ABSTRACT
We propose here a hypothesis of the cause of cancer that brings together fundamental changes in methyl-group metabolism resulting in methionine dependence and global DNA hypomethylation which destabilizes the genome leading to aneuploid karyotypes which evolve and stabilize into autonomous cancer. Experimental support for this hypothesis is that methioine dependence is a general metabolic defect in caner. Methionine dependence is due to excess use of methionene for aberrant transmethylation reactions that apparently divert methyl groups from DNA. The resulting global DNA hypomethylation is also a general phenomena in cancer. Global hypomethylation leads to an unstable genomes and aneuploid karyotypes, another general phenomena in cancer. The excessive and aberrant use of methionine in cancer is strongly observed in [11C]methionine PET imaging, where high uptake of [11C]methionine results in a very strong and selective tumor signal compared with normal tissue background. [11C]methionine is superior to [18C] fluorodeoxyglucose (FDG)-PET for PET imaging, suggesting methionine dependence is more tumor-specific than glucose dependence.
Methionine dependence and altered transmethylation in cancer cells
The first hint that methyl metabolism is perturbed in cancer came almost 60 years ago when Sugimura et al.Citation1 observed that rat tumor growth was slowed by giving the rats a defined diet depleted in methionine. Approximately 45 years ago, it was observed that L5178Y mouse leukemia cells in culture required very high levels of methionine to proliferate.Citation2 Subsequently, most cancer cell lines were found to be methionine dependent.Citation3,4 These cell lines were derived from various cancer types including liver, pancreatic ovarian, submaxillary, brain, lung, bladder, prostate, breast, kidney, cervical, colon, fibrosarcoma, osteosarcoma, rhabdomyosarcoma, leiomyosarcoma, neuroblastoma, glioblastoma and melanoma. The occurrence of methionine dependence among these diverse cancer types suggests that methionine dependence may be a general phenomena in cancer.
Human patient tumors, including tumors of the colon, breast, ovary, prostate, and a melanoma, were also found to be methionine dependent in Gelfoam® histoculture.Citation5 Normal unestablished cell strains, thus far characterized, grow well in methionine depleted medium.Citation3,6
Methionine-dependent cancer cells synthesize normal amounts of methionine
Multiple lines of evidence indicated that methionine-dependent cancer cells synthesize large amounts of methionine endogenously through the reaction catalyzed by N5-methyltetrahydropteroyl-l-glutamate: l-homocysteine S-methyltransferase (EC 2.1.1.13).Citation7 For example, the activity of the methyltransferase involved in methionine biosynthesis was comparable in extracts of methionine-dependent malignant and normal methionine-independent cells. In addition, the uptake of radioactive label from [5−14C]methyltetrahydropteroyl-l-glutamic acid (N5-methyl-H4PteGlu) was at least as great in the methionine-dependent malignant cells as in the normal methionine-independent cells and was nearly totally dependent on the addition of homocysteine, the methyl acceptor. The majority of the labeled methyl groups incorporated by methionine-dependent cancer cells was recovered as methionine. These results indicated that the methionine auxotrophy (dependence) of the malignant cells does not result simply from the inability to synthesize and incorporate methionine from homocysteine and N5-methyl-H4PteGlu.Citation8
Low levels of endogenous methionine and S-adenosyl methionine in cancer cells under methionine-limiting conditions
We subsequently observed that although methionine-dependent cancer cells synthesized a normal amount of endogenous methionine, the level of free methionine and S-adenosylmethionine (AdoMET), the universal methyl donor, were very low in methionine-dependent cancer cells in methionine (MET) depleted homocysteine (HCY)-supplemented medium (MET−HCY+), but is normal in MET+HCY− medium. We determined that a low AdoMET/AdoHCY ratio probably limits growth of methionine-dependent cells in MET−HCY+ medium.Citation9
Enhanced rates of transmethylation in cancer cells
