ABSTRACT
Chronic environmental exposure to metal toxicants such as chromium and arsenic is closely related to the development of several types of common cancers. Genetic and epigenetic studies in the past decade reveal that post-translational modifications of histones play a role in metal carcinogenesis. However, exact molecular mechanisms of metal carcinogenesis remain to be elucidated. In this study we found that As2O3, an environmental metal toxicant, upregulated overall modifications of many cellular proteins by SUMO2/3. Sumoylated proteins from arsenic-treated cells constitutively expressing His6-SUMO2 were pulled down by Ni-IDA resin under denaturing conditions. Mass spectrometric analysis revealed over 100 proteins that were potentially modified by sumoylation. Mus81, a DNA endonuclease involved in homologous recombination repair, was among the identified proteins whose sumoylation was increased after treatment with As2O3. We further showed that K10 and K524 were 2 lysine residues essential for Mus81 sumoylation. Moreover, we demonstrated that Mus81 sumoylation is important for normal mitotic chromosome congression and that cells expressing SUMO-resistant Mus81 mutants displayed compromised DNA damage responses after exposure to metal toxins such as Cr(VI) and arsenic.
Introduciton
Chronic exposure to arsenic is associated with increased incidences of cancer of skin, bladder, lung, kidney, and liver.Citation1-7 Despite extensive studies in the past, the mechanism of arsenic carcinogenesis remains largely unclear. Recent studies reveal that that epigenetic effects including DNA methylation and histone post-translational modifications may also play a role in carcinogenesis induced by metals including arsenic.Citation8-10 On the other hand, it remains unclear whether arsenic exposure induces post-translational modifications, especially by sumoylation and ubiquitination, of non-histone components during oncogenic transformation and tumor development.
The SUMO (small ubiquitin-related modifier) pathway resembles the ubiquitin pathway. It consists of 3 enzymatic components: the E1 activating enzyme (SAE1/2), the E2 conjugating enzyme (UBC9), and a series of E3 ligases that promote sumoylation in a substrate-specific manner.Citation11,12 In general, sumoylation occurs on lysine residues immediately after amino acids of a hydrophobic nature. Mammalian cells have 3 major structural homologs: SUMO1, SUMO2, and SUMO3. Similar to other types of post-translational modifications, sumoylation is reversed by the proteolytic cleavage of isopeptidases of the SENP family.Citation12 Extensive research has uncovered important functions of sumoylation that control the subcellular localization, stability, and enzyme activities of target proteins. Sumoylation is also crucial in the development and cell biology as its disruption causes abnormalities in growth, differentiation, and embryogenesis.Citation13
In this study, we report that arsenic (As2O3) treatment substantially increased overall modifications of many cellular proteins by SUMO2/3. Mass spectrometric analysis identified Mus81, a DNA endonuclease involved in homologous recombination repair, as one of those proteins whose sumoylation was enhanced by arsenic treatment. Further studies revealed that K10 and K524 of Mus81 are 2 main lysine residues for sumoylation. Significantly, Mus81 sumoylation is important for normal mitotic chromosome congression. Cells expressing SUMO-resistant Mus81 mutants displayed compromised DNA damage responses after treatment with Cr(VI) or arsenic.
Results
As2O3 increases overall sumoylation in cells
It has been reported that As2O3 treatment induces oligomerization and sumoylation of PML and PML/PARA, leading to degradation of these proteins.Citation14,15 To study how arsenic affects overall cellular sumoylation, we treated HeLa cells with increasing concentrations of As2O3 (ATO) for 24 h, after which equal amounts of cell lysates were blotted with an antibody to SUMO2/3. We observed that sumoylated protein signals were greatly increased in a concentration-dependent manner after ATO treatment (). As arsenic is known to induce a mild mitotic arrest,Citation16,17 we included nocodazole-treated cell lysates as control. Intriguingly, nocodazole treatment did not increase overall sumoylation signals within the cell, suggesting that arsenic-induced overall sumoylation is unlikely due to a change in the cell cycle status.
Figure 1. Arsenic upregulates overall protein sumoylation in cells. (A) HeLa cells were treated with arsenic trioxide (ATO) at indicated concentrations for 24 h, after which cells were collected and lysed. Equal amounts of cell lysates were blotted with antibodies to SUMO2/3, cyclin B, UBC9 and ß-actin. Protein lysates from cells treated with nocodazole overnight were also used as control. (B) HeLa cells were treated with ATO (5 µM) for various times as indicated, after which cells were collected and lysed. Equal amounts of cell lysates were blotted with antibodies to SUMO2/3, UBC9 and ß-actin. (C) HCT116 cells were treated with ATO at indicated concentrations for 24 h, after which cells were collected and lysed. Equal amounts of cell lysates were blotted with antibodies to SUMO2/3, p53, and ß-actin. (D) HL-60 cells were treated with ATO at indicated concentrations for 24 h, after which cells were collected and lysed. Equal amounts of cell lysates were blotted with antibodies to SUMO2/3 and ß-actin.
