ABSTRACT
Retinoic acid receptor γ (RARγ), a unique member of the nuclear receptor superfamily, plays an important role in the progression of several cancers such as hepatocellular carcinoma, esophageal cancer, and cholangiocarcinoma. However, little is known about the regulatory mechanism of the RARγ expression in colorectal cancer (CRC) progression. In the present study, we found that RARγ was frequently overexpressed in human CRC specimens and CRC cell lines, and it mainly resided in the cytoplasm in CRC specimens. Tissue microarrays showed that RARγ indicated vital clinical significance in CRC. RARγ knockdown neither affected CRC cell proliferation nor blocked the cell cycle of CRC cells. However, RARγ knockdown increased the sensitivity of CRC cells to chemotherapeutics through downregulation of multi-drug resistance 1(MDR1). Further studies suggested that RARγ knockdown resulted in downregulation of MDR1, in parallel with suppression of the Wnt/β-catenin pathway. Moreover, a significantly positive association between RARγ and MDR1 was demonstrated in CRC tissue microarrays. Collectively, these results suggested that overexpression of RARγ contributed to the multidrug chemoresistance of CRC cells, at least in part due to upregulation of MDR1 via activation of the Wnt/β-catenin pathway, indicating that RARγ might serve as a potential therapeutic target for chemoresistant CRC patients.
Introduction
Colorectal cancer (CRC) is the third malignant tumors with the third most common cause of cancer-related mortality around the world.Citation1 CRC is characterized by poor prognosis of early stage, early metastasis, remarkably high malignancy, easy to relapse of surgical removal and multidrug resistance (MDR).Citation2 Therefore, chemoradiotherapy offers the unique possibility of cure for the majority of CRC patients. However, radiation therapy and currently available chemotherapeutics have limited efficacy and can barely improve patient survival because of MDR.Citation3 Hence, there is imperative to have a better understanding of the molecular mechanism involved in CRC progression, which is important for development of novel therapeutic strategies for the treatment of CRC.
Retinoic acid receptor γ (RARγ) is a member of the nuclear receptor superfamily that is essential in embryonic development, maintenance of differentiated cellular phenotypes, metabolism, and cell death.Citation4 There are 3 RAR subtypes, α, β, and γ. Among them, RARγ plays unique and uncharacterized roles in many physiologic processes including carcinogenesis. RARγ is a critical physiologic and pharmacological regulator of the balance between haematopoietic stem cell self-renewal and differentiation.Citation5 It interacts with Wnt/β-catenin to regulate chondrocyte function and matrix turnover.Citation6 Retinoic acid (RA), a RARγ ligand, increases death of SH-SY5Y neuroblastoma cells and induces apoptosis of pancreatic cancer cells depending on RARγ,Citation7,8 indicating the suitability of targeting RARγ for cancer therapy. Consistently, the oncogenic potential of RARγ has been demonstrated. RARγ is overexpressed in multiple human cancers such as hepatocellular carcinoma, esophageal cancer, and cholangiocarcinoma and plays vital roles in promoting the progression of tumors through multiple signaling pathways including PI3K/Akt, NF-κB, and Wnt/β-catenin.Citation9-11 However, the expression profile of RARγ in CRC and its function remained unknown.
Multidrug resistance (MDR) is a major obstacle to develop effective therapeutic drugs for the treatment of cancers.Citation12 The typical mechanism contributing to MDR is mainly associated with reduced intracellular drug concentration and an increased drug efflux.Citation13 Upregulation of drug transporters results in an active efflux, thus inducing cancer cells to resist chemotherapeutic drugs.Citation14 ATP-binding cassette transporters such as Multidrug resistance 1 (MDR1/P-gp/ ABCB1),Citation15 have been implicated in the multidrug resistance of most human tumors including CRC and constitute an important cause of therapeutic failure.Citation12,16-18
In the present study we demonstrated that RARγ is frequently overexpressed in human CRC tissues and CRC cell lines when compared with their respective controls. RARγ knockdown increased the sensitivity of CRC cells to chemotherapeutic drugs. Moreover, RARγ knockdown suppressed MDR1 expression in CRC cells via the Wnt/β-catenin pathway. In addition, there is a significant correlation between RARγ level and the MDR1expression in CRC tissues. Our findings suggested that RARγ might serve as a new molecular target for chemoresistant CRC patients.
