ABSTRACT
Notch signaling has been reported to play an essential role in tumorigenesis. Several studies have suggested that Notch receptors could be oncoproteins or tumor suppressors in different types of human cancers. Emerging evidence has suggested that Notch pathway regulates cell growth, apoptosis, cell cycle, and metastasis. In the current study, we explore whether Notch-1 could regulate the cell invasion and migration as well as EMT (epithelial-mesenchymal transition) in prostate cancer cells. We found that overexpression of Notch-1 enhanced cell migration and invasion in PC-3 cells. However, downregulation of Notch-1 retarded cell migration and invasion in prostate cancer cells. Importantly, we observed that overexpression of Notch-1 led to EMT in PC-3 cells. Notably, we found that EMT-type cells are associated with EMT markers change and cancer stem cell phenotype. Taken together, we concluded that downregulation of Notch-1 could be a promising approach for inhibition of invasion in prostate cancer cells, which could be useful for the treatment of metastatic prostate cancer.
Introduction
Prostate cancer is one of the most common malignant in men and the second leading cause of cancer death for males in the United States.Citation1 Over 161,360 prostate cancer cases will be expected to occur and 26,730 patients will die from prostate cancer in 2017.Citation1 Although routine screening with the PSA (prostate-specific antigen) test is helpful for early diagnosis of prostate cancer, high rates of over-diagnosis by PSA test contribute to screen-detected cancers.Citation2 Currently, several treatments include surgery, chemotherapy, and radiation therapy.Citation3 In addition, hormonal ablation therapy is also often used for prostate cancer patients. Androgen deprivation is initially useful to shrink the tumor volume.Citation3 However, many patients exhibit resistance to androgen deprivation therapy, resulting in mCRPC (metastatic castrate-resistant prostate cancer).Citation4 The patients with mCRPC have poor survival, suggesting that understanding the mechanism of prostate cancer development and progression is pivotal for discovery of new therapies of prostate cancer.
Multiple studies have revealed that cellular signaling cascades such as Akt, mTOR (mammalian target of rapamycin), Wnt, and Shh (sonic hedgehog) are critically involved in pathological progression of prostate cancer.Citation5-8 Recently, Notch signaling pathway was characterized as a potential driver in prostate cancer development.Citation9,10 Notch receptors (Notch 1–Notch 4) and their ligands (Jagged-1, Jagged-2, Delta-1, Delta-3, and Delta-4) have been identified.Citation11,12 When ligand binds to its receptor, metalloproteinase and gamma secretase will cleave Notch receptor, leading to releasing ICN (intracellular domain of Notch) from the plasma membrane and subsequent translocating into nucleus.Citation13,14 Thus, ICN forms a complex with CSL (CBF1/Su(H)/Lag-1) and triggers the transcription of its targets such as cyclin D, Hey family and Hes (hairy enhancer of split) family.Citation15 Deregulated Notch signaling has been observed in a variety of human cancers including prostate cancer.Citation16-19 For example, Jagged-1 expression is associated with prostate cancer metastasis and recurrence.Citation20 Similarly, another study showed that elevated Jagged-1 and Notch-1 expression was found in high grade and metastatic prostate cancers.Citation21 Moreover, depletion of Notch-1 inhibited proliferation and induced apoptosis in PC-3 cells.Citation22 Additionally, downregulation of CSL