We observed that cancer cells have enhanced overall rates of transmethylation compared with normal cells. Transmethylation rates were measured by blocking AdoHCY hydrolase and measuring AdoHCY, which accumulates as a result of transmethylation. The enhanced transmethylation rates may be the basis of the methionine dependence of cancer cells which explains the low levels of free methionine and the low AdoMET/AdoHCY ratio in cancer cells under methionine deprivation, despite high rates of methionine synthesis.Citation10 The elevated methionine use in cancer cells has been termed the “Hoffman effect.”Citation11
Methionine-dependent cancer cells arrest in S/G2 phase of the cell cycle under methionine deprivation
Growth arrest of methionine-dependent cancer cells in MET −HCY+ medium resulted in a reduction in the percentage of mitotic cells in the cell population. Cell cycle analysis demonstrated that the cells are arrested in the S/G2 phases of the cell cycle in MET −HCY+ medium.Citation12,13 This is in contrast to a G1-phase accumulation of cells, which occurs only in methionine-supplemented medium at very high cell densities and is similar to the G1 block seen in cultures of normal fibroblasts at high density.Citation12,13
Methionine independent revertants
Rare cells from methionine-dependent cancer cell lines regained the normal ability to grow in MET−HCY+ medium. These lines were termed methionine-independent revertants.Citation14 Methionine-independent revertants also had much lower basal transmethylation rates than parental methionine-dependent cell lines.Citation15 These results further suggested that methionine dependence is due to an increase in the rate of transmethylation reactions.
We then demonstrated that methionine-independent revertants cells concomitantly reverted for characteristics associated with cancer and became less malignant. Thus, the methionine-independent revertants become more normal-like indicating further a relationship between altered methionine metabolism and oncogenic transformation.Citation16
Diversion of methyl groups in methionine-dependent cancer cells
When a methionine-dependent cancer cell line was cultured under conditions of methionine depletion, methylation of nucleic acids was decreased. However, there was increased methylation of both an endogenous substrate and Escherichia coli tRNA. These results indicated that methyl groups were being diverted from their normal sites, including DNA, in methionine-dependent cancer cells. Please see below for the implications of this abnormality.
Discovery and description of DNA hypomethylation in cancer cells
Genome-wide DNA hypomethylation of human cancer cells was first described by us in the early eighties,Citation17 and has been found in almost all types of cancers.Citation18 Other groups subsequently confirmed our discovery.Citation19-24
Perucho et al.Citation25 used a biochemical quantitative assay to estimate the percentage of methylated sites in DNA in normal and cancer gastric tissues from patients with gastric cancer and patients with high-risk gastritis (HRG) caused by H. pylori infection. The initiation and incidence and extent of somatic genome-wide hypomethylation was investigated to determine its chronology in the progression of gastric carcinogenesis.Citation25 Genome-wide hypomethylation was found in the gastric mucosa of gastric cancer patients and HRG patients as well as global DNA hypomethylation in tumors, suggesting an epigenetic field defect of DNA hypomethylation in gastric cancer, with H. pylori infection.Citation26 The degree of DNA hypomethylation in primary tumors was significantly associated with stage with the extent of invasion through the stomach layers.Citation25 One group of gastric cancer patients had much greater demethylation than the majority of the patients. The latter group was termed enhanced somatic hypomethylation (ESH). ESH tumors had a more aggressive phenotype, including invasion through the gastric wall and into adjacent lymph nodes.Citation25 Liteplo and KerbelCitation27 found that high-metastatic variants of a melanoma had more DNA hypomethylation than the low-metastatic parental cells, similar to the pattern Perucho et al.Citation25 observed.