Materials and methods
Reagents
Methyl thiazolyl tetrazolium (MTT) and propidium iodide (PI) were purchased from Sigma (St. Louis, MO, USA). Vincristine sulfate (VCR) and 5-fluorouracil (5-FU) were purchased from Sigma-Aldrich (Indianapolis, IN, United States). Polyclonal antibodies against RARγ and MDR1 were from Abcam Ltd. (Cambridge, United Kingdom). Goat anti-rabbit and anti-mouse secondary antibodies conjugated to horseradish peroxidase and Lipofectamine 2000 from Invitrogen (Carlsbad, CA, USA). PVDF membrane was from Millipore (Billerica, MA, USA). The EliVision Plus kit was obtained from Maixin Bio (Fuzhou, China).
Patients and tumor specimens
Tumorous and their adjacent noncancerous colorectal cancer tissues were collected from patients who underwent surgery at the First Affiliated Hospital of Xiamen University. Written informed consent was obtained from each patient and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institute Research Ethics Committee of the First Affiliated Hospital of Xiamen University. Fresh surgical samples from colorectal cancer tissues were collected between 2011 and 2014.
Construction of lentivirus-based shRNA expression vector
PLL3.7 vector mediated shRNA was performed by constructing RARγ targeting sequence (5′-GCTACCAAGTGCATCATCA-3′) into pLL3.7-neo vector to generate pLL3.7-shRARγ. The sequence (5′-TTCTCCGAACGTGTCACGT-3′) was used for control shRNA. Lentivirus packaging and infection were performed as described previously.Citation19
Cell culture and stable cell lines
HT29 and HCoEpiC were cultured in RPMI1640. HCT116, RKO and SW480 were grown in Dulbecco's modified Eagle's medium (DMEM). Both medium was supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 U/mL streptomycin at 37°C in a 5% CO2 incubator. After lentivirus infection with pLL3.7-shRARγ or with pLL3.7-shCtrl, stably transfected HT29, HCT116 and RKO cells were established by treatment with geneticin (250 μg/mL) for 3 weeks.
Cell proliferation assay
Cell proliferation was analyzed by MTT assay, as described previously.Citation20 A total of 2 × 103 cells were seeded in each well of 96-well plates, and MTT was added to each well every 24 h. The plates were incubated for 4 h before addition of 100 μL DMSO. The absorbance was measured at 490 nm with a microplate reader (Model 680; Bio-Rad, Hercules, CA, USA)
Cell cycle analysis
ShRARγ-RKO and shCtrl-RKO cells were synchronized by serum starvation 24 h and then cultured by an exchange of 10% fetal bovine serum for 24 h. And then the cells were harvested, and fixed in 75% ethanol at 4°C for 30 min. Cells were incubated with RNase A at 37°C for 30 min, and then stained with propidium iodide for 30 min. Cell cycle was measured by flow cytometry. The data were analyzed with the ModFit 3.3 (Verity Software House, Topsham, ME, USA) software.
Quantitative PCR
Quantitative real-time PCR (qPCR) was performed using the ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control. The primers for PCR reactions are as follows: GAPDH, forward: GAAACTTCTGGATGCTGGTG, reverse: TACGTGAATGTGGCCTGT; RARγ, forward: AAAACTGTATCATCAACAAGG, reverse: CTTCACCTCTTTCTTCTTCTTG. MRP primers are as described previously.Citation21
Western blotting
Equal amounts of protein lysates were electrophoresed on 10% SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membrane. The membrane was then incubated with primary and secondary antibodies, and the signal was finally detected using an enhanced chemiluminescence (ECL) system.
IHC (Immunohistochemistry)
Paraffine-embedded human CRC tissue sections were immunostained with antibody against RARγ (1:200) or MDR1 (1:200) and detected with corresponding secondary antibody. The slides were stained with DAB for 5 min and then costained with hematoxylin to visualize nuclei.
Dual-luciferase reporter assays
The cells (1.0 × 104 cells / well) were seeded in 96-well plates for 24 h before transfection. Then cells in each well were cotransfected with 100 ng pTOPFlash or pFOPFlash reporter plasmid and 20 ng Renilla luciferase expression vector using Lipofectamine 2000 for another 24 h and subsequently with Wnt3a (50 ng/mL) for 6 h. The activities of both firefly luciferase and renilla luciferase were measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA). The firefly luciferase activity was normalized to renilla luciferase activity.