activity suppressed cell proliferation in prostate cancer cells.Citation23 CSL regulated Akt to mediate androgen- independence in prostate cancer progression.Citation24 Furthermore, it has been found that Notch-3 is activated and contributes to the progression of prostate cancer.Citation25 High expression of Notch signaling pathway stimulated cell proliferation in prostate luminal epithelial cells.Citation26 Notably, Notch signaling pathway could play a role especially in the formation of PIN (prostatic intraepithelial neoplasia) structures.Citation27 Strikingly, Notch promoted tumor metastasis in a prostate-specific Pten (phosphatase and tensin homolog)-null mouse model.Citation28 Interestingly, phosphorylation of Notch-1 by Pim (proviral insertion in murine) kinases promoted oncogenic signaling in prostate cancer cells.Citation29 Recently, one study identified that inhibition of Notch pathway arrested PTEN-deficient advanced prostate cancer via enhancing p27-driven cellular senescence.Citation30
Studies investigated the function of Notch signaling pathway in prostate cancer.Citation31 However, it is unclear whether Notch pathway is associated with EMT in prostate cancer. Therefore, in the current study, we explore the role of Notch in regulation of EMT in prostate cancer cells. We found that overexpression of Notch-1 enhanced cell migration and invasion in PC-3 cells, whereas downregulation of Notch-1 retarded cell migration and invasion in prostate cancer cells. Moreover, overexpression of Notch-1 led to EMT in PC-3 cells. Notably, we found that EMT-type cells are associated with EMT markers change and cancer stem cell (CSC) phenotype. Taken together, our results indicated that activation of Notch signaling is associated with EMT characteristics of prostate cancer cells. These findings demonstrated that Notch pathway could be a promising target for the treatment of metastatic prostate cancer.
Results
Activation of Notch-1 in PC-3 cells
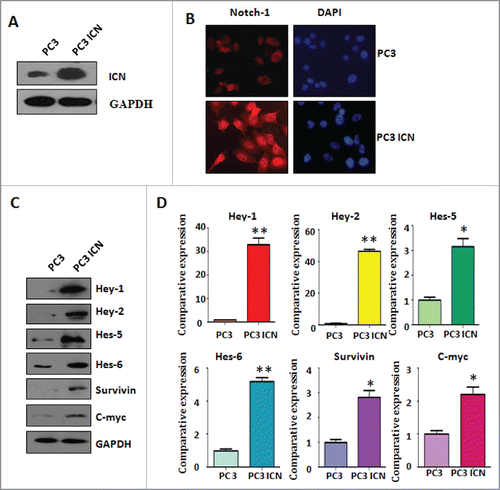
To explore the function of Notch-1 in prostate cancer cells, PC-3 cells were transfected with human ICN plasmid or pcDNA3 as control group. Western blotting analysis was used to detect the efficacy of plasmid transfection in PC-3 cells. We found that ICN transfection in PC-3 (PC-3 ICN) cells led to higher expression of Notch-1 (). To further confirm the transfection efficacy of ICN plasmid, immunofluorescence was performed in PC-3 cells with ICN transfection. We observed that PC-3 ICN cells displayed an increased activation of Notch-1 (). To validate whether PC-3 ICN could activate the downstream targets of Notch-1, Western blotting analysis was conducted to measure the expression of Notch-1 targets. We found that multiple targets of Notch-1 are highly expressed in PC-3 ICN cells, including Hey-1, Hey-2, Hes-5, Hes-6, Survivin, and c-Myc (). Our real-time RT-PCR (reverse transcription polymerase chain reaction) results showed that the mRNA levels of these genes are also increased in PC-3 ICN cells (). Our results demonstrated that Notch-1 is highly activated in PC-3 ICN cells.
Figure 1. Activation of Notch-1 in PC-3 cells. (A) Western blotting was used to detect the expression of Notch-1 in prostate cancer cells. PC-3 ICN: PC-3 with ICN plasmid transfection. (B) Immunofluorescence was performed in PC-3 cells with ICN transfection. (C) Western blotting analysis was conducted to measure the expression of Notch-1 targets in prostate cancer cells. (D) Real-time RT-PCR was performed to detect the mRNA levels of Notch-1 targets in prostate cancer cells. *P<0.05; **P<0.01 vs PC-3 cells.