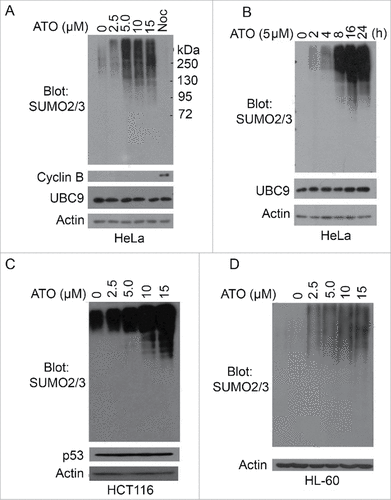
Further studies revealed that arsenic-induced increase of protein sumoylation was also time-dependent, peaking at about 16 h post treatment (). As UBC9 is the only SUMO E2 enzyme in mammalian cells, we also blotted with the antibody to this enzyme and found that its protein level was unchanged after arsenic treatment ( and ). This observation suggests that increased overall sumoylation is not due to the modulation of UBC9 protein level. To exclude that As2O3-induced sumoylation is HeLa cell-specific, we treated HCT116 (colon carcinoma) expressing wild-type p53 with increased concentrations of ATO for 24 h and observed that arsenic also induced overall protein sumoylation in these cells (). In the clinic, As2O3 is an effective drug for the treatment of acute promyelocytic leukemia.Citation18,19 We then treated HL-60, a promyelocytic leukemia cell line, with increasing concentrations of As2O3. We observed that arsenic treatment also caused an increase of overall protein sumoylation in these cells, strongly suggesting that ATO-induced SUMO-modifications of cellular proteins are a general phenomenon in vivo.
Mus81 is sumoylated and its sumoylation is modulated by arsenic exposure
To identify protein targets that were SUMO2/3-modified after arsenic treatment, we obtained HeLa cells that constitutively expressed transfected His6-SUMO2 (S2 cells thereafter) and treated these cells with or without ATO (5 μM) for 16 h. Equal amounts of cell lysates from different treatments were incubated with Ni-IDA resin and proteins bound to the resin were eluted under a denaturing condition. Mass spectrometric analysis of proteins specifically bound to Ni-IDA resin led to the identification of over 100 potential sumoylation targets. These proteins are involved in regulating the cell cycle, transcription, DNA damage responses and repair, and the immune system ().
Table 1. Potential proteins with enhanced SUMO2-modification after As2O3 treatment.
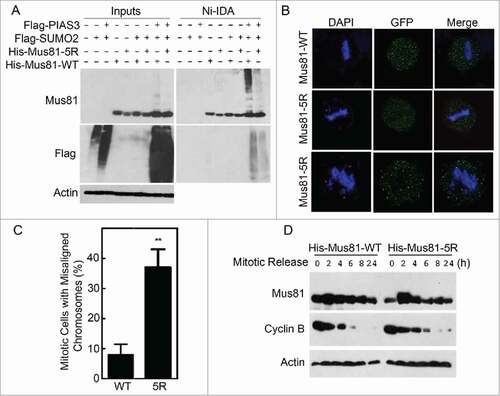
Among putative SUMO-targets is Mus81, a structure-specific endonuclease. Mus81 plays a critical role in the homologous recombination (HR) DNA repair pathway.Citation20,21 Mus81 is also reported to co-localize with FANCD2,Citation22,23 which in turn plays a role in suppressing chromosome mis-segregation and chromosomal instability.Citation23,24 We then selected Mus81 for further characterization. After arsenic treatment, several slow mobility bands, along with the primary band, were detected by the Mus81 antibody in HeLa cells and the slow-mobility bands were significantly enriched in the nuclear fraction (). The cellular fractionation efficiency was confirmed by blotting with PARP-1, a nuclear protein. We named these slow-mobility bands as Mus81-S (SUMO-modified Mus81). Arsenic treatment greatly increased the intensity of Mus81-M in both total cell lysates and the nuclear fraction. Mus81 antibody was specific as siRNAs to Mus81 greatly reduced its level in the cell ().
Figure 2. Enhanced Mus81 sumoylation after arsenic treatment. (A) HeLa cells were treated with ATO (5 µM) for 24 h after which cells were collected for lysis and fractionations. Equal amounts of cytoplasmic and nuclear fractions, along with total cell lysates, were blotted with antibodies to Mus81, PARP1 and ß-actin. Mus81-S denotes SUMO-modified Mus81 bands. (B) HeLa cells were transfected with Mus81 siRNAs or luciferase siRNAs for 24 h after which equal amounts of cell lysates were blotted for Mus81 and ß-actin. (C) HeLa cells were co-transfected with plasmids expressing Flag-SUMO2, Flag-UBC9 and/or His6-Mus81 for 48 h after which cells were lysed. Equal amounts of lysates were incubated with Ni-IDA resin. Proteins bound to the resin, along with lysate inputs, were blotted with antibodies to Mus81, Flag, and ß-actin. (D) HeLa S2 cells that constitutively expressed transfected SUMO2 were transfected with a plasmid expressing GPF-Mus81 or GFP alone for 48 h after which equal amounts of lysates were immunoprecipitated with the anti-GFP antibody. GFP precipitates, along with lysate inputs, were blotted with antibodies to GFP, Mus81, and/or ß-actin. (E) HeLa cells were transfected with various plasmids as indicated for 48 h after which equal amoutns of lysates were precipitated with the anti-Mus81 antibody. Mus81 precipitates, along with lysate inputs, were blotted with antibodies to GFP, Mus81, and/or ß-actin.
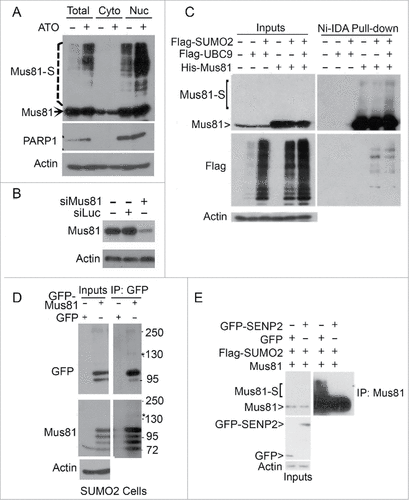
To further characterize Mus81 sumoylation, we co-transfected HeLa cells with Flag-SUMO2, Flag-UBC9, and His6-Mus81 for 24 h, after which cell lysates were incubated with Ni-IDA resin. Proteins pulled-down by Ni-IDA resin, along with lysate inputs, were blotted with antibodies to Mus81 and the Flag tag. We observed that Mus81-S was enriched in cells co-transfected with UBC9 and SUMO2 ().
