ABSTRACT
Tyrosine phosphorylation is rare, representing only about 0.5% of phosphorylations in the cell under basal conditions. While mitogenic tyrosine kinase signaling has been extensively explored, the role of phosphotyrosine signaling across the cell cycle and in particular during mitosis is poorly understood.
Two recent, independent studies tackled this question from different angles to reveal exciting new insights into the role of this modification during cell division. Caron et al.Citation1 exploited mitotic phosphoproteomics data sets to determine the extent of mitotic tyrosine phosphorylation, and St-Denis et al.Citation2 identified protein tyrosine phosphatases from all subfamilies as regulators of mitotic progression or spindle formation. These studied collectively revealed that tyrosine phosphorylation may play a more prominent and active role in mitotic progression than previously appreciated.
Protein phosphorylation is well recognized as a major mode of post-translational regulation. The human genome encodes over 23,000 proteins; more than two-thirds phosphorylated.Citation3 Phosphorylation is a crucial component of signal transduction, regulating pathways that control processes from cell division to cell death. At the molecular level, reversible phosphorylation can lead to changes in protein structure and stability, protein–protein interactions, enzyme activation, or subcellular localization. Phosphorylation is most dynamic during the mitotic stage of the cell cycle,Citation4 and many mitotic phosphoproteome studies have resulted in a wealth of data on serine (Ser) and threonine (Thr) phosphorylation events. However, phosphotyrosine (pTyr) sites are relatively rare, and are underrepresented even in large scale mitosis-specific phosphoproteome studies. Seminal work from many groups in the 1980s, 1990s and early 2000s has revealed remarkable insight into how tyrosine (Tyr) phosphorylation regulates many aspects of growth and development. It is an integral component of cell surface signaling by receptors such as receptor tyrosine kinases, and integrins which signal in part via intracellular kinases such as the Src-family kinases (SFKs).Citation5 These cell surface signals are then propagated throughout the cytoplasm and nucleus through signaling networks often involving feedbacks and crosstalks to finally elicit the appropriate response. A number of tissue-specific pTyr screens have been reported (e.g. Citation6-8) but little is known about Tyr phosphorylation dynamics throughout the cell cycle, leaving a significant gap in our understanding of cell cycle control.
The best understood pTyr site regulated during mitosis is arguably the inhibitory phosphorylation of cyclin-dependent kinase (CDK1) at Tyr,Citation15 which is catalyzed by WEE1 and MYT1, and whose dephosphorylation serves as the basis for the switch-like activity of CDK1/cyclin B at mitotic entry.Citation9 Tyr phosphorylation at the spindle was clearly demonstrated almost 20 years ago when pTyr-containing epitopes, detected by the monoclonal 4G10 pTyr antibody, were observed at kinetochores and centrosomes in the rat cell line PtK1.Citation10 More recent work showed that inhibition of SFKs or another intracellular Tyr kinase, ABL, resulted in spindle and mitotic progression defects.Citation11-13 Moreover, activation of the cell surface EGFR has been shown to determine the timing of centrosome separation,Citation14 and can selectively activate downstream signaling components, including Src, during mitosis.Citation15 Thus, while pTyr signaling appears relevant to spindle formation and function, the mitotic substrates and signaling pathways regulated by Tyr phosphorylation in mitotic human cells are not yet defined.
Collectively, these observations prompted us to identify and explore Tyr phosphorylation in the human mitotic phosphosignalling space.Citation1 Our integrated approach revealed extensive Tyr phosphorylation during mitosis, particularly at the spindle and associated structures. We first determined the localization of pTyr signals in mitotic HeLa cells and the non-cancerous immortalized RPE1 cells, and we verified the presence of prominent spindle-associated Tyr phosphorylation during mitosis using a panel of different anti-pTyr antibodies. Tyr phosphorylation signals were augmented in the presence of phosphatase inhibitors and lost after phosphatase pretreatment of permeablised cells, demonstrating specificity of the signals. To help evaluate the extent and role of pTyr during mitosis, we turned to quantitative, high-resolution and phosphoproteome-wide cell cycle studies that have also identified a number of Tyr phosphorylation sites during mitosis.Citation16,17 We collated 1950 pTyr sites on 1344 proteins in HeLa cells from large-scale phosphoproteomics studies in mitotic cell extracts. Using an integrated approach, we identified a number of subnetworks that are likely regulated by Tyr phosphorylation, including a subnetwork enriched for SFKs. Importantly, we defined for the first time a dedicated kinetochore/spindle subnetwork of pTyr containing proteins, in agreement with immunofluorescence observations. Bioinformatics confirmed enrichment of SFK phosphorylation motifs at the spindle, which was verified by chemical inhibition of SFKs.