Overexpression of Notch enhanced cell migration and invasion
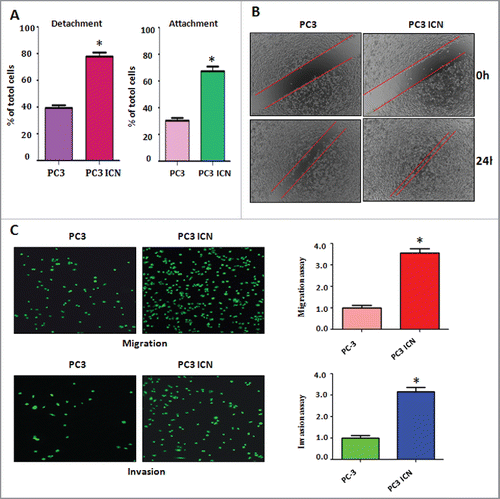
To examine whether Notch-1 could contribute to migratory activity of PC-3 cells, cell detachment and attachment were measured in PC-3 ICN cells. We found that PC-3 ICN cells exhibited enhanced capacity for attachment and detachment (). To evaluate whether Notch-1 could promote cell migration, wound healing assay was applied for detection of cell migration. We found that PC-3 ICN cells had more wound healing capacity compared with PC-3 cells (). Matrigel migration and invasion chamber assays were performed to examine the invasive activity of PC-3 cells transfected with ICN. We found that PC-3 ICN cells had increased cell migration (). We also observed that PC-3 ICN cells showed a higher level of penetration through the Matrigel-coated membrane ().
Figure 2. Overexpression of Notch enhanced cell migration and invasion. (A) Cell detachment and attachment were measured in PC-3 ICN cells. *P<0.05 vs PC-3 cells. (B) Wound healing assay was applied for detection of cell migration in PC-3 cells with ICN transfection. (C) Left panel: Matrigel migration and invasion chamber assays were performed to examine the invasive activity of PC-3 cells transfected with ICN. Right panel: quantitative results are illustrated for left panel. *P<0.05 vs PC-3 cells.
Down-regulation of Notch-1 retarded cell migration and invasion
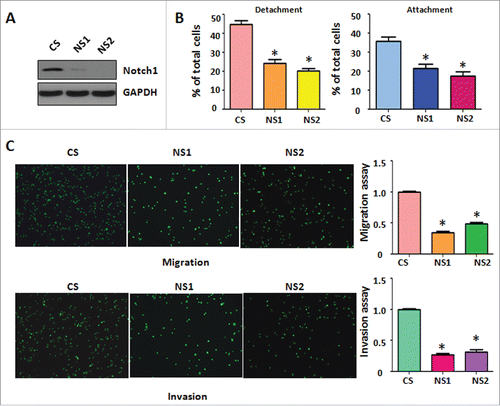
To further determine whether Notch-1 regulates cell migratory activity, we used Notch-1 siRNA for downregulation of Notch-1 in PC-3 cells. The efficacy of Notch-1 siRNA for knocking down Notch-1 was validated by Western blotting. We found that Notch-1 expression was barely detectable in Notch-1 siRNA transfected PC-3 cells (). Moreover, our cell detachment and attachment analysis showed that downregulation of Notch-1 by its siRNA inhibited cell detachment and attachment in PC-3 cells (). Furthermore, our migration and invasion assays demonstrated that depletion of Notch-1 retarded cell migration and invasion in PC-3 cells (). Therefore, our results suggest that downregulation of Notch-1 suppressed cell migration and invasion.
Figure 3. Down-regulation of Notch-1 retarded cell migration and invasion. (A) Western blotting was conducted to measure the efficacy of Notch-1 siRNA for knocking down Notch-1 in PC-3 cells. CS: control siRNA; NS: Notch-1 siRNA. (B) Cell detachment and attachment were measured in PC-3 cells transfected with Notch-1 siRNA. *P<0.05 vs PC-3 cells. (C) Left panel: Matrigel migration and invasion chamber assays were conducted to detect the invasive activity of PC-3 cells transfected with Notch-1 siRNA. Right panel: quantitative results are illustrated for left panel. *P<0.05 vs control.