We then transfected HeLa S2 cells with either GFP-Mus81 or GFP alone for 24 h, after which cell lysates were subjected to pull-down analysis by Ni-IDA resin. Pulldown proteins, along with lysate puts, were blotted with antibodies to GFP and Mus81. We observed that a modified band migrating at about 140 kDa was detected by antibodies to both GFP and Mus81 (). Mus81-S was also abolished after expression of GFP-SENP2, a SUMO-specific isopeptidase (), again indicating that Mus81 is a sumoylated protein.
SUMO E3 ligase PIAS3 facilitates Mus81 sumoylation
Although UBC9 was capable of boosting Mus81 sumoylation, its signals remained rather weak. To identify potential SUMO E3 ligase(s) of Mus81, we co-transfected HEK293T cells with plasmids expressing His6-Mus81, Flag-SUMO2, and several SUMO E3 ligases including Flag-PIAS1, Flag-PIAS3, Flag-PIASx, or Flag-PIASy for 48 h. Total lysates were then immunoblotted with Mus81 and Flag antibodies. We observed that PIAS1 and PIAS3 promoted Mus81 sumoylation (), suggesting that they might be SUMO E3 ligases mediating Mus81 sumoylation.
Figure 3. PIAS1 and PIAS3 promote Mus81 sumoylation. (A) HEK293 cells co-transfected with plasmids expressing Flag-SUMO2, His6-Mus81, and PIAS1 (or other family member) for 48 after which equal amounts of cell lysates were blotted with antibodies to Mus81, Flag, and ß-actin. (B) HEK293 cells were transfected with plasmids expressing various plasmid constructs as indicated for 48 h after which cells were lysed. Equal amounts of cell lysates were incubated with Ni-IDA resin after which proteins bound to the resin were eluted and blotted with the anti-Mus81 antibody. (C) HEK293 cells were co-transfected with plasmids expressing Flag-SUMO2, His6-Mus81, and Flag-PIAS3 (2 different concentrations) for 48 h after which equal amounts of cell lysates were incubated with Ni-IDA resin. Proteins bound to the resin, along lysate inputs, were blotted with antibodies to Mus81, Flag, and/or ß-actin.
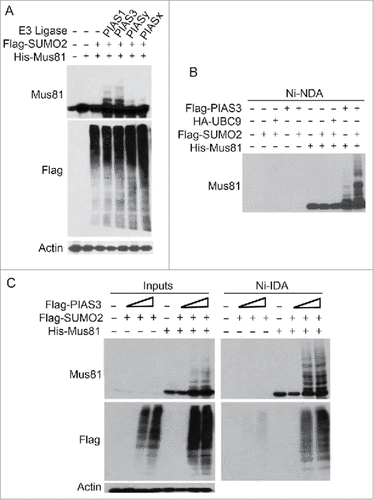
To confirm that PIAS3 is a SUMO E3 ligase for Mus81, we transfected HEK293T cells with plasmid constructs expressing His6-Mus81, Flag-SUMO2, HA-UBC9 and/or Flag-PIAS3 for 48 h. Cell lysates were subjected to affinity pull-down with Ni-IDA resin. Immunoblotting revealed that co-transfection of His6-Mus81 and PIAS3 is sufficient to promote Mus81 sumoylation (). Co-transfection with Flag-SUMO2 and PIAS3 further boosted Mus81 sumoylation. To further validate PIAS3 is a SUMO E3 ligase of Mus81, HEK293T cells were transfected with increasing concentrations of PIAS3 expression plasmid for 48 h. We observed that expression of PIAS3 promoted Mus81 sumoylation in a concentration-dependent manner (). Blotting with the anti-Flag antibody showed that PIAS3 expression was efficient. Combined, our studies show that PIAS3 is a Mus81 SUMO E3 ligase, positively regulating Mus81 sumoylation.
Lysines 10 and 524 are important residues for mediating Mus81 sumoylation
To identify the site(s) that are important for sumoyation, we analyzed Mus81 amino acid structure using the criteria available at Abgent Inc. Five lysine residues (K10, K123, K170, K232, and K524) conformed to the consensus site optimal for sumoylation (). We first made Mus81 mutants that harbored a single lysine residue. We co-transfected HEK293K cells with plasmids expressing Myc-PIAS3, Flag-SUMO2, and a Mus81 mutant (or WT Mus81) for 24 h, after which equal amounts of cell lysates were subjected to pull-down analysis with Ni-IDA resin. We observed that mutation of K10 or K524 alone reduced Mus81 sumoylation as compared with the wild-type control ( and ). To confirm the importance of K10 and K524 (as well as other 3 lysine residues) in mediating Mus81 sumoylation, we also made a mutant that replaced 5 lysines with arginines, which was named Mus81–5R. As expected, expression of Mus81–5R largely abolished sumoylation ().
Figure 4. Lysines 10 and 524 are essential for Mus81 sumoylation. (A) Schematic presentation of Mus81 with 5 lysine residues that largely conform to sumoylation consensus motif. (B) HEK293 cells were transfected with various expression plasmid constructs as indicated for 48 h after which equal amounts of cell lysates were incubated with Ni-IDA resin. Proteins specifically bound to the resin were eluted and blotted, along with lysate inputs, for Mus81, Flag (SUMO2), Myc (PIAS3), and/or ß-actin. (C) HEK293 cells were transfected with various expression plasmid constructs as indicated for 48 h after which equal amounts of cell lysates were incubated with Ni-IDA resin. Proteins specifically bound to the resin were eluted and blotted, along with lysate inputs, for Mus81, Flag, and/or ß-actin.