DNA hypomethylation and aneuploidy
Suzuki et al. observed that hypomethylation preceded diploidy loss in a significant subset of gastrointestinal cancers, and had a stronger association with genetic damage and poorer prognosis than hypermethylation.Citation28 Widespread DNA hypomethylation in gastric cancer associates with chromosomal instability and poor prognosis. Compare et al.Citation29 also observed that global demethylation is an early event in the gastritis-cancer pathway. Preneoplastic lesions had decreased DNA methylation over time despite the eradication of H. pylori. Perucho et al.Citation25 proposed that DNA hypomethylation contributes to the initial stages of the carcinogenesis process by destabilizing the genome. The association between DNA hypomethylation and aneuploidy had been previously reported.Citation30-34
Jaenish et al.Citation33 generated transgenic mice with depleted DNA methyltransferase 1 (DNMT1) which resulted in genome-wide hypomethylation in all tissues. The mutant mice developed T cell lymphomas which had a high frequency of chromosome 15 trisomy. In another study, DNMT-deficient HCT-116 colon cancer cells had a high degree of DNA hypomethylation and genome instability leading to aneuploidy, including many novel chromosomal translocations.Citation34 These results indicate that DNA hypomethylation plays a causal role in chromosomal instability, aneuploidy, and subsequent cancer.Citation33
We suggest here that the pre-malignant DNA hypomethylation observed by Perucho et al.Citation25 results from the unbalanced global transmethylation observed in methionine dependence.
Results from Duesberg and colleaguesCitation35-41 suggest that autonomous cancers result from speciation based on selection of existing aneuploid karyotypes that convert mortal somatic cells to immortality and eventually to independence and cancer. It should be noted that BoveriCitation42 more than 100 years ago implicated altered karyotypes in the generation of malignancy.
Deprivation of methionine leads to cancer
Copeland and Salmon observed the development of liver, lung, and other cancers in a significant percentage of rats on a choline-deficient diet which results in depleted methionine.Citation42 Subsequently, Ghoshal and FarberCitation43 observed that Fischer 344 male rats fed a choline-methionine-deficient diet developed a 100% incidence of preneoplastic hepatocyte nodules, whereby 51% of the rats developed hepatocellular carcinoma. Supplement of the diet with 0.8% choline chloride prevented the development of both the precancerous nodules and subsequent cancer.Citation44 The cancers resulting from methionine/choline-deprived diets in the rats were probably due to methyl shortage and subsequent DNA hypomethylation, since methionine is the source of methyl groups for DNA methylation. DNA hypomethylation appears to be an early event in choline/methionine-deficient diets, which is irreversibleCitation44-47 and mediated by a deficiency in S-adenosylmethionine.Citation48
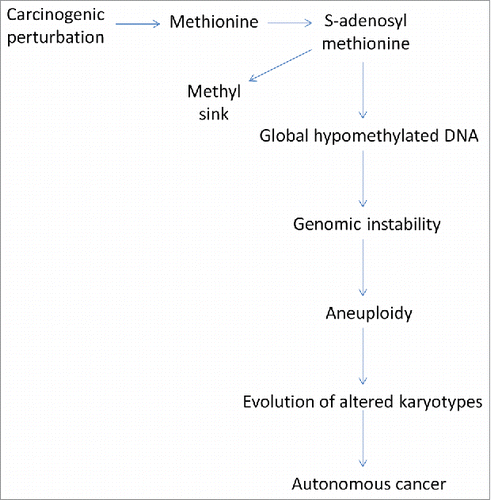
From perturbed methionine metabolism to DNA hypomethylation to a destabilized genome to aneuploidy to cancer ()
We suggest here that the pre-malignant DNA hypomethylation such as that observed by Perucho et al.Citation25 results from the unbalanced excess transmethylation which diverts methyl groups into where they are not available for normal methylation processes such as DNA methylation. The excess transmethylation rate explains the “methionine dependence” of cancer cells. Hypomethylated DNA then results in the initiation of aneuploidy and subsequent speciation to autonomous cancer.