One of our most striking observations was the identification of pTyr sites in the vicinity of Ser-Pro motifs, known to be the minimal motif for phosphorylation by mitogen activated protein kinases and CDKs. Phosphorylation on the neighboring Ser-Pro and Tyr motifs were identified in the vast majority of cases, and in a number of instances, peptides doubly phosphorylated at both the Pro-directed Ser as well as the Tyr were identified in the collated data set. These observations raise the intriguing possibility of cross-talk between CDKs and tyrosine kinases during mitosis, an idea which will require further investigation.
Another interesting observation was the preponderance of Tyr phosphorylation in kinase domains in general, and Tyr kinase domains in particular, highlighting the importance of crosstalk and autoregulation, respectively. Of particular interest, the key centrosome and kinetochore kinase polo-like kinase 1 (PLK1) was found to be phosphorylated at Tyr217 in the P+1 loop of the kinase domain. Substitution of the native Tyr with a phosphomimetic amino acid completely abrogated PLK1 activity and subsequent downstream PLK1 signaling, suggesting that phosphorylation at this site is incompatible with catalysis. Considering that PLK1 activity is critical to multiple mitotic events, it is unlikely that phosphorylation of this site occurs with significant stoichiometry, but may instead serve as a mechanism to selectively and rapidly inhibit sub-populations of PLK1 during rapid mitotic transitions. Nevertheless, a Tyr at this position is highly conserved among many Ser/Thr kinase subfamilies, and the identification of inhibitory phosphorylation at this position in other Ser/Thr kinases again illustrates that this kind of regulatory cross-talk between kinase families maybe more widespread than previously appreciated.Citation18
An independent study looking at the flip-side of the coin -phosphatases- arrived to similar conclusions. St-Denis et al. used a combination of phosphatase interaction screens and a follow-up phenotypic screen to explore the cellular functions of protein phosphatase.Citation2 To specifically target mitosis, the authors performed an endoribonuclease-generated small interfering RNA (esiRNA) screen targeting 151 phosphatases and 77 known phosphatase regulatory subunits to determine mitotic phenotypes associated with depletion of these phosphatases. After a secondary orthogonal screen to verify the primary hits, 33 phosphatases or their regulatory units were found to have an effect on mitotic progression, spindle formation or both. Strikingly,13 of these belonged to the protein tyrosine phosphatase (PTP) family, and every PTP subgroup was represented in the data set. Individual PTPs were found to play distinct roles in mitotic progression, including microtubule spindle stability (CDC14A), cell-matrix attachment (PTPRF), and mitotic exit (DUSP19). Of particular interst in this study was the identification of the atypical PTP DUSP23 (also known as VHZ) as a regulator of centriole duplication. DUSP23 likely elicits this function through association with PLK4 activity, a maste regulator centrosome biogenesis, and modification of its catalytic activity.Citation2
Future challenges in studying pTyr phospohryaltion during mitosis
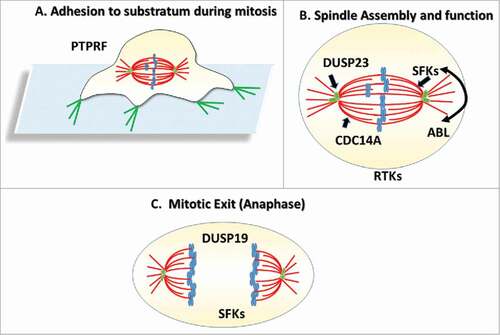
All in all, the observations from these two publications argue strongly that the regulation of Tyr phosphorylation is an important aspect of faithful mitotic cell division (). Clearly, much work remains to be done to more fully appreciate the extent and importance of pTyr modification during mitosis.