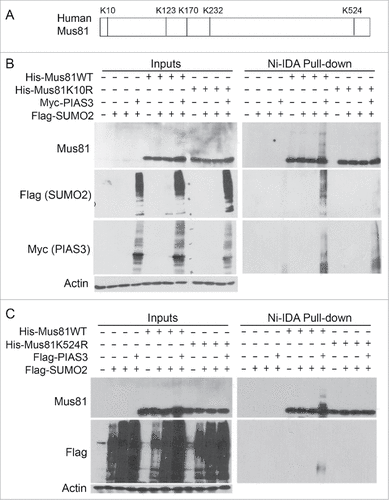
Figure 5. Expression of sumoylation-resistant Mus81 mutant induces lagging chromosomes and delays mitotic progression. (A) HEK293 cells were co-transfected with various expression plasmids as indicated for 48 h after which equal amounts of cell lysates were incubated with Ni-IDA resin. Proteins bound to the resin were eluted and blotted, along with lysate inputs, for Mus81, Flag, and/or ß-actin. (B) HeLa cells transfected with GFP-Mus81 or GFP-Mus81–5R for 48 h. Cells were then fixed and stained with DAPI. Mitotic cells were analyzed for the presence of mis-aligned/lagging chromosomes. (C) Mitotic cells with mis-aligned or lagging chromosomes (denoted with red star) after expression of either Mus81 or its sumoylation mutant as shown in B were counted. Data were summarized from 3 independent experiments. (D) HeLa cells transfected with His6-Mus81 or His6-Mus81–5R for 24 h after which cells were treated with nocodazole for 16 h. Mitotic cells were collected by shake-off and re-cultured in fresh medium for various times as indicated. Equal amounts of cell lysates were blotted for Mus81, cyclin B, and ß-actin.
SUMO-resistant mutant induces misaligned chromosomes in mitotic cells
Sumoylation plays a role in regulating the subcellular localization of target proteinsCitation25-29 To determine whether sumoylation played a role in regulating subcellular localization of Mus81, HeLa cells were transfected with GFP-tagged WT Mus81 or its Mus81–5R for 48 h. Cells were then fixed and stained with antibody against GFP. Fluorescence microscopy showed that both GFP-Mus81 and GFP-Mus81–5R was primarily located to the nucleus, and when the expression level was high, GFP-Mus81 and GFP-Mus81–5R mutant were enriched in nucleoli during interphase (). During mitosis, GFP-Mus81 and GFP-Mus81–5R formed discrete foci (). However, cells expressing GFP-Mus81–5R displayed a significant higher percentage of misaligned chromosomes in mitotic cells ( and )
To study whether Mus81 sumoylation played a role in regulating mitotic progression, we transfected HeLa cells with His6-Mus81 and His6-Mus81–5R for 24 h followed by treatment with nocodazole for 16 h. Mitotic cells were collected and re-cultured in fresh medium for mitotic release. We observed that cyclin B levels were decreased at a slower rate than WT Mus81 during mitotic release (). These observations suggest that expression of the Mus81 mutant induces a mitotic delay.
Compromised DNA damage response in cells expressing Mus81–5R
To further confirm that Mus81 sumoylation was upregulated after arsenic exposure, we co-transfected HEK293 cells with His6-Mus81, Flag-PIAS3 and Flag-SUMO2 for 24 h, after which transfected cells were treated with or without arsenic for additional 24 h. We observed that sumoylated Mus81 was significantly higher in cells treated with arsenic (). We further determined the upregulation of Mus81 sumoylation using HeLa S2 cells that constitutively expressed His6-SUMO2. We observed that Flag-Mus81 was SUMO2-modified and that its modification increased in a time-dependent manner ().
Figure 6. Cells expressing sumoylation-resistant mutant of Mus81 have compromised DNA damage responses to metal toxins. (A) HeLa cells were co-transfected with plasmids expressing for Flag-PIAS3, Flag-SUMO2, His6-Mus81 for 48 h after which equal amounts of cell lysates were incubated with Ni-IDA resin. Proteins specifically bound to the resin, along with lysate inputs, were blotted for Mus81 and/or ß-actin. (B) HeLa S2 cells were transfected with Mus81 expression construct for 24 h after which cells were treated with ATO for various times as indicated. Equal amounts of lysates were incubated with Ni-IDA resin. Protein specifically bound to the resin were eluted and blotted, along with lysate inputs, with antibodies to Mus81, Flag, and ß-actin. (C) HEK293 cells transfected with a plasmid construct expressing either Mus81 or Mus81–5R for 48 h after which cells were treated with ATO for various times. Equal amounts of cell lysates were blotted with antibodies to Mus81, γ-H2AX, p-ATM, Geminin, Emi1, PARP-1, and ß-actin. (D) HEK293 cells transfected with a plasmid construct expressing either Mus81 or Mus81–5R for 48 h after which cells were treated with Cr(VI) for various times as indicated. Equal amounts of cell lysates were then blotted with antibodies to Mus81, γ-H2AX, p-ATM, PARP-1, and ß-actin.
