The conserved Cdc7 kinase regulates various chromosome events. Among them, regulation of initiation of DNA replication at origins is the most conserved and important function of Cdc7. Cdc7 phosphorylates subunits of Mcm in the pre-Replicative Complex, which triggers its association with Sld3-Cdc45 to facilitate formation of the CMG helicase. Cdc7 has also been implicated in cellular responses to replication/ genotoxic stress. In yeast, Dbf4, the activation subunit, is a target of replication checkpoint. Phosphorylation of Dbf4 by Rad53 checkpoint kinase is a part of mechanism that inhibits replication initiation in response to replication stress.Citation1,2 On the other hand, roles of Cdc7 in checkpoint activation also have been shown. Among them, Mrc1/Claspin is a potential target of Cdc7 kinase in replication checkpoint activation.Citation3,4
In a recent issue of Cell Cycle, Tudzarova and her colleagues reported a novel genotoxic stress checkpoint that involves Cdc7.Citation5 Authors found that Cdc7 protein level is significantly reduced in response to genotoxic stress (irradiation or Doxorubicin) in release from serum starvation of IMR90 fibroblast cells. Notably, this reduction depends on p53 function. Furthermore, both transcriptional and post-transcriptional (protein stability) pathways operate for the p53-dependent inhibition of Cdc7 expression. The former is mediated by the p53-miR-192/215-Cdc7, whereas the latter by p53-Fbxw7β (ubiquitin ligase)-p53 pathway. Activation of these pathways inhibited the entry into S phase in the presence of DNA damages.
p53 plays a major role in protection of genome integrity from genotoxic stress. The well known p53-p21 pathway downregulates Cyclin-dependent kinase. The p53-Cdc7 link discovered in this study provides another cellular strategy by which cells protect its genome by inhibiting the S phase entry in the presence of DNA damages. Authors have also shown the presence of a cross-talk between the two pathways. The inhibition of Cdk activity by the p53-p21 pathway leads to the inhibition of phosphorylation of potential Cdc4/Fbxw7β phosphodegron sequences on Cdc7 (most notably T376) that stimulates Cdc7 degradation by inducing GSKβ-mediated Cdc7 phosphorylation and subsequent association of Fbxw7β (E3 ubiquitin ligase).
Added finding in this report is the Cdc7-mediated repression of p53 expression, which results in unrestrained S phase entry and the generation of DNA damages. This could partially explain the genomic instability of cancer cells which often overexpress Cdc7.
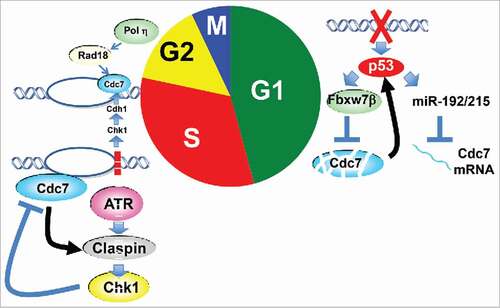
In summary, Cdc7, which plays a central role for initiation of DNA replication, plays crucial roles also in cellular responses to replication and genotoxic stress through multiple mechanisms (). During G1 phase, it serves as a target of p53-mediated arrest of S phase entry. The same authors previously reported the presence of p53-dependent “origin activation checkpoint” in human cells in which replication initiation is inhibited.Citation6 It is the safest strategy to inhibit the cell cycle before the start of DNA synthesis, when there are lesions on the genome. During S phase, Cdc7-Dbf4 serves as both an activator and a target of replication checkpoint response. Cdc7 activates ATR-Claspin-Chk1 (Rad3-Mrc1-Cds1 in fission yeast) pathway most likely through regulating Claspin/Mrc1. Cdc7-Dbf4 are hyperphosphorylated by checkpoint effector kinases in response to replication stress, which may somehow inhibit further origin firing. It was also reported in human cells that genotoxic stress during S phase results in stabilization of Cdc7 to facilitate the lesion bypass by Rad18-Polη.Citation7 Theses strategies are needed to preserve ongoing replication forks and ensure the completion of the genome replication.
Figure 1. Cellular responses to replication/ genotoxic stress involving Cdc7 kinase. During G1, p53 downregulates Cdc7 level through two pathways to prevent entry into S phase. p53 also inhibits Cdk through p21 and this facilitates Cdc7 destruction (not shown in figure). During S, Cdc7 plays roles as both replication stress checkpoint activator and target. Cdc7 also recruits lesion bypass machinery at the stalled fork.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Zegerman P, Diffley JF. Checkpoint-dependent inhibition of DNA replication initiation by Sld3 and Dbf4 phosphorylation. Nature 2010; 467:474-478; PMID:20835227; http://dx.doi.org/10.1038/nature09373
- Lopez-Mosqueda J, Maas NL, Jonsson ZO, Defazio-Eli LG, Wohlschlegel J, Toczyski DP. Damage-induced phosphorylation of Sld3 is important to block late origin firing. Nature 2010; 467:479-483; PMID:20865002; http://dx.doi.org/10.1038/nature09377
- Kim JM, Kakusho N, Yamada M, Kanoh Y, Takemoto N, Masai H. Cdc7 kinase mediates Claspin phosphorylation in DNA replication checkpoint. Oncogene 2008; 27:3475-3482; PMID:18084324; http://dx.doi.org/10.1038/sj.onc.1210994
- Matsumoto S, Shimmoto M, Kakusho N, Yokoyama M, Kanoh Y, Hayano M, Russell P, Masai H. Checkpoint-independent Regulation of Origin Firing by Mrc1 through Interaction with Hsk1 kinase. Cell Cycle 2010; 9:4627-4637; PMID:21099360; http://dx.doi.org/10.4161/cc.9.23.13937
- Tudzarova S, Mulholland P, Dey A, Stoeber K, Okorokov AL, Williams GH. p53 controls CDC7 levels to reinforce G1 cell cycle arrest upon genotoxic stress. Cell Cycle 2016; 15:2958-2972; PMID:27611229; http://dx.doi.org/10.1080/15384101.2016.1231281
- Tudzarova S, Trotter MW, Wollenschlaeger A, Mulvey C, Godovac-Zimmermann J, Williams GH, Stoeber K. Molecular architecture of the DNA replication origin activation checkpoint. EMBO J 2010; 29:3381-3394; PMID:20729811; http://dx.doi.org/10.1038/emboj.2010.201
- Yamada M, Masai H, Bartek J. Regulation and roles of Cdc7 kinase under replication stress. Cell Cycle 2014; 13:1859-1866; PMID:24841992; http://dx.doi.org/10.4161/cc.29251