PC-3 ICN cells have the morphologic changes
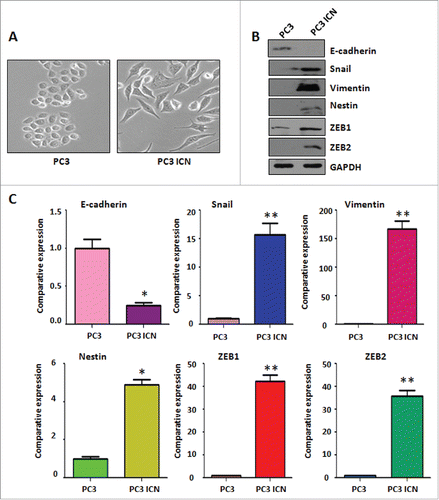
It has been reported that overexpression of Notch-1 is associated with EMT in human cancer cells.Citation32 Therefore, we determined whether Notch-1 could contribute to EMT in prostate cancer cells. PC-3 cells are typical of an epithelial cobblestone appearance. However, we found that PC-3 ICN cells displayed elongated, irregular fibroblastoid morphology (). These changes in phenotype implied that PC-3 ICN cells could undergo the EMT. It has been documented that EMT-type cells have more invasive characteristics. In line with this, our PC-3 ICN cells have increased migratory and invasive ability. These results indicated that PC-3 ICN cells underwent EMT with more cell migratory characteristics.
Figure 4. PC-3 ICN cells have the morphologic changes and EMT feature. (A) The PC-3 and PC-3 ICN cells were photographed under the microscope. (B) Western blotting was conducted to measure the protein levels of EMT markers in PC-3 ICN cells. (C) Real-time RT-PCR was performed to detect the mRNA levels of EMT markers in prostate cancer cells. *P<0.05; **P<0.01 vs PC-3 cells.
PC-3 ICN cells have the EMT marker changes
To further validate whether PC-3 cells with overexpression of Notch-1 underwent EMT, the expression of EMT markers was detected by real-time RT-PCR and Western blotting analysis in PC-3 ICN cells. Our Western blotting data demonstrated that the protein level of EMT markers in PC-3 ICN cells is consistent with EMT characteristics (). Consistently, our real-time RT-PCR results showed that E-cadherin expression was significantly decreased, whereas the expression of Vimentin, Slug, ZEB1 and ZEB2 was increased (). Moreover, immunofluorescence data confirmed our Western blotting results (), suggesting that PC-3 ICN cells underwent EMT change. To further validate the role of Notch signaling pathway in these EMT type cells, PC-3 ICN cells were treated with Notch-1 siRNAs, which inactivates Notch activation. We found that PC-3 ICN cells were reversed from EMT to MET after treatment with Notch-1 siRNA (). Importantly, we found that Notch-1 siRNA treatments inhibited the expression of mesenchymal markers including ZEB1, Vimentin, Nestin, and Snail in PC-3 ICN cells ().
Figure 5. PC-3 ICN cells have the EMT marker changes. Immunofluorescence was conducted to measure the expression of EMT markers in prostate cancer cells.