Statistical analysis
Statistical analysis was performed by using GraphPad Prism 6 (San Diego, CA, USA). Data are represented as means ± SD from at least 3 independent experiments. One-way analysis of variance (ANOVA) or t-test was used for comparison of 2 and more than 2 data sets. P value ≤ 0.05 was considered statistically significant.
Results
RARγ expression is frequently upregulated in human CRC tissues and cell lines
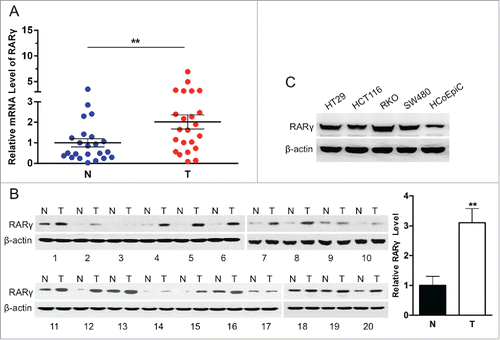
To evaluate the expression of RARγ in CRC, we performed qPCR and western blotting to assess the mRNA and protein level of RARγ in a set of 23 tumor and adjacent non-tumorous colorectal tissues and protein in paired 20. The mRNA and protein levels of RARγ were significantly upregulated in tumor specimens versus the surrounding non-tumorous colorectal tissues (). In addition, RARγ expression was also significantly increased in CRC cell lines such as HT29, HCT116, RKO, and SW480 compared with normal colonic epithelium HCoEpiC (). Therefore, overexpression of RARγ in CRC specimens as well as in CRC cell lines suggested that RARγ might play a role in CRC progression.
Figure 1. RARγ is overexpressed in human CRC tissues and cell lines. (A) Expression of RARγ mRNA in 23 human CRC samples and their surrounding non-tumorous tissue samples. (B) Expression of RARγ protein in 20 human CRC specimens and their adjacent specimens (Left). Quantitative analysis of RARγ protein in CRC specimens (Right). (C) Western blot analysis of expression of RARγ protein in normal colon epithelium cells and 4 CRC cell lines. **P < 0.01.
Clinical significance of RARγ in CRC
To further assess expression of RARγ, immunohistochemistry (IHC) was used to analyze RARγ expression on 90 paraffin-embedded CRC tissues and paired paracarcinoma tissues on tissue microarrays (TMAs). The IHC results showed that RARγ, predominantly present in the cell cytoplasm, was strongly stained in CRC tissues but weakly or not stained in paired adjacent colorectal tissues (). The staining intensity of RARγ protein was categorized as high (2+, 3+) and low (±, +) (), and the high-expression rate of RARγ protein in CRC tissues (73.3%) was much higher than that in adjacent non-tumorous colorectal tissues (20%) (P < 0.001) ().
Figure 2. Clinical significance of RARγ in CRC. (A) Immunohistochemical analysis of RARγ expression on tissue microarrays. T: tumor tissue, P: paracarcinoma tissue. (B) Representative staining intensity of tumors classified as 4 different grades. (C) Correlations of the RARγ protein expression with overall survival rate.
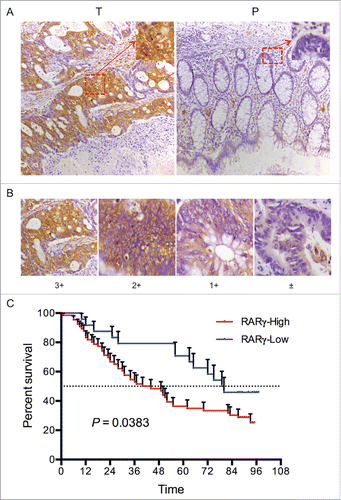
Table 1. Distribution of RARγ classifications on CRC and paracarcinoma tissues.
The clinical data in show that RARγ overexpression was significantly associated with pathological differentiation, T classification and clinical stages (P < 0.05), whereas no association was found with age, sex, tumor size, lymph node metastasis and distant metastasis (P > 0.05). Furthermore, the postoperative overall survival rate in patients with high RARγ expression was lower than that in patients with low RARγ expression (P < 0.05) (). These results indicated the clinical significance of RARγ in the diagnosis and prognosis of CRC patients.
Table 2. Relationships between RARγ and clinical features in CRC.