Figure 1. Phosphotyrosine writers and erasures during mitosis. (A) During early mitosis, retraction of actin-rich focation adhesiosn results in retraction -like fibers which may be regulated by the PTPRF phosphatase. (B) During mitosis, the Src Family Kinases (SFKs), and the CDC14A phosphatase contribute to proper spindle formation. SFKs and th ABL kinase have also both been implicated in spindle positioning and spindle orientation, whereas DUSP23 may regulate centriole biogenesis in S- phase thereby contributing to spindle bipolarity. (C) DSUP19 is thought to regulate the duration of anaphase whereas SFKs may play a role in cytokinesis.
An obvious fellow-up that emergesfrom these studies will be the identification of sites specifically up-and downregulated during cell division. This will initially require meticulous and quantitative mass spectrometry approaches to identify these sites and their dynamics, which is unlikely to be trivial considering the general scarcity of this modification. The recent improvement in immunoaffinity methods and the development of novel pTyr enrichment approaches will undoubtedly play an important role in this process.Citation19,20
An related challenge will be matching these pTyr site to their physiological readers and writers, as well as determining their functional significance to mitotic fidelity. Current literature strongly point to the pleiotropic SFKs as good candidates for the writers and the mitotic phosphatase screen revealed unexpected input from various but poorly understood PTPs. The phenotypic phosphatase screen revealed important insights into mitotic events that are regulated by tyrosine phosphatases which will direct future functional endeavors. Finally, what is the extent of cross-talk between Tyr kinases and the predominantly SeréThr kinases that drive mitosis, and when and where is this cross-talk occuring? Clearing, exciting times are ahead for kinase signaling in mitosis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
I would like to thank Michael Schwab and Terence Capellini for assistance with the title.
Funding
Work in the Elowe lab has been supported by grants from the Canadian Institutes for Health research (CIHR; #244442), the National Sciences and Engineering Research Council (#05841), and the Canadian Cancer Society (#19140). SE holds a CIHR New Investigator award.
References
- Caron D, Byrne DP, Thebault P, Soulet D, Landry CR, Eyers PA, Elowe S. Mitotic phosphotyrosine network analysis reveals that tyrosine phosphorylation regulates Polo-like kinase 1 (PLK1). Sci Signal 2016; 9:rs14; PMID:27965426; http://dx.doi.org/10.1126/scisignal.aah3525
- St-Denis N, Gupta GD, Lin ZY, Gonzalez-Badillo B, Veri AO, Knight JD, Rajendran D, Couzens AL, Currie KW, Tkach JM, et al. Phenotypic and interaction profiling of the human phosphatases identifies diverse mitotic regulators. Cell Rep 2016; 17:2488-501; PMID:27880917; http://dx.doi.org/10.1016/j.celrep.2016.10.078
- Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 2006; 127:635-48; PMID:17081983; http://dx.doi.org/10.1016/j.cell.2006.09.026
- Olsen JV, Vermeulen M, Santamaria A, Kumar C, Miller ML, Jensen LJ, Gnad F, Cox J, Jensen TS, Nigg EA, et al. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci Signal 2010; 3:ra3; PMID:20068231; http://dx.doi.org/10.1126/scisignal.2000475
- Pawson T, Scott JD. Protein phosphorylation in signaling – 50 Years and counting. Trends Biochem Sci 2005; 30:286-90; PMID:15950870; http://dx.doi.org/10.1016/j.tibs.2005.04.013