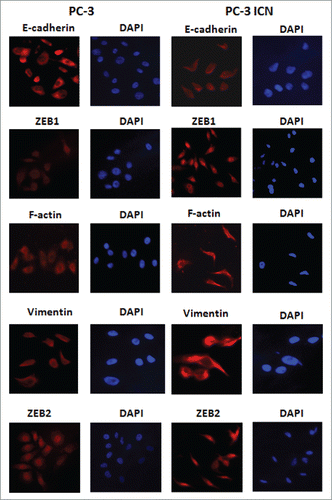
Figure 6. PC-3 ICN cells have increased the formation of prostate cancer spheres. (A) The PC-3 ICN cells with Notch-1 siRNA transfection were photographed under the microscope. CS: control siRNA; NS: Notch-1 siRNA. (B) Western blotting was conducted to measure the protein levels of CSC markers in PC-3 ICN cells after Notch-1 siRNA transfection. (C) The formation of sphere was photographed under the microscope in PC-3 ICN cells. (D) The formation of sphere was photographed in PC-3 ICN cells with Notch-1 siRNA transfection. (E) Left panel: Real-time RT-PCR was performed to detect the mRNA levels of CSC markers in prostate cancer cells. *P<0.05; **P<0.01 vs PC-3 cells. Right panel: Western blotting analysis was used to measure the protein levels of CSC markers in prostate cancer cells.
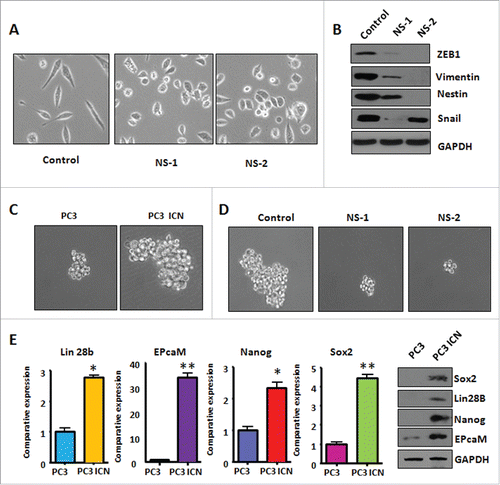
PC-3 ICN cells have increased the formation of prostate cancer spheres
Sphere forming assay was performed to investigate the capacity of CSC self-renewal in PC-3 ICN cells. We found that PC-3 ICN cells showed increased formation of prostate cancer spheres (). Moreover, inhibition of Notch-1 by its siRNA decreased formation of prostate cancer spheres (). Mechanistically, the expression of CSC surface markers including EpCAM, Lin28B, Nanog, and Sox2 was increased in PC-3 ICN cells by our real-time RT-PCR and Western blotting analysis (). Our results suggest that overexpression of Notch-1 could enhance the formation of prostate cancer sphere associated with CSC markers change. Our findings further indicated that Notch-1 plays a key role in the regulation of CSC phenotype.
Discussion
Accumulating evidence has demonstrated that EMT is involved in tumor aggressiveness due to enhanced cancer cell metastasis.Citation33 It has been documented that during EMT dynamic process, epithelial cells undergo morphologic changes from epithelial cobblestone phenotype to elongated fibroblastic phenotype.Citation34 In other words, epithelial cells obtain mesenchymal cell features. Epithelial markers including E-cadherin and γ-catenin are downregulated, while mesenchymal markers such as Vimentin, Snail, Slug, ZEB1, and ZEB2 are increased.Citation35 Multiple studies have revealed that EMT-type cells acquire enhanced migration and invasion capacity.Citation36 Therefore, exploring the mechanism of EMT development is required to discover new approaches for the treatment of cancer metastasis.
EMT is triggered by the interplay of extracellular signals and secreted factors including transforming growth factor-β (TGF-β), hepatocyte growth factor (HGF), fibroblast growth factor (FGF), and epidermal growth factor (EGF).Citation37,38 Recently, Notch signaling pathway was validated to trigger EMT in human cancer cells. For example, it has been reported that Notch promoted EMT during cardiac development and oncogenic transformation.Citation39 Moreover, Notch-mediated EMT is associated with upregulation of Snail transcription factor.Citation40 Furthermore, one study demonstrated that concomitant Notch activation and p53 deletion led to EMT and metastasis in mouse gut.Citation41 Notably, Notch and TGF-β form a positive regulatory loop and regulate EMT in epithelial ovarian cancer cells.Citation42 Strikingly, Notch was found to promote stemness and EMT in colorectal cancer.Citation43 Similarly, Notch signaling triggered EMT, leading to enhanced invasion and metastasis in adenoid cystic carcinoma.Citation44 One study showed that aberrant expression of EMT markers E-cadherin, Vimentin, and Notch-1 was observed in prostate cancer specimen and bone metastasis tissues, suggesting that Nocth-1 could play a role in bone metastasis via regulation of EMT.Citation45 In line with these reports, we found that Notch-1 induced EMT in prostate cancer cells, resulting in increased cell migration and invasion.
