Collective cell migration requires groups of cells to establish a hierarchy of cellular identities that coordinates their movement.Citation1 During new blood vessel branching by angiogenesis, this takes the form of a single endothelial “tip cell” that leads nascent vessels and is followed by endothelial “stalk cells”.Citation2 Tip cells are highly motile and navigate sprouting vessels by sensing chemotactic stimuli such as vascular endothelial growth factor (VEGF). In contrast, stalk cells are less motile and form the trunk of developing vessels. As new vessels grow, migrating endothelial cells must also proliferate,Citation3,4 which presents tip cells with a problem, as each daughter cell must acquire a different migratory profile dependent on their resultant position. The more distal daughter takes on tip cell identity, while the more proximal daughter becomes the trailing stalk cell. Rapid re-establishment of this leader-follower hierarchy is critical to ensuring collective cell migration is not disrupted during proliferative growth, but how this occurs is unclear.
In our recent publication,Citation5 we used live imaging in zebrafish embryos to address this problem and revealed that dividing tip cells use a form of asymmetric cell division to promptly generate daughters with distinct tip or stalk cell identities. After tip cell division, the distal daughter immediately acquired tip cell-like motility whereas the proximal daughter displayed motility near identical to a stalk cell. Mathematical modeling suggested that this seamless re-establishment of the tip-stalk hierarchy might be driven by asymmetries in daughter cell size, which would differentially partition Vegf signaling components between daughter cells. Supporting in-silico observations, live imaging revealed that dividing tip cells generated a distal daughter at the tip cell position that was on average 1.8–1.9 times larger that the more proximal daughter at the stalk cell position. Moreover, the motility of each daughter cell after division was positively correlated with their size, suggesting that asymmetries in cell size underpinned post-mitotic differences in daughter cell behavior. Consistent with the model, we confirmed that the larger distal daughter of tip cell division inherited a greater proportion of the Vegf signaling machinery and displayed higher levels of Vegf signaling, establishing it as the leading tip cell. Importantly, asymmetries in Vegf signaling following division were essential for normal vessel formation, as in the absence of differential Vegfr activity the tip-stalk arrangement of daughters was disrupted and cells display symmetric motilities. Hence, post-mitotic asymmetries in cell size and signaling offers a simple way of introducing heterogeneity, which re-establishes the tip-stalk hierarchy and maintains collective movement during proliferative tissue growth.
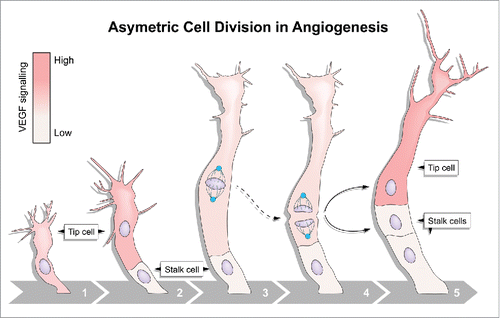
But what are the molecular mechanisms determining asymmetric cell division during angiogenesis? Some key insights can be gleaned from examples of asymmetric divisions in other systems that also generate daughter cells of distinct sizes, for example in Drosophila neuroblasts and C. elegans zygotes. In these systems, asymmetric positioning of the mitotic spindle dictates the site of cleavage, which in turn controls resulting daughter cell size.Citation6 Similar to these other systems, during endothelial tip cell division in zebrafish embryos the mitotic spindle was displaced toward the proximal pole before anaphase. Positioning of the plane of division away from the volumetric center of the cell resulted in 2 daughter cells with clearly unequal dimensions (). Hence, asymmetric positioning of the mitotic spindle likely underpins post-mitotic asymmetries in cell size and Vegf signaling.
Figure 1. Asymmetric endothelial cell division. Highly motile endothelial tip cells sprout from parental vessels (1) and lead stalk cells (2). Upon tip cell division, the mitotic spindle (3) is displaced to the proximal pole of the cell (4) before anaphase. This introduces cell size asymmetry and generates daughter cells with distinct Vegf signaling levels and behaviors (5).
Currently the mechanisms via which the spindle of dividing endothelial cells is asymmetrically positioned are unknown. However, in most cells, spindle positioning is regulated by the attachment of astral microtubules to the cell cortex, generating decisive pulling forces. Consequently, unequal cell sizes can be the result of differential cortical forces derived from asymmetrically distributed motor proteins.Citation6 A canonical set of proteins is known to generate the membrane associated pulling force that act upon mitotic spindles. Partner of Inscuteable (Pins) (LGN in Drosophila and GPR1/2 in C.elegans), anchored at the membrane by Gαi, binds to NuMA (Mud in Drosophila and LIN-5 in C.elegans), which in turn binds to the dynein/dynactin complex, this then pulls on the plus ends of astral microtubules.Citation7 Asymmetric enrichment of this complex can cause the spindle to be pulled toward one size of the cell and generates daughter cell size asymmetry. However, it remains to be seen whether such asymmetries in the spindle orienting machinery are involved in positioning the mitotic spindle of angiogenic endothelial cells.
In conclusion, asymmetric division of endothelial cells seamlessly integrates cell proliferation with maintained collective cell migration and, as such, acts as a driving force that guarantees correct blood vessel morphogenesis and growth. Considering the key role collective cell migration plays in the development and remodelling of many tissues,Citation1 it will be key to determine whether a similar role for asymmetric divisions is conserved in other cell systems.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Mayor R, Etienne-Manneville S. The front and rear of collective cell migration. Nat Rev Mol Cell Biol 2016; 17:97-109; PMID:26726037; http://dx.doi.org/10.1038/nrm.2015.14
- Herbert SP, Stainier DYR. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol 2011; 12:551-64; PMID:21860391; http://dx.doi.org/10.1038/nrm3176
- Zeng G, Taylor SM, McColm JR, Kappas NC, Kearney JB, Williams LH, Hartnett ME, Bautch VL. Orientation of endothelial cell division is regulated y VEGF signaling during blood vessel formation. Blood 2007; 109:1345-52; PMID:17068148; http://dx.doi.org/10.1182/blood-2006-07-037952
- Aydogan V, Lenard A, Denes AS, Sauteur L, Belting HG, Affolter M. Endothelial cell division in angiogenic sprouts of differing cellular architecture. Biol Open 2015; 4:1259-69; PMID:26369932; http://dx.doi.org/10.1242/bio.012740
- Costa G, Harrington KI, Lovegrove HE, Page DJ, Chakravartula S, Bentley K, Herbert SP. Asymmetric division coordinates collective cell migration in angiogenesis. Nat Cell Biol 2016; 18:1292-301; PMID:27870831; http://dx.doi.org/10.1038/ncb3443
- Kiyomitsu T. Mechanisms of daughter cell-size control during cell division. Trends Cell Biol 2015; 25:286-95; PMID:25548067; http://dx.doi.org/10.1016/j.tcb.2014.12.003
- Bergstralh DT, Haack T, St Johnston D. Epithelial polarity and spindle orientation: intersecting pathways. Philos Trans R Soc Lond B Biol Sci 2013; 368:20130291; PMID:24062590; http://dx.doi.org/10.1098/rstb.2013.0291