RARγ knockdown enhances the sensitivity of CRC cells to chemotherapeutics
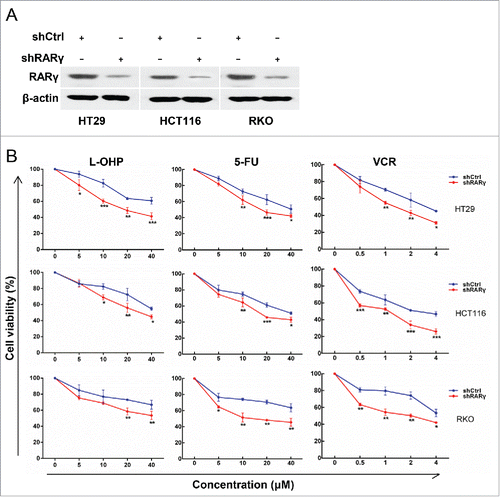
To investigate the role of RARα in the growth of CRC cells, MTT assay was performed and showed that stable knockdown of RARγ in these cells resulted in no significant changes in cell proliferation (supplementary )), while cell cycle analysis was obtained consistent results. The cell cycle of RARγ knockdown cells did not arrest in any phase of the cell cycle (supplementary ). These data demonstrated that RARγ knockdown did not affect CRC cell growth.
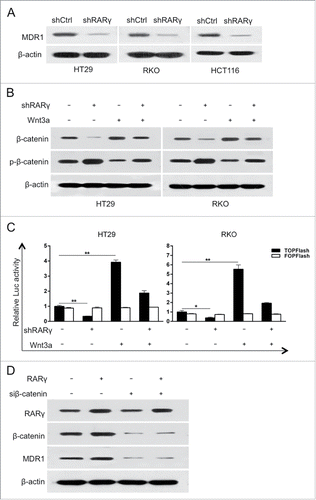
To establish stable RARγ knockdown cells for further study, RARγ-specific shRNA, but not control shRNA, efficiently reduced the levels of the endogenous RARγ protein in these cells (). We further explored whether RARγ modulates the resistance of CRC to chemotherapeutic drugs. RARγ knockdown enhanced the sensitivity of all 3 CRC cell lines to L-OHP, 5-FU and VCR (). These data indicated that RARγ plays an important role in cancer drug resistance.
RARγ upregulates MDR1 via Wnt/β-catenin pathway
To determine the mechanism by which RARγ knockdown sensitizes CRC cells to chemotherapeutics, the mRNA levels of the MDR associated proteins were assessed. RARγ knockdown resulted in the repression of MDR1, but not MRP1, MRP2 or MRP3 mRNA (supplementary ). Indeed, the protein expression of MDR1 decreased in RARγ knockdown CRC cells compared with the control cells (). In addition, western blotting analysis showed that the expression of β-catenin was decreased in RARγ knockdown CRC cells, along with phosphorylation of β-catenin at ser33/37 was increased. Moreover, Wnt3a-induced accumulation of β-catenin markedly decreased in RARγ knockdown CRC cells (). Wnt3a-induced activity of the TCF/LEF reporter gene was attenuated by RARγ knockdown (). Furthermore, RARγ-induced increase of MDR1 protein was abolished by siRNA against β-catenin (). Collectively, these data demonstrated that RARγ might serve as an essential coactivator for Wnt/β-catenin pathway activation and thus enhances its transcriptional activity for MDR1.
Figure 4. RARγ knockdown inhibited the Wnt/β-catenin pathway. (A) The protein levels of MDR1 in RARγ-knockdown CRC cells. (B) Protein levels of phosphorylated β-catenin (p-β-catenin) and β-catenin in CRC cells assessed by western blot. (C) T-cell factor / lymphoid enhancing factor (TCF/LEF)-responsive luciferase activity assessed by dual-luciferase reporter assay. A representative experiment of 3 independent experiments is shown. (D) Western blot analysis of the indicated proteins after transfection for 24 h.