Recent studies have discovered that EMT is associated with drug resistance.Citation46 EMT conferred drug resistance characteristics to cancer cells against chemotherapeutic drugs.Citation47 For instance, gemcitabine-resistant pancreatic cancer cells acquired EMT phenotype and exhibited higher activation of Notch signaling pathway.Citation32 Silencing of Notch-1 enhanced docetaxel induced mitotic arrest and apoptosis in prostate cancer cells.Citation48 Moreover, suppression of acquired docetaxel resistance in prostate cancer is through depletion of Notch-dependent tumor-initiating cells.Citation49 GSI inhibitor suppressed Notch pathway and enhanced the antitumor effect of docetaxel in prostate cancer.Citation50 Recently, one study validated that activation of Notch-1 synergized with multiple pathways in promoting castration resistant prostate cancer.Citation51 Therefore, identification of precise mechanisms that regulate EMT process would likely be useful for exploring novel therapeutic approaches for the treatment of human cancers. It has been reported that EMT is associated with CSCs in human cancers.Citation52 CSCs possess the ability to self-renew and differentiate.Citation53 Moreover, CSCs have been validated and isolated from many human cancers including prostate cancer.Citation54-56 Several cell markers including Oct4, Nanog, Sox2, CD44 and Nestin could be prostate CSCs markers.Citation54 In line with this concept, we found that overexpression of Notch-1-induced EMT is associated with CSCs in prostate cancer cells. Our findings suggest that targeting EMT could eliminate CSCs in prostate cancer.
In conclusion, our findings clearly demonstrated that overexpression of Notch-1 enhanced cell migration and invasion, whereas downregulation of Notch-1 retarded cell migratory activity in prostate cancer cells. Overexpression of Notch-1 induced EMT and exhibited CSC feature in prostate cancer cells, which are associated with EMT and CSC markers change. Interestingly, studies have shown that Notch signaling could play an anti-tumor role in prostate cancer.Citation57,58 Multiple studies suggest Notch is a tumor suppressor in context dependent.Citation59,60 Therefore, deeper investigation is required to explore the function of Notch-1 using transgenic mouse model in the near future.
Experimental procedures
Cell culture, reagents and antibodies
Human PC-3 cells were grown in RPMI (Roswell park memorial institute)-1640 medium supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin in 5% CO2. Primary antibodies against Notch-1, Hey-1, Hey-2, Hes-5, and Hes-6 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β-actin antibody was purchased from Cell Signaling Technology (Danvers, MA). Antibodies against vimentin and nestin were purchased from Abcam (Cambridge, MA).
Plasmids and transfections
PC-3 cells were transfected with human Notch-1 ICN or vector alone (pcDNA3) by Lipofectamine 2000. PC-3 cells were transfected with Notch-1 siRNA and control siRNA, respectively, using Lipofectamine 2000.Citation61
Wound healing assay
The cells were allowed to grow to 90–95% confluency in 6-well plates. Then, the wound was created by scratching the surface of the plates using a pipette tip. After 20 h, the cells were photographed under the microscope.Citation61
Cell migration and invasion assay
The 24-well inserts (BD Biosciences, Bedford, MA) with 8μm pores was used to detect the cell migration following the manufacturer's protocol. The cell invasive activity was measured using the BD BioCoat Tumor Invasion Assay System.