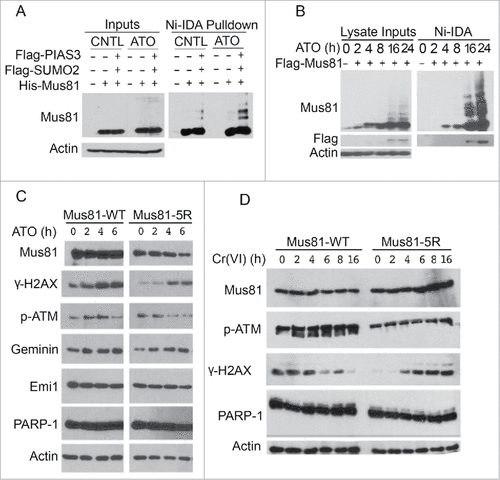
It has been reported that Mus81 plays a critical role in telomere maintenance.Citation30-32 It has been also reported that As2O3 treatment leads to damage to telomeres.Citation33 We then studied whether Mus81 sumoylation was involved in DNA damage responses after exposure to metal toxins including arsenic and chromium. Consistent with an early report,Citation33 ATO exposure triggered DNA damage responses manifested as elevated levels of phosphorylated H2AX (γ-H2AX) and phosphorylated ATM (p-ATM); however, cells expressing transfected His6-Mus81–5R plasmid displayed a reduced level of γ-H2AX compared with cells transfected with the WT counterpart (). His6-Mus81–5R was significantly decreased after ATO exposure whereas WT His6-Mus81 was rather stable during the treatment. PARP-1 cleavage was not observed in these cells, suggesting that ectopic expression of either WT Mus81 or its sumoylation-resistant mutant did not trigger apoptosis.
It has been shown that after chromium treatment Mus81 deficient cells exhibited elevated levels of γ-H2AX compared with wild-type cells.Citation34 To study whether Mus81 sumoylation had any effect on chromium responses, we transfected HEK293T cells with a plasmid expressing WT His6-Mus81 or His6-Mus81–5R followed by treatment with Cr(VI) for various times. Immunoblotting revealed that cells transfected with His6-Mus81–5R contained a lower level of γ-H2AX than those transfected with the WT counterpart (). Exposure to Cr(VI) induced a moderate increase of p-ATM in cells transfected with WT His6-Mus81 plasmid, which was coupled with decreased γ-H2AX signals 6 h post treatment (). On the other hand, cells transfected with His6-Mus81–5R contained a low level of γ-H2AX before Cr(VI) exposure. Cr(VI) treatment increased γ-H2AX levels 4 h after Cr(VI) treatment ().
Discussion
In this study, we demonstrated that Mus81 is covalently modified by SUMO2 and that K10 and K524 are 2 residues important for mediating sumoylation. We also show that PIAS3 is a SUMO E3 ligase that promotes Mus81 sumoylation although PIAS1 appears to be able to stimulate Mus81 sumoylation as well. It would be interesting to further study whether PIAS1 and PIAS3 regulate Mus81 sumoylation at different sites as Mus81 undergoes SUMO-modifications at multiple sites. SUMO2 moiety appears to be a limiting factor in mediating Mus81 sumoylation as HeLa S2 cells that constitutively express transfected SUMO2 contain much higher levels of sumoylated Mus81 than the parental cells either in the presence or absence of arsenic. Moreover, SENP2 is capable of desumoylating Mus81, suggesting that sumoylation of Mus81 is a reversible process.
Our current study shows that Mus81 is involved in regulating genomic stability. Ectopic expression of sumoylation-resistant mutant of Mus81 leads to a high rate of misaligned chromosomes in mitosis. Given chromosomal stability is a hallmark of transformed cells, defects in Mus81 sumoylation and its regulatory pathways can contribute to malignant transformation. Moreover, it is well known that Mus81 has a main function in DNA double-strand repair process. We have observed that cells expressing sumoylation-resistant mutants display an elevated level of DNA damage markers as compared with that of WT Mus81 after exposure to Cr(VI) for more than 6 h. These results suggest that Mus81 sumoylation may directly regulate its DNA repair activity. A compromised activity of Mus81–5R in DNA repair leads to the accumulation of double-strand breaks.
It has been shown that AS2O3 induces cell toxicity by reducing telomerase activity in glioblastoma cells.Citation35,36,37 We have shown that arsenic up-regulates sumoylation of many protein targets including Mus81. Sumoylation-deficient Mus81 is less stable in the presence of arsenic compared with wild-type Mus81, which may explain the weakened DNA damage response in mutant Mus81 cells. It is known that arsenic does not directly perturb the genome. Increased γ-H2AX signals are likely to be a result of arsenic-induced oxidative stress which in turns causes DNA strand breaks. It has been shown that As2O3 induces cell toxicity by reducing telomerase activity in glioblastoma cells.Citation37 It will be of great interest to determine if aberrant Mus81 sumoylation contributes to malignant transformation induced by arsenic exposure.
Materials and methods
Cell culture and chemicals
HeLa, HEK293T, HCT116, and HL60 cells were obtained from the American Type Culture Collection (Manassas, VA). HeLa SUMO stable cell lines constitutively expressing HIS6-SUMOs were kindly provided by Ronald Hay at University of Dundee in UK. Cells were cultured in dishes or on Lab-Tek II chamber slides (Fisher Scientific, Pittsburgh, PA) in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) with 5% CO2 at 37°C. Na2CrO4 (Sigma Chemical Co.,St. Louis, MO) was dissolved in 0.9% (w/v) NaCl at the concentration of 0.5 M as a stock solution. Nocodazole (Sigma Chemical Co.) was dissolved in dimethyl sulphoxide (DMSO). Maximal final concentration of DMSO used in cell culture was kept below 0.1%. HeLa SUMO2 stable cell lines were cultured in DMEM with 10% FBS with the selection of puromycin at a final concentration of 2.5 ug/ml. Arsenic trioxide was dissolved in 1N NaOH at the concentration of 1M as a stock solution. Various final concentrations were diluted in DMEM in each experiment as indicated. And the same final concentration of NaOH was added to each control group.