RARγ associates with chemoresistance in CRC specimens
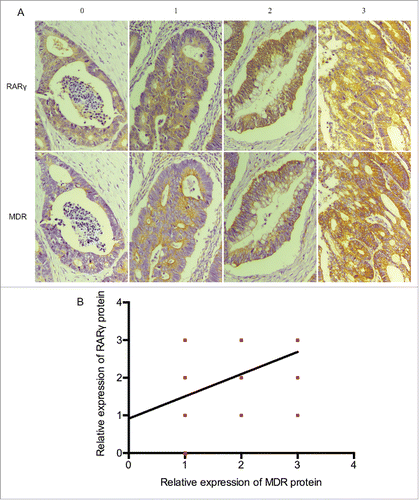
To determine whether RARγ-regulated MDR1 expression in CRC cell lines has clinical implications, tissue microarrays from 90 patients with CRC who had undergone resection were examined via immunostaining with RARγ and MDR1 antibodies. Tissue microarray analysis revealed a strong correlation between RARγ and MDR1 levels (). Statistical t-test indicated a very significant (P < 0.0001) positive correlation (r = 0.6148) (). These data suggested that RARγ might contribute to chemoresistance of CRC patients.
Discussion
Like other nuclear receptors, RARγ is known to play vital genomic roles through regulating the transcription of target genes by binding to DNA response elements.Citation4 Accumulating evidence, however, indicated that RARγ may also have extranuclear actions, which shows non-genomic regulatory effects. Aberrant RARγ expression was been considered as a common phenomenon in multiple human cancers. But, the expression profile and the role of RARγ in CRC remained elusive. In the present study, we found that the overexpression of RARγ was detected in most of the CRC tissues and all CRC cell lines examined, suggesting that RARγ may play a role in CRC progression. Our results are consistent with previous studies regarding the expression profile of RARγ in hepatocellular carcinoma, esophageal cancer, and cholangiocarcinoma.Citation9-11
Additionally, we found that RARγ was abnormally expressed in the cytoplasm of human CRC specimens, which is in concert with previous studies that RARγ often resided in the cytoplasm of cancer cells.Citation9,22 Arguably the most fundamental trait of cancer cells involves their capability to sustain proliferation signaling.Citation23 Our data presented here showed that RARγ knockdown did not affect CRC cells proliferation.
The most significant finding of our study is that lentiviral vectors carrying shRARγ-mediated RARγ silencing effectively sensitized CRC cells to chemotherapeutics, as reflected by a strong inhibition of MDR1 rather than MRP1, MRP2 or MRP3, which is in agreement with our previous studies.Citation11 The poor prognosis of CRC patients is largely due to development of chemoresistance.Citation24 Resistance to chemotherapeutic drugs is a major obstacle in the treatment of cancers, and such resistance has been related to the expression of multidrug-efflux transporters.Citation25 One of the best characterized mechanisms of MDR is overexpression of drug efflux transporters including ATP-binding cassette (ABC) drug transporter protein MDR1, and multidrug resistance-associated protein MRP1, MRP2 and MRP3, which decreases drug uptake and increases drug efflux in cancer cells.Citation14,26 Previous studies showed that MDR1 serves as an important MDR protein of CRC.Citation27,28 Of particular interest, the molecular mechanism of the way regulating MDR1 in CRC is still unclear.
The results from the present study demonstrated that Wnt/β-catenin pathway was involved in the RARγ-induced upregulation of MDR1 in CRC. The aberrant activation of Wnt/β-catenin signaling pathway is vital the development of cancers, including CRC.Citation29-31 Importantly, RARγ was required for the transduction of Wnt/β-catenin signaling and the activation of MDR1 in CRC cells, suggesting that RARγ mediates the chemoresistance of CRC. What's more, RARγ expression in CRC tissues is positively correlated with expression of MDR1.