Cell attachment and detachment assay
Cells were seeded in 24-well plates. After 1-h incubation, unattached cells were removed, and the attached cells were counted after trypsinization. For cell detachment assay, after cells were seeded for 24 h incubation, the medium was removed and the cells were incubated with 0.05% trypsin for 3 min to detach the cells from the culture plates. Then, we counted the detached and attached cells.
Real-time RT-PCR analysis for gene expression
The total RNA from cells was isolated by Trizol (Invitrogen, Carlsbad, CA) and purified by RNeasy Mini Kit and RNase-free DNase Set (QIAGEN, Valencia, CA) according to the manufacturer's protocols. The primers and the PCR reaction are described previously.Citation32
Protein extraction and Western blotting
Cells were lysed and the protein concentrations were determined using the Bio-Rad protein assay kit (Bio-Rad Laboratories, CA). The lysates were then resolved by SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) and immunoblotted with indicated antibodies.
Immunofluorescence microscopy
Cells were fixed with 4% paraformaldehyde for 10 min, permeabilized in 0.5% Triton X-100 for 10 min, and incubated in PBS and 10% goat serum blocking solution for 1 h. The cells were incubated for 2 h with anti-Notch-1, anti-E-cadherin, anti-vimentin, anti-ZEB1, anti-ZEB2, anti-F-actin, in 5% goat serum and were analyzed.
Sphere formation assay
Single cell suspensions of cells were seeded on 6-well plate at 1,000 cells/well in sphere formation medium (1:1 RPMI-1640/F12 medium supplemented with B-27 and N-2, Invitrogen). Fresh sphere formation medium was added every 3 d. After 10 d of incubation, the spheres were photographed under the microscope.
Data analysis
Results were analyzed using GraphPad Prism 4.0 (Graph pad Software, La Jolla, CA). All data were expressed as mean ± SD (standard deviation). Statistical comparisons were done using Student test. P<0.05 was considered to be statistically significant.
Disclosure of potential conflicts of interest
The authors declare that they have no conflict of interest.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67:7-30; PMID:28055103; http://dx.doi.org/10.3322/caac.21387
- Jemal A, Fedewa SA, Ma J, Siegel R, Lin CC, Brawley O, Ward EM. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. Jama 2015; 314:2054-61; PMID:26575061; http://dx.doi.org/10.1001/jama.2015.14905
- Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016; 66:271-89; PMID:27253694; http://dx.doi.org/10.3322/caac.21349
- McCrea E, Sissung TM, Price DK, Chau CH, Figg WD. Androgen receptor variation affects prostate cancer progression and drug resistance. Pharmacol Res 2016; 114:152-62; PMID:27725309; http://dx.doi.org/10.1016/j.phrs.2016.10.001
- Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer 2015; 15:701-11; PMID:26563462; http://dx.doi.org/10.1038/nrc4016
- Chen H, Zhou L, Wu X, Li R, Wen J, Sha J, Wen X. The PI3K/AKT pathway in the pathogenesis of prostate cancer. Front Biosci (Landmark Ed) 2016; 21:1084-91; PMID:27100493; http://dx.doi.org/10.2741/4443
- Fisher KW, Montironi R, Lopez Beltran A, Moch H, Wang L, Scarpelli M, Williamson SR, Koch MO, Cheng L. Molecular foundations for personalized therapy in prostate cancer. Curr Drug Targets 2015; 16:103-14; PMID:25547910; http://dx.doi.org/10.2174/1389450115666141229154500