Plasmids, mutagenesis, and transfection
The original template plasmid for cloning of His6-MUS81 was obtained from Dr. Stephen West at London Research Institute in UK. Mus81 mutants (His6-Mus81–5R, His6-Mus81K10R, His6-Mus81K123R, His6-MUS81K170R, His6-MUS81K232R, and His6-MUS81K524R) was performed using the QuickChange Lightning Multi-Site-directed Mutagenesis kit (Stratagene). Individual mutations were confirmed by DNA sequencing (Seqwright). Transfections of plasmids or siRNAs were performed using Lipofectamine 2000 or Fugene HD (Invitrogen & Promega) as described in our previous studies.Citation38, 39 Transfections of siRNAs were performed using Lipofectamine 2000 according to the instructions provided by the supplier (Invitrogen).Western blot: SDS-PAGE was performed using the mini gel system from Bio-Rad. Proteins were transferred to PVDF membranes. After blocking with 1xTBS/T buffer containing 5% nonfat dry milk for 1 h, the membranes were incubated overnight with primary antibodies including cyclin B (Cell Signaling), β-actin (Cell Signaling), Flag (Cell Signaling), HA (Abcam), GFP (Santa Cruz), SUMO2/3 (Abcam), Mus81 (Abcam), PARP-1 (Cell Signaling), UBC9 (Cell Signaling), P53 (Santa Cruz), gamma-H2AX (Cell signaling), EME1 (Santa Cruz), PML (Abcam), p-ATM (Cell Signaling), followed by incubation with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling) for 1 h at room temperature. After thorough washing with 1X TBS/T buffer, signals on the membranes were developed with an enhanced chemiluminescent system (Millipore).
Ni-IDA Pull Down Assay. For denaturing pull-down assays with Ni-IDA resin (Clonetech), HeLa cells or HEK293T cells were transfected with indicated plasmids. Then cells were collected and lysed in a lysis buffer (8 M urea, 300 mM NaCl, 50 mM sodium phosphate, 20 mM imidazole, 30 mM NEM, 0.5% Triton X-100, pH 7.4). Equal amounts of total lysates were incubated with Ni-IDA agarose resin for 3 hours (Qiagen). The resin was successively washed at room temperature with washing buffer (8 M Urea, 300 mM NaCl, 50 mM sodium phosphate, 40 mM imidazole, 30 mM NEM, 0.5% Triton X-100, pH 7.4) for 3 times. After last wash, His6-tagged products were eluted in the following buffer (8 M urea, 300 mM NaCl, 50 mM sodium phosphate, 300 mM imidazole, 30 mM NEM, 0.5% Triton X-100, pH 7.4). Samples were Western-blotted for Mus81 and flag antibodies.
Fluorescence microscopy
Fluorescence microscopy was performed as described in our early studies.Citation40, 41 Briefly, HeLa cells seeded on chamber slides were transfected with various expression constructs for 48 h. At the end of transfection, cells were fixed with 4% paraformaldehyde in PBS for 20 min at room temperature. After permeabilization by incubation with 0.5% Triton X-100 in PBS for 20 min, cells were incubated with 2% bovine serum albumin (BSA) in PBS for 1 h, followed by incubating overnight with GFP antibody (Santa Cruz). Cells were stained with Fluor 444-conjugated goat anti-mouse IgG (Invitorgen) for 1 h. Cellular DNA was finally stained with 4-diamidino-2-phenylindole (DAPI, Molecular Probe, Eugene, OR). Fluorescence signals were detected on a Leica TCS SP5 confocal microscope or on a Leica AF6000 fluorescence microscope.
Cell fractionation
HeLa cell whole lysates were lysed in Harvest buffer (10 mM HEPES, pH 7.9, 50 mM NaCl, 0.5 M sucrose, 0.1 mM EDTA, 0.5% Triton X-100, Proteinase inhibitors and Phosphatase inhibitors were freshly added before use) for 5 min on ice. Centrifuge the lysates at the speed of 500 g for 5min. The supernatant was then collected as cytoplasm fraction. The rest of the pellets were lysed in urea lysis buffer (8 M Urea, 50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole, 0.5% Triton X-100, 20 mM N-Ethylmaleimide). Samples were analyzed by Western blotting.
Statistical analysis
The Student's t test was used to evaluate the difference between 2 groups. A value of p < 0.05 was considered to be statistically significant. Data were summarized from 3 independent experiments.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank coworkers in the laboratory for valuable discussions during the course of the study.
Funding
This work was supported in part by US Public Service Awards to WD (ES020591) and NIEHS Center grant (ES000260), and LL (EY022364).
References
- Cohen SM, Ohnishi T, Arnold LL, Le XC. Arsenic-induced bladder cancer in an animal model. Toxicol Appl Pharmacol 2007; 222(3):258-63; PMID:17109909; http://dx.doi.org/10.1016/j.taap.2006.10.010
- Chen CJ, Chen CW, Wu MM, Kuo TL. Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br J Cancer 1992; 66(5):888-92; PMID:1419632; http://dx.doi.org/10.1038/bjc.1992.380
- Chen CJ, Chuang YC, You SL, Lin TM, Wu HY. A retrospective study on malignant neoplasms of bladder, lung and liver in blackfoot disease endemic area in Taiwan. Br J Cancer 1986; 53(3):399-405; PMID:3964542; http://dx.doi.org/10.1038/bjc.1986.65
- Chiou HY, Hsueh YM, Liaw KF, Horng SF, Chiang MH, Pu YS, Lin JS, Huang CH, Chen CJ. Incidence of internal cancers and ingested inorganic arsenic: a seven-year follow-up study in Taiwan. Cancer Res 1995; 55(6):1296-300; PMID:7882325
- Burns FJ, Uddin AN, Wu F, Nadas A, Rossman TG. Arsenic-induced enhancement of ultraviolet radiation carcinogenesis in mouse skin: a dose-response study. Environ Health Prospect 2004; 112:599-603.
