidenko ZN, Li F, Blagosklonny MV. Exploring long-term protection of normal human fibroblasts and epithelial cells from chemotherapy in cell culture. Oncotarget 2011;2:222-33; PMID: 21447859; http://dx.doi.org/10.18632/oncotarget.248
- Blagosklonny MV. Tissue-selective therapy of cancer. Br J Cancer 2003;89:1147-51; PMID: 14520435; http://dx.doi.org/10.1038/sj.bjc.6601256
- Wilson EL, Gartner M, Campbell JAH, Dowdle EB. Growth and behaviors of human melanomas in nude mice: effects of fibroblasts. In Immuno-Deficient Animals. 4th Int. Workshop on Immune-Deficient Animals in Exp. Res., Chexbres 1982; 357-361 (Karger, Basel, 1984).
- Kojima M, Higuchi Y, Yokota M, Ishii G, Saito N, Aoyagi K, Sasaki H, Ochiai A. Human subperitoneal fibroblast and cancer cell interaction creates microenvironment that enhances tumor progression and metastasis. PLoS One 2014; 9(2):e88018.