Thus, we propose that carcinogenesis is initiated by perturbation of methionine metabolism, for example, rats on a chronic methionine-depleted diet, and altered transmethylation (“metabolic reprogramming”) resulting in DNA hypomethylation thereby destabilizing the karyotype, which sets off a chain reaction of aneuploidizations as Duesberg proposes, which generate ever-more abnormal karyotypes and eventually cancer-specific combinations.Citation49
Every hypothesis should be testable and be able to be negated. The present hypothesis predicts that premalignancy such as observed in the stomach with H. pylori infection or SV40 infection, other infectious agents such as human papiloma virus, or Epstein-Barr virus,Citation50 or carcinogens such as nitrosourea or cigarette smoke, should have an elevated methionine requirement and excess and altered transmethylation leading to DNA hypomethylation as shown in the stomach after H. pylori infection by Perucho et al.Citation25 The hypothesis predicts that chronic perturbation of methionine metabolism should be carcinogenic, such as a chronic methionine-depleted diet.Citation42,43
The current hypothesis predicts that deprivation of methionine should be therapeutic for cancer and in mouse models, this is the case.Citation7,51-55
Our hypothesis accommodates the role of altered genes that may influence the behavior of cancers, but with possibly rare exceptions, individual gene alterations do not account for the global changes of cancer that methionine dependence, global DNA hypomethylation and altered karotype evolution do.
The hypothesis also explains why the use of [11C] methionine is so effective in positron emission tomography (MET-PET) imaging, since the cancers use excessive methionine for their aberrant excess transmethylation and therefore take up excess [11C] methionine, compared to normal tissue.Citation56-60
In a comparison of MET-PET and fluorodeoxyglucose (FDG)-PET, MET-PET was found to be superior for gliomaCitation61 suggesting that cancer may have a greater abnormal requirement for methionine than glucose.
Recently, a paper appeared with the title “The new anticancer era: tumor metabolism targeting.Citation62 The present review suggests this “new anticancer era” started in 1959 with the observation of Sugimura et al.Citation1 that depriving cancer of methionine arrested tumor growth. It is our hope that this era will continue and lead to more effective cancer treatments.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Sugimura T, Birnbaum SM, Winitz M, Greenstein JP. Quantitative nutritional studies with water-soluble, chemically defined diets. VIII. The forced feeding of diets each lacking in one essential amino acid. Arch Biochem Biophys 1959; 81:448-55; PMID:13638009; http://dx.doi.org/10.1016/0003-9861(59)90225-5
- Chello PL, Bertino JR. Dependence of 5-methyltetrahydrofolate utilization by L5178Y murine leukemia cells in vitro on the presence of hydroxycobalamin and transcobalamin II. Cancer Res 1973; 33:1898-904; PMID:4737200
- Mecham JO, Rowitch D, Wallace CD, Stern PH, Hoffman RM. The metabolic defect of methionine dependence occurs frequently in human tumor cell lines. Biochem Biophys Res Commun 1983; 117:429-34; PMID:6661235; http://dx.doi.org/10.1016/0006-291X(83)91218-4
- Tan Y, Xu M, Hoffman RM. Broad selective efficacy of recombinant methioninase and polyethylene glycol-modified recombinant methioninase on cancer cells in vitro. Anticancer Res 2010; 30:1041-6; PMID:20530407
- Guo HY, Herrera H, Groce A, Hoffman RM. Expression of the biochemical defect of methionine dependence in fresh patient tumors in primary histoculture. Cancer Res 1993; 53:2479-83; PMID:8495409
- Hoffman RM. Altered methionine metabolism, DNA methylation and oncogene expression in carcinogenesis. A review and synthesis. Biochim Biophys Acta 1984; 738:49-87; PMID:6204687
- Hoffman RM. Development of recombinant methioninase to target the general cancer-specific metabolic defect of methionine dependence: A 40-year odyssey. Expert Opin Biol Ther 2015; 15:21-31; PMID:25439528; http://dx.doi.org/10.1517/14712598.2015.963050
- Hoffman RM, Erbe RW. High in vivo rates of methionine biosynthesis in transformed human and malignant rat cells auxotrophic for methionine. Proc Natl Acad Sci USA 1976; 73:1523-7; PMID:179090; http://dx.doi.org/10.1073/pnas.73.5.1523
- Coalson DW, Mecham JO, Stern PH, Hoffman RM. Reduced availability of endogenously synthesized methionine for S-adenosylmethionine formation in methionine dependent cancer cells. Proc Natl Acad Sci USA 1982; 79:4248-51; http://dx.doi.org/10.1073/pnas.79.14.4248
- Stern PH, Hoffman RM. Elevated overall rates of transmethylation in cell lines from diverse human tumors. In Vitro - Rapid Commun in Cell Biology 1984; 20:663-70
- Murakami T, Li S, Han Q, Tan Y, Kiyuna T, Igarashi K, Kawaguchi K, Hwang HK, Miyaki K, Singh AS, et al. Recombinant methioninase effectively targets a Ewing's sarcoma in a patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget, in press.