- Chen CL, Hsu LI, Chiou HY, Hsueh YM, Chen SY, Wu MM, Chen CJ, Blackfoot Disease Study Group. Ingested arsenic, cigarette smoking, and lung cancer risk: a follow-up study in arseniasis-endemic areas in Taiwan. JAMA 2004; 292(24):2984-90; PMID:15613666; http://dx.doi.org/10.1001/jama.292.24.2984
- Chen CJ, Chuang YC, Lin TM, Wu HY. Malignant neoplasms among residents of a blackfoot disease-endemic area in Taiwan: high-arsenic artesian well water and cancers. Cancer Res 1985; 45(11 Pt 2):5895-9; PMID:4053060
- Martinez-Zamudio R, Ha HC. Environmental epigenetics in metal exposure. Epigenetics 2011; 6(7):820-7; PMID:21610324; http://dx.doi.org/10.4161/epi.6.7.16250
- Fragou D, Fragou A, Kouidou S, Njau S, Kovatsi L. Epigenetic mechanisms in metal toxicity. Toxicol Mech Methods 2011; 21(4):343-52; PMID:21495872; http://dx.doi.org/10.3109/15376516.2011.557878
- Arita A, Costa M. Epigenetics in metal carcinogenesis: nickel, arsenic, chromium and cadmium. Metallomics 2009; 1(3):222-8; PMID:20461219; http://dx.doi.org/10.1039/b903049b
- Palancade B, Doye V. Sumoylating and desumoylating enzymes at nuclear pores: underpinning their unexpected duties? Trends Cell Biol 2008; 18(4):174-83; PMID:18313922; http://dx.doi.org/10.1016/j.tcb.2008.02.001
- Di Bacco A, Gill G. SUMO-specific proteases and the cell cycle. An essential role for SENP5 in cell proliferation. Cell Cycle 2006; 5(20):2310-3; PMID:17102611; http://dx.doi.org/10.4161/cc.5.20.3367
- Dawlaty MM, Malureanu L, Jeganathan KB, Kao E, Sustmann C, Tahk S, Shuai K, Grosschedl R, van Deursen JM. Resolution of sister centromeres requires RanBP2-mediated SUMOylation of topoisomerase IIalpha. Cell 2008; 133(1):103-15; PMID:18394993; http://dx.doi.org/10.1016/j.cell.2008.01.045
- Lallemand-Breitenbach V, Jeanne M, Benhenda S, Nasr R, Lei M, Peres L, Zhou J, Zhu J, Raught B, de Thé H. Arsenic degrades PML or PML-RARalpha through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat Cell Biol 2008; 10(5):547-55; PMID:18408733; http://dx.doi.org/10.1038/ncb1717
- Zhang XW, Yan XJ, Zhou ZR, Yang FF, Wu ZY, Sun HB, Liang WX, Song AX, Lallemand-Breitenbach V, Jeanne M, et al. Arsenic trioxide controls the fate of the PML-RARalpha oncoprotein by directly binding PML. Science 2010; 328(5975):240-3; PMID:20378816; http://dx.doi.org/10.1126/science.1183424
- Halicka HD, Smolewski P, Darzynkiewicz Z, Dai W, Traganos F. Arsenic trioxide arrests cells early in mitosis leading to apoptosis. Cell Cycle 2002; 1(3):201-9; PMID:12429934; http://dx.doi.org/10.4161/cc.1.3.126
- Xie S, Wu H, Wang Q, Kunicki J, Thomas RO, Hollingsworth RE, Cogswell J, Dai W. Genotoxic stress-induced activation of Plk3 is partly mediated by Chk2. Cell Cycle 2002; 1(6):424-9; PMID:12548019; http://dx.doi.org/10.4161/cc.1.6.271
- Zhang P, Wang S, Hu L, Qiu F, Yang H, Xiao Y, Li X, Han X, Zhou J, Liu P. [Seven years' summary report on the treatment of acute promyelocytic leukemia with arsenic trioxide–an analysis of 242 cases]. Zhonghua Xue Ye Xue Za Zhi 2000; 21(2):67-70; PMID:11876960
- Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood 1996; 88(3):1052-61; PMID:8704214
- Ciccia A, McDonald N, West SC. Structural and functional relationships of the XPF/MUS81 family of proteins. Annu Rev Biochem 2008; 77:259-287; PMID:18518821; http://dx.doi.org/10.1146/annurev.biochem.77.070306.102408
- Ciccia A, McDonald N, West SC. Structural and functional relationships of the XPF/MUS81 family of proteins. Annu Rev Biochem 2008; 77:259-87; PMID:18518821; http://dx.doi.org/10.1146/annurev.biochem.77.070306.102408
- Naim V, Wilhelm T, Debatisse M, Rosselli F. ERCC1 and MUS81-EME1 promote sister chromatid separation by processing late replication intermediates at common fragile sites during mitosis. Nat Cell Biol 2013; 15(8):1008-15; PMID:23811686; http://dx.doi.org/10.1038/ncb2793
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144(5):646-74; PMID:21376230; http://dx.doi.org/10.1016/j.cell.2011.02.013
- Naim V, Rosselli F. The FANC pathway and BLM collaborate during mitosis to prevent micro-nucleation and chromosome abnormalities. Nat Cell Biol 2009; 11(6):761-8; PMID:19465921; http://dx.doi.org/10.1038/ncb1883