In conclusion, our study has for the first time demonstrated RARγ upregulates MDR1 expression via Wnt/β-catenin pathway and mediating MDR of CRC cells. RARγ could represent a potential therapeutic target for chemoresistant CRC patients.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
1295180_Supplemental_Material.docx
Download MS Word (624.8 KB)Funding
This work was supported by Grants from the National Nature Science Foundation of China Grant Nos. 81572394 and 81402309; and the National Science Foundation for Fostering Talents in Basic Research of the National Natural Science Foundation of China Grant No. J1310027.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5-29; PMID:25559415; http://dx.doi.org/10.3322/caac.21254
- Davies JM, Goldberg RM. Treatment of metastatic colorectal cancer. Semin Oncol 2011; 38:552-60; PMID:21810514; http://dx.doi.org/10.1053/j.seminoncol.2011.05.009
- Dai Y, Wilson G, Huang B, Peng M, Teng G, Zhang D, Zhang R, Ebert MP, Chen J, Wong BC, et al. Silencing of Jagged1 inhibits cell growth and invasion in colorectal cancer. Cell Death Dis 2014; 5:e1170; PMID:24722295; http://dx.doi.org/10.1038/cddis.2014.137
- Gronemeyer H, Gustafsson J-Å, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov 2004; 3:950-64; PMID:15520817; http://dx.doi.org/10.1038/nrd1551
- Purton LE, Dworkin S, Olsen GH, Walkley CR, Fabb SA, Collins SJ, Chambon P. RARgamma is critical for maintaining a balance between hematopoietic stem cell self-renewal and differentiation. J Exp Med 2006; 203:1283-93; PMID:16682494; http://dx.doi.org/10.1084/jem.20052105
- Yasuhara R, Yuasa T, Williams JA, Byers SW, Shah S, Pacifici M, Iwamoto M, Enomoto-Iwamoto M. Wnt/beta-catenin and retinoic acid receptor signaling pathways interact to regulate chondrocyte function and matrix turnover. J Biol Chem 2010; 285:317-27; PMID:19858186; http://dx.doi.org/10.1074/jbc.M109.053926
- Goranov BB, Campbell Hewson QD, Pearson AD, Redfern CP. Overexpression of RARgamma increases death of SH-SY5Y neuroblastoma cells in response to retinoic acid but not fenretinide. Cell Death Differ 2006; 13:676-9; PMID:16341128; http://dx.doi.org/10.1038/sj.cdd.4401824
- Pettersson F, Dalgleish AG, Bissonnette RP, Colston KW. Retinoids cause apoptosis in pancreatic cancer cells via activation of RAR-gamma and altered expression of Bcl-2/Bax. Br J Cancer 2002; 87:555-61; PMID:12189556; http://dx.doi.org/10.1038/sj.bjc.6600496
- Yan TD, Wu H, Zhang HP, Lu N, Ye P, Yu FH, Zhou H, Li WG, Cao X, Lin YY, et al. Oncogenic potential of retinoic acid receptor-gamma in hepatocellular carcinoma. Cancer Res 2010; 70:2285-95; PMID:20197465; http://dx.doi.org/10.1158/0008-5472.CAN-09-2968
- Kumar A, Kaur J, Chattopadhyay TK, Mathur M, Ralhan R. Differential expression of retinoic acid receptors in normal and malignant esophageal tissues. J Exp Ther Oncol 2004; 4:1-8; PMID:15255287
- Huang GL, Luo Q, Rui G, Zhang W, Zhang QY, Chen QX, Shen DY. Oncogenic activity of retinoic acid receptor gamma is exhibited through activation of the Akt/NF-kappaB and Wnt/beta-catenin pathways in cholangiocarcinoma. Mol Cell Biol 2013; 33:3416-25; PMID:23798555; http://dx.doi.org/10.1128/MCB.00384-13
- Zhu H, Liu Z, Tang L, Liu J, Zhou M, Xie F, Wang Z, Wang Y, Shen S, Hu L, et al. Reversal of P-gp and MRP1-mediated multidrug resistance by H6, a gypenoside aglycon from Gynostemma pentaphyllum, in vincristine-resistant human oral cancer (KB/VCR) cells. Eur J Pharmacol 2012; 696:43-53; PMID:23051672; http://dx.doi.org/10.1016/j.ejphar.2012.09.046
- Rocchi E, Khodjakov A, Volk EL, Yang CH, Litman T, Bates SE, Schneider E. The product of the ABC half-transporter gene ABCG2 (BCRP/MXR/ABCP) is expressed in the plasma membrane. Biochem Biophys Res Commun 2000; 271:42-6; PMID:10777678; http://dx.doi.org/10.1006/bbrc.2000.2590
- Kimura Y, Morita SY, Matsuo M, Ueda K. Mechanism of multidrug recognition by MDR1/ABCB1. Cancer Sci 2007; 98:1303-10; PMID:17608770; http://dx.doi.org/10.1111/j.1349-7006.2007.00538.x