- Hoffman RM, Jacobsen SJ. Reversible growth arrest in simian virus 40-transformed human fibroblasts. Proc Natl Acad Sci USA 1980; 77:7306-10; PMID:6261250; http://dx.doi.org/10.1073/pnas.77.12.7306
- Yano S, Li S, Han Q, Tan Y, Bouvet M, Fujiwara T, Hoffman RM. Selective methioninase-induced trap of cancer cells in S/G2 phase visualized by FUCCI imaging confers chemosensitivity. Oncotarget 2014; 5:8729-36; PMID:25238266; http://dx.doi.org/10.18632/oncotarget.2369
- Hoffman RM, Jacobsen SJ, Erbe RW. Reversion to methionine independence by malignant rat and SV40-transformed human fibroblasts. Biochem Biophys Res Commun 1978; 82:228-34; PMID:208554; http://dx.doi.org/10.1016/0006-291X(78)90600-9
- Judde JG, Ellis M, Frost P. Biochemical analysis of the role of transmethylation in the methionine dependence of tumor cells. Cancer Res 1989; 49:4859-65; PMID:2503245
- Hoffman RM, Jacobsen SJ, Erbe RW. Reversion to methionine independence in simian virus 40-transformed human and malignant rat fibroblasts is associated with altered ploidy and altered properties of transformation. Proc Natl Acad Sci USA 1979; 76:1313-17; PMID:220612; http://dx.doi.org/10.1073/pnas.76.3.1313
- Diala ES, Hoffman RM. Hypomethylation of HeLa cell DNA and the absence of 5-methylcytosine in SV40 and adenovirus (type 2) DNA: analysis by HPLC. Biochem Biophys Res Commun 1982; 107:19-26; PMID:6289818; http://dx.doi.org/10.1016/0006-291X(82)91663-1
- Diala ES, Cheah MSC, Rowitch D, Hoffman RM. Extent of DNA methylation in human tumor cells. J. Natl. Cancer Inst 1983; 71:755-64; PMID:6578371
- Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer 2004; 4:143-53; PMID:14732866; http://dx.doi.org/10.1038/nrc1279
- Park JH, Park J, Choi JK, Lyu J, Bae MG, Lee YG, Bae JB, Park DY, Yang HK, Kim TY, et al. Identification of DNA methylation changes associated with human gastric cancer. BMC Med Genomics 2011; 4:82; PMID:22133303; http://dx.doi.org/10.1186/1755-8794-4-82
- Yoshida T, Yamashita S, Takamura-Enya T, Niwa T, Ando T, Enomoto S, Maekita T, Nakazawa K, Tatematsu M, Ichinose M, et al. Alu and Satα hypomethylation in Helicobacter pylori-infected gastric mucosae. Int J Cancer 2011; 128:33-9; PMID:20602342; http://dx.doi.org/10.1002/ijc.25534
- Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature 1983; 301:89-92; PMID:6185846; http://dx.doi.org/10.1038/301089a0
- Feinberg AP, Vogelstein B. Hypomethylation of ras oncogenes in primary human cancers. Biochem Biophys Res Commun 1983; 111:47-54; PMID:6187346; http://dx.doi.org/10.1016/S0006-291X(83)80115-6
- Khan R, Zhang XY, Supakar PC, Ehrlich KC, Ehrlich M. Human methylated DNA-binding protein. Determinants of a pBR322 recognition site. J Biol Chem 1988; 263:14374-83; PMID:3170549
- Leodolter A, Alonso S, González B, Ebert MP, Vieth M, Röcken C, Wex T, Peitz U, Malfertheiner P, Perucho M. Somatic DNA hypomethylation in H. pylori-associated high-risk gastritis and gastric cancer: Enhanced somatic hypomethylation associates with advanced stage cancer. Clin Transl Gastroenterol 2015; 6:e85; PMID:25928808; http://dx.doi.org/10.1038/ctg.2015.14