- Gill G. SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev 2004; 18(17):2046-59; PMID:15342487; http://dx.doi.org/10.1101/gad.1214604
- Bassi C, Ho J, Srikumar T, Dowling RJ, Gorrini C, Miller SJ, Mak TW, Neel BG, Raught B, Stambolic V. Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science 2013; 341(6144):395-9; PMID:23888040; http://dx.doi.org/10.1126/science.1236188
- Yang F, Yao Y, Jiang Y, Lu L, Ma Y, Dai W. Sumoylation is important for stability, subcellular localization, and transcriptional activity of SALL4, an essential stem cell transcription factor. J Biol Chem 2016; 291(1):428; PMID:26724314; http://dx.doi.org/10.1074/jbc.A112.391441
- Yang F, Yao Y, Jiang Y, Lu L, Ma Y, Dai W. Sumoylation is important for stability, subcellular localization, and transcriptional activity of SALL4, an essential stem cell transcription factor. J Biol Chem 2012; 287(46):38600-8; PMID:23012367; http://dx.doi.org/10.1074/jbc.M112.391441
- Huang Y, Yao Y, Xu HZ, Wang ZG, Lu L, Dai W. Defects in chromosome congression and mitotic progression in KIF18A-deficient cells are partly mediated through impaired functions of CENP-E. Cell Cycle 2009; 8(16):2643-9; PMID:19625775; http://dx.doi.org/10.4161/cc.8.16.9366
- Saint-Leger A, Koelblen M, Civitelli L, Bah A, Djerbi N, Giraud-Panis MJ, Londoño-Vallejo A, Ascenzioni F, Gilson E. The basic N-terminal domain of TRF2 limits recombination endonuclease action at human telomeres. Cell Cycle 2014; 13(15):2469-74; PMID:25483196; http://dx.doi.org/10.4161/cc.29422
- Olivier M, Da Ines O, Amiard S, Serra H, Goubely C, White CI, Gallego ME. The Structure-Specific Endonucleases MUS81 and SEND1 Are Essential for Telomere Stability in Arabidopsis. Plant Cell 2016; 28(1):74-86; PMID:26704385
- Wan B, Yin J, Horvath K, Sarkar J, Chen Y, Wu J, Wan K, Lu J, Gu P, Yu EY, et al. SLX4 assembles a telomere maintenance toolkit by bridging multiple endonucleases with telomeres. Cell Rep 2013; 4(5):861-9; PMID:24012755; http://dx.doi.org/10.1016/j.celrep.2013.08.017
- Cheng Y, Li Y, Ma C, Song Y, Xu H, Yu H, Xu S, Mu Q, Li H, Chen Y, et al. Arsenic trioxide inhibits glioma cell growth through induction of telomerase displacement and telomere dysfunction. Oncotarget 2016; 7(11):12682-92; PMID:26871293
- Tamblyn L, Li E, Sarras H, Srikanth P, Hande MP, McPherson JP. A role for Mus81 in the repair of chromium-induced DNA damage. Mutat Res 2009; 660(1–2):57-65; PMID:19026666; http://dx.doi.org/10.1016/j.mrfmmm.2008.10.013
- de Lange T. How telomeres solve the end-protection problem. Science 2009; 326(5955):948-52; PMID:19965504; http://dx.doi.org/10.1126/science.1170633
- van Steensel B, Smogorzewska A, de Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell 1998; 92(3):401-13; PMID:9476899; http://dx.doi.org/10.1016/S0092-8674(00)80932-0
- Jiao Y, Zhang W, Liu J, Ni W, Xu W, Jin J, Qian W. Telomere attrition and chromosome instability via downregulation of TRF2 contributes to arsenic trioxide-induced apoptosis of human T-Cell leukemia cell line molt-4 cells. Cancer Biol Ther 2007; 6(8):1186-92; PMID:17643074; http://dx.doi.org/10.4161/cbt.6.8.4381
- Huang X, Ruan Q, Fang Y, Traganos F, Darzynkiewicz Z, Dai W. Physical and functional interactions between mitotic kinases during polyploidization and megakaryocytic differentiation. Cell Cycle 2004; 3(7):946-51; PMID:15190214; http://dx.doi.org/10.4161/cc.3.7.973
- Yang Y, Wang X, Dai W. Human Sgo1 is an excellent target for induction of apoptosis of transformed cells. Cell Cycle 2006; 5(8):896-901; PMID:16628005; http://dx.doi.org/10.4161/cc.5.8.2691
- Liu XS, Zhao XD, Wang X, Yao YX, Zhang LL, Shu RZ, Ren WH, Huang Y, Huang L, Gu MM, et al. Germinal Cell Aplasia in Kif18a Mutant Male Mice Due to Impaired Chromosome Congression and Dysregulated BubR1 and CENP-E. Genes Cancer 2010; 1(1):26-39; PMID:20981276; http://dx.doi.org/10.1177/1947601909358184
- Wang X, Yang Y, Duan Q, Jiang N, Huang Y, Darzynkiewicz Z, Dai W. sSgo1, a major splice variant of Sgo1, functions in centriole cohesion where it is regulated by Plk1. Dev Cell 2008; 14(3):331-41; PMID:18331714; http://dx.doi.org/10.1016/j.devcel.2007.12.007