- Kruger M, Kratchmarova I, Blagoev B, Tseng YH, Kahn CR, Mann M. Dissection of the insulin signaling pathway via quantitative phosphoproteomics. Proc Natl Acad Sci U S A 2008; 105:2451-6; PMID:18268350; http://dx.doi.org/10.1073/pnas.0711713105
- Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007; 131:1190-203; PMID:18083107; http://dx.doi.org/10.1016/j.cell.2007.11.025
- Rush J, Moritz A, Lee KA, Guo A, Goss VL, Spek EJ, Zhang H, Zha XM, Polakiewicz RD, Comb MJ. Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat Biotechnol 2005; 23:94-101; PMID:15592455; http://dx.doi.org/10.1038/nbt1046
- Lindqvist A, Rodríguez-Bravo V, Medema RHH. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J Cell Biol 2009; 185:193-202; PMID:19364923; http://dx.doi.org/10.1083/jcb.200812045
- Taagepera S, Campbell MS, Gorbsky GJ. Cell-cycle-regulated localization of tyrosine and threonine phosphoepitopes at the kinetochores of mitotic chromosomes. Exp Cell Res 1995; 221:249-60; PMID:7589252; http://dx.doi.org/10.1006/excr.1995.1373
- Nakayama Y, Matsui Y, Takeda Y, Okamoto M, Abe K, Fukumoto Y, Yamaguchi N. c-Src but not Fyn promotes proper spindle orientation in early prometaphase. J Biol Chem 2012; 287:24905-15; PMID:22689581; http://dx.doi.org/10.1074/jbc.M112.341578
- Klinghoffer RA, Sachsenmaier C, Cooper JA, Soriano P. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J 1999; 18:2459-71; PMID:10228160; http://dx.doi.org/10.1093/emboj/18.9.2459
- Okamoto M, Nakayama Y, Kakihana A, Yuki R, Yamaguchi N, Yamaguchi N. Fyn accelerates M phase progression by promoting the assembly of mitotic spindle microtubules. J Cell Biochem 2016; 117:894-903; PMID:26365631; http://dx.doi.org/10.1002/jcb.25373
- Mardin BR, Isokane M, Cosenza MR, Krämer A, Ellenberg J, Fry AM, Schiebel E. EGF-induced centrosome separation promotes mitotic progression and cell survival. Dev Cell 2013; 25:229-40; PMID:23643362; http://dx.doi.org/10.1016/j.devcel.2013.03.012
- Wee P, Shi H, Jiang J, Wang Y, Wang Z. EGF stimulates the activation of EGF receptors and the selective activation of major signaling pathways during mitosis. Cell Signall 2015; 27:638-51; http://dx.doi.org/10.1016/j.cellsig.2014.11.030
- Kettenbach AN, Schweppe DK, Faherty BK, Pechenick D, Pletnev AA, Gerber SA. Quantitative phosphoproteomics identifies substrates and functional modules of Aurora and Polo-like kinase activities in mitotic cells. Sci Signal 2011; 4:rs5; PMID:21712546; http://dx.doi.org/10.1126/scisignal.2001497
- Sharma K, D'Souza RC, Tyanova S, Schaab C, Wiśniewski JR, Cox J, Mann M. Ultradeep human phosphoproteome reveals a distinct regulatory nature of Tyr and Ser/Thr-based signaling. Cell Rep 2014; 8:1583-94; PMID:25159151; http://dx.doi.org/10.1016/j.celrep.2014.07.036
- Lai S, Pelech S. Regulatory roles of conserved phosphorylation sites in the activation T-loop of the MAP kinase ERK1. Mol Biol Cell 2016; 27:1040-50; PMID:26823016; http://dx.doi.org/10.1091/mbc.E15-07-0527
- Bian Y, Li L, Dong M, Liu X, Kaneko T, Cheng K, Liu H, Voss C, Cao X, Wang Y, et al. Ultra-deep tyrosine phosphoproteomics enabled by a phosphotyrosine superbinder. Nat Chem Biol 2016; 12:959-66; PMID:27642862; http://dx.doi.org/10.1038/nchembio.2178
- Ding SJ, Qian WJ, Smith RD. Quantitative proteomic approaches for studying phosphotyrosine signaling. Expert Rev Proteomics 2007; 4:13-23; PMID:17288512; http://dx.doi.org/10.1586/14789450.4.1.13