- Ushijima T, Hattori N. Molecular pathways: involvement of Helicobacter pylori-triggered inflammation in the formation of an epigenetic field defect, and its usefulness as cancer risk and exposure markers. Clin Cancer Res 2012; 18:923-9; PMID:22205689; http://dx.doi.org/10.1158/1078-0432.CCR-11-2011
- Liteplo RG, Kerbel RS. Reduced levels of DNA 5-methylcytosine in metastatic variants of the human melanoma cell line MeWo. Cancer Res 1987; 47:2264-7; PMID:3567919
- Suzuki K, Suzuki I, Leodolter A, Alonso S, Horiuchi S, Yamashita K, Perucho M. Global DNA demethylation in gastrointestinal cancer is age dependent and precedes genomic damage. Cancer Cell 2006; 9:199-207; PMID:16530704; http://dx.doi.org/10.1016/j.ccr.2006.02.016
- Compare D, Rocco A, Liguori E, D'Armiento FP, Persico G, Masone S, Coppola-Bottazzi E, Suriani R, Romano M, Nardone G. Global DNA hypomethylation is an early event in Helicobacter pylori-related gastric carcinogenesis. J Clin Pathol 2011; 64:677-82; PMID:21617174; http://dx.doi.org/10.1136/jcp.2010.087858
- Kamiyama H, Suzuki K, Maeda T, Koizumi K, Miyaki Y, Okada S, Kawamura YJ, Samuelsson JK, Alonso S, Konishi F, Perucho M. DNA demethylation in normal colon tissue predicts predisposition to multiple cancers. Oncogene 2012; 31:5029-37
- Rodriguez J, Frigola J, Vendrell E, Risques RA, Fraga MF, Morales C, Moreno V, Esteller M, Capellà G, Ribas M, et al. Chromosomal instability correlates with genome-wide DNA demethylation in human primary colorectal cancers. Cancer Res 2006; 66:8462-9468; PMID:16951157; http://dx.doi.org/10.1158/0008-5472.CAN-06-0293
- Eden A, Gaudet F, Waghmare A, Jaenisch R. Chromosomal instability and tumors promoted by DNA hypomethylation. Science 2003; 300:455; PMID:12702868; http://dx.doi.org/10.1126/science.1083557
- Gaudet F, Hodgson JG, Eden A, Jackson-Grusby L, Dausman J, Gray JW, Leonhardt H, Jaenisch R. Induction of tumors in mice by genomic hypomethylation. Science 2003; 300:489-92; PMID:12702876; http://dx.doi.org/10.1126/science.1083558
- Karpf AR, Matsui S. Genetic disruption of cytosine DNA methyltransferase enzymes induces chromosomal instability in human cancer cells. Cancer Res 2005; 65:8635-9; PMID:16204030; http://dx.doi.org/10.1158/0008-5472.CAN-05-1961
- Bloomfield M, Duesberg P. Karyotype alteration generates the neoplastic phenotypes of SV40-infected human and rodent cells. Mol Cytogenet 2015; 8:79; PMID:26500699; http://dx.doi.org/10.1186/s13039-015-0183-y
- Bloomfield M, McCormack A, Mandrioli D, Fiala C, Aldaz CM, Duesberg P. Karyotypic evolutions of cancer species in rats during the long latent periods after injection of nitrosourea. Mol Cytogenet 2014; 7:71; PMID:25614763; http://dx.doi.org/10.1186/s13039-014-0071-x
- Duesberg PH. Does aneuploidy destabilize karyotypes automatically? Proc Natl Acad Sci USA 2014; 111:E974; PMID:24569866
- Li L, McCormack AA, Nicholson JM, Fabarius A, Hehlmann R, Sachs RK, Duesberg PH. Cancer-causing karyotypes: chromosomal equilibria between destabilizinganeuploidy and stabilizing selection for oncogenic function. Cancer Genet Cytogenet 2009; 188:1-25; PMID:19061776; http://dx.doi.org/10.1016/j.cancergencyto.2008.08.016
- Duesberg PH. Are cancers dependent on oncogenes or on aneuploidy? Cancer Genet Cytogenet 2003 May; 143(1):89-91.