- Sharom FJ. ABC multidrug transporters: Structure, function and role in chemoresistance. Pharmacogenomics 2008; 9:105-27; PMID:18154452; http://dx.doi.org/10.2217/14622416.9.1.105
- Balcerczak E, Panczyk M, Piaskowski S, Pasz-Walczak G, Salagacka A, Mirowski M. ABCB1/MDR1 gene polymorphisms as a prognostic factor in colorectal cancer. Int J Colorectal Dis 2010; 25:1167-76; PMID:20533057; http://dx.doi.org/10.1007/s00384-010-0961-2
- Zhang H, Zhang X, Wu X, Li W, Su P, Cheng H, Xiang L, Gao P, Zhou G. Interference of Frizzled 1 (FZD1) reverses multidrug resistance in breast cancer cells through the Wnt/beta-catenin pathway. Cancer Lett 2012; 323:106-13; PMID:22484497; http://dx.doi.org/10.1016/j.canlet.2012.03.039
- Hu T, To KK, Wang L, Zhang L, Lu L, Shen J, Chan RL, Li M, Yeung JH, Cho CH. Reversal of P-glycoprotein (P-gp) mediated multidrug resistance in colon cancer cells by cryptotanshinone and dihydrotanshinone of Salvia miltiorrhiza. Phytomedicine 2014; 21:1264-72; PMID:25172788; http://dx.doi.org/10.1016/j.phymed.2014.06.013
- Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, Rooney DL, Zhang M, Ihrig MM, McManus MT, et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet 2003; 33:401-6; PMID:12590264; http://dx.doi.org/10.1038/ng1117
- Huang GL, Zhang W, Ren HY, Shen XY, Chen QX, Shen DY. Retinoid X receptor alpha enhances human cholangiocarcinoma growth through simultaneous activation of Wnt/beta-catenin and nuclear factor-kappaB pathways. Cancer Sci 2015; 106:1515-23; PMID:26310932; http://dx.doi.org/10.1111/cas.12802
- Huang GL, Shen DY, Cai CF, Zhang QY, Ren HY, Chen QX. Beta-escin reverses multidrug resistance through inhibition of the GSK3beta/beta-catenin pathway in cholangiocarcinoma. World J Gastroenterol 2015; 21:1148-57; PMID:25632187; http://dx.doi.org/10.3748/wjg.v21.i4.1148
- Han YH, Zhou H, Kim JH, Yan TD, Lee KH, Wu H, Lin F, Lu N, Liu J, Zeng JZ, et al. A unique cytoplasmic localization of retinoic acid receptor-gamma and its regulations. J Biol Chem 2009; 284:18503-14; PMID:19416983; http://dx.doi.org/10.1074/jbc.M109.007708
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/10.1016/j.cell.2011.02.013
- Ng L, Wan T, Chow A, Iyer D, Man J, Chen G, Yau TC, Lo O, Foo CC, Poon JT, et al. Osteopontin overexpression induced tumor progression and chemoresistance to oxaliplatin through induction of stem-like properties in human colorectal cancer. Stem Cells Int 2015; 2015:247892; PMID:26106421; http://dx.doi.org/10.1155/2015/247892
- Kim KJ, Moon SM, Kim SA, Kang KW, Yoon JH, Ahn SG. Transcriptional regulation of MDR-1 by HOXC6 in multidrug-resistant cells. Oncogene 2013; 32:3339-49; PMID:22907429; http://dx.doi.org/10.1038/onc.2012.354
- Ozben T. Mechanisms and strategies to overcome multiple drug resistance in cancer. FEBS Lett 2006; 580:2903-9; PMID:16497299; http://dx.doi.org/10.1016/j.febslet.2006.02.020
- Mochida Y, Taguchi K, Taniguchi S, Tsuneyoshi M, Kuwano H, Tsuzuki T, Kuwano M, Wada M. The role of P-glycoprotein in intestinal tumorigenesis: Disruption of mdr1a suppresses polyp formation in Apc(Min/+) mice. Carcinogenesis 2003; 24:1219-24; PMID:12807720; http://dx.doi.org/10.1093/carcin/bgg073
- Yasunaga M, Matsumura Y. Role of SLC6A6 in promoting the survival and multidrug resistance of colorectal cancer. Sci Rep 2014; 4:4852; PMID:24781822; http://dx.doi.org/10.1038/srep04852
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004; 20:781-810; PMID:15473860; http://dx.doi.org/10.1146/annurev.cellbio.20.010403.113126
- Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012; 487:330-7; PMID:22810696; http://dx.doi.org/10.1038/nature11252
- Voloshanenko O, Erdmann G, Dubash TD, Augustin I, Metzig M, Moffa G, Hundsrucker C, Kerr G, Sandmann T, Anchang B, et al. Wnt secretion is required to maintain high levels of Wnt activity in colon cancer cells. Nat Commun 2013; 4:2610; PMID:24162018; http://dx.doi.org/10.1038/ncomms3610