- Fabarius A, Hehlmann R, Duesberg PH. Instability of chromosome structure in cancer cells increases exponentially with degrees of aneuploidy. Cancer Genet Cytogenet 2003; 143:59-72; PMID:12742157
- Rasnick D, Duesberg PH. How aneuploidy affects metabolic control and causes cancer. Biochem J 1999; 340(Pt 3):621-30; PMID:10359645
- Copeland DH, Salmon WD. The occurrence of neoplasms in the liver, lungs, and other tissues of rats as a result of prolonged choline deficiency. Am J Pathol 1946; 22:1059-79; PMID:20999314
- Ghoshal AK, Farber E. The induction of liver cancer by dietary deficiency of choline and methionine without added carcinogens. Carcinogenesis 1984; 5:1367-70; PMID:6488458; http://dx.doi.org/10.1093/carcin/5.10.1367
- Christman JK, Chen M-L, Sheikhnejad G, Dizik M, Abileah S, Wainfan E. Methyl deficiency, DNA methylation, and cancer: Studies on the reversibility of the effects of a lipotrope-deficient diet. J Nutr Biochem 1993; 4:672-80; http://dx.doi.org/10.1016/0955-2863(93)90106-7
- Wainfan E, Dizik M, Stender M, Christman JK. Rapid appearance of hypomethylated DNA in livers of rats fed cancer-promoting, methyl-deficient diets. Cancer Res 1989; 49:4094-7; PMID:2743304
- Tsujiuchi T, Tsutsumi M, Sasaki Y, Takahama M, Konishi Y. Hypomethylation of CpG sites and c-myc gene overexpression in hepatocellular carcinomas, but not hyperplastic nodules, induced by a choline-deficient L-amino acid-defined diet in rats. Jpn J Cancer Res 1999; 90:909-13; PMID:10551317
- Shimizu K, Onishi M, Sugata E, Sokuza Y, Mori C, Nishikawa T, Honoki K, Tsujiuchi T. Disturbance of DNA methylation patterns in the early phase of hepatocarcinogenesis induced by a choline-deficient L-amino acid-defined diet in rats. Cancer Sci 2007; 98:1318-22; PMID:17640295; http://dx.doi.org/10.1111/j.1349-7006.2007.00564.x
- Shivapurkar N, Poirier LA. Tissue levels of S-adenosylmethionine and S-adenosylhomocysteine in rats fed methyl-deficient, amino acid-defined diets for one to five weeks. Carcinogenesis 1983; 4:1051-7; PMID:6872150; http://dx.doi.org/10.1093/carcin/4.8.1051
- Duesberg P, Li R. Multistep carcinogenesis: a chain reaction of aneuploidizations. Cell Cycle 2003; 2:202-10; PMID:12734426; http://dx.doi.org/10.4161/cc.2.3.382
- Kanai Y, Hirohashi S. Alterations of DNA methylation associated with abnormalities of DNA methyltransferases in human cancers during transition from a precancerous to a malignant state. Carcinogenesis 2007; 28:2434-42; PMID:17893234; http://dx.doi.org/10.1093/carcin/bgm206
- Kokkinakis DM, Schold SC Jr, Hori H, Nobori T. Effect of long-term depletion of plasma methionine on the growth and survival of human brain tumor xenografts in athymic mice. Nutrition and Cancer 1997; 29:195-204; PMID:9457739; http://dx.doi.org/10.1080/01635589709514624
- Kokkinakis DM, von Wronski MA, Vuong TH, Brent TP, Schold SC Jr. Regulation of O6-methylguanine-DNA methyltransferase by methionine in human tumour cells. British J Cancer 1997; 75:779-88; PMID:9062396; http://dx.doi.org/10.1038/bjc.1997.141
- Kokkinakis DM, Hoffman RM, Frenkel EP, Wick JB, Han Q, Xu M, Tan Y, Schold SC. Synergy between methionine stress and chemotherapy in the treatment of brain tumor xenografts in athymic mice. Cancer Res 2001; 61:4017-23; PMID:11358820
- Tan Y, Sun X, Xu M, Tan X-Z, Sasson A, Rashidi B, Han Q, Tan X-Y, Wang X, An Z, et al. Efficacy of recombinant methioninase in combination with cisplatin on human colon tumors in nude mice. Clin Cancer Res 1999; 5:2157-63; PMID:10473100
- Yoshioka T, Wada T, Uchida N, Maki H, Yoshida H, Ide N, Kasai H, Hojo K, Shono K, Maekawa R, et al. Anticancer efficacy in vivo and in vitro, synergy with 5-fluorouracil, and safety of recombinant methioninase. Cancer Res 1998; 58:2583-7; PMID:9635582
- Grosu AL, Weber WA, Riedel E, Jeremic B, Nieder C, Franz M, Gumprecht H, Jaeger R, Schwaiger M, Molls M. L-(methyl-11C) methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int J Radiat Oncol Biol Phys 2005; 63:64-74; PMID:16111573; http://dx.doi.org/10.1016/j.ijrobp.2005.01.045
- Glaudemans AW, Enting RH, Heesters MA, Dierckx RA, van Rheenen RW, Walenkamp AM, Slart RH. Value of 11C-methionine PET in imaging brain tumours and metastases. Eur J Nucl Med Mol Imaging 2013; 40:615-35; PMID:23232505; http://dx.doi.org/10.1007/s00259-012-2295-5
- Tsuyuguchi N, Takami T, Sunada I, Iwai Y, Yamanaka K, Tanaka K, Nishikawa M, Ohata K, Torii K, Morino M, et al. Methionine positron emission tomography for differentiation of recurrent brain tumor and radiation necrosis after stereotactic radiosurgery–in malignant glioma. Ann Nucl Med 2004; 18:291-6; PMID:15359921; http://dx.doi.org/10.1007/BF02984466
- Nariai T, Tanaka Y, Wakimoto H, Aoyagi M, Tamaki M, Ishiwata K, Senda M, Ishii K, Hirakawa K, Ohno K. Usefulness of L-[methyl-11C] methionine-positron emission tomography as a biological monitoring tool in the treatment of glioma. J Neurosurg 2005; 103:498-507; PMID:16235683; http://dx.doi.org/10.3171/jns.2005.103.3.0498
- Tamura K, Yoshikawa K, Ishikawa H, Hasebe M, Tsuji H, Yanagi T, Suzuki K, Kubo A, Tsujii H. Carbon-11-methionine PET imaging of choroidal melanoma and the time course after carbon ion beam radiotherapy. Anticancer Res 2009; 29:1507-14; PMID:19443358
- Singhal T, Narayanan TK, Jacobs MP, Bal C, Mantil JC. 11C-methionine PET for grading and prognostication in gliomas: a comparison study with 18F-FDG PET and contrast enhancement on MRI. J Nucl Med 2012; 53:1709-15; PMID:23055534; http://dx.doi.org/10.2967/jnumed.111.102533
- Borriello A, Della Ragione F. The new anticancer era: Tumor metabolism targeting. Cell Cycle 2017; 16:310-1; PMID:28055312; http://dx.doi.org/10.1080/15384101.2016.1271635