- Wadosky KM, Koochekpour S. Molecular mechanisms underlying resistance to androgen deprivation therapy in prostate cancer. Oncotarget 2016; 7:64447-70; PMID:27487144; http://dx.doi.org/10.18632/oncotarget.10901
- Su Q, Xin L. Notch signaling in prostate cancer: Refining a therapeutic opportunity. Histol Histopathol 2016; 31:149-57; PMID:26521657; http://dx.doi.org/10.14670/HH-11-685
- Flores AN, McDermott N, Meunier A, Marignol L. NUMB inhibition of NOTCH signalling as a therapeutic target in prostate cancer. Nat Rev Urol 2014; 11:499-507; PMID:25134838; http://dx.doi.org/10.1038/nrurol.2014.195
- Andersson ER, Lendahl U. Therapeutic modulation of Notch signalling–are we there yet? Nat Rev Drug Discov 2014; 13:357-78; PMID:24781550; http://dx.doi.org/10.1038/nrd4252
- Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: A little bit of everything but not all the time. Nat Rev Cancer 2011; 11:338-51; PMID:21508972; http://dx.doi.org/10.1038/nrc3035
- Takebe N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, Yang SX, Ivy SP. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: Clinical update. Nat Rev Clin Oncol 2015; 12:445-64; PMID:25850553; http://dx.doi.org/10.1038/nrclinonc.2015.61
- Guruharsha KG, Kankel MW, Artavanis-Tsakonas S. The Notch signalling system: Recent insights into the complexity of a conserved pathway. Nat Rev Genet 2012; 13:654-66; PMID:22868267; http://dx.doi.org/10.1038/nrg3272
- Marignol L, Rivera-Figueroa K, Lynch T, Hollywood D. Hypoxia, notch signalling, and prostate cancer. Nat Rev Urol 2013; 10:405-13; PMID:23712204; http://dx.doi.org/10.1038/nrurol.2013.110
- Deng G, Ma L, Meng Q, Ju X, Jiang K, Jiang P, Yu Z. Notch signaling in the prostate: Critical roles during development and in the hallmarks of prostate cancer biology. J Cancer Res Clin Oncol 2016; 142:531-47; PMID:25736982; http://dx.doi.org/10.1007/s00432-015-1946-x
- Leong KG, Gao WQ. The Notch pathway in prostate development and cancer. Differentiation 2008; 76:699-716; PMID:18565101; http://dx.doi.org/10.1111/j.1432-0436.2008.00288.x
- Carvalho FL, Simons BW, Eberhart CG, Berman DM. Notch signaling in prostate cancer: A moving target. Prostate 2014; 74:933-45; PMID:24737393; http://dx.doi.org/10.1002/pros.22811
- Alcolea MP, Jones PH. Cell competition: Winning out by losing notch. Cell Cycle 2015; 14:9-17; PMID:25551772; http://dx.doi.org/10.4161/15384101.2014.988027
- Santagata S, Demichelis F, Riva A, Varambally S, Hofer MD, Kutok JL, Kim R, Tang J, Montie JE, Chinnaiyan AM, et al. JAGGED1 expression is associated with prostate cancer metastasis and recurrence. Cancer Res 2004; 64:6854-7; PMID:15466172; http://dx.doi.org/10.1158/0008-5472.CAN-04-2500
- Zhu H, Zhou X, Redfield S, Lewin J, Miele L. Elevated Jagged-1 and Notch-1 expression in high grade and metastatic prostate cancers. Am J Transl Res 2013; 5:368-78; PMID:23634247
- Ye QF, Zhang YC, Peng XQ, Long Z, Ming YZ, He LY. Silencing Notch-1 induces apoptosis and increases the chemosensitivity of prostate cancer cells to docetaxel through Bcl-2 and Bax. Oncol Lett 2012; 3:879-84; PMID:22741011; http://dx.doi.org/10.3892/ol.2012.572
- Yong T, Sun A, Henry MD, Meyers S, Davis JN. Down regulation of CSL activity inhibits cell proliferation in prostate and breast cancer cells. J Cell Biochem 2011; 112:2340-51; PMID:21520243; http://dx.doi.org/10.1002/jcb.23157
- Wang H, Zhang L, Fu Y, Fang F, Jiang Y, Dong Y, Zhu W. CSL regulates AKT to mediate androgen independence in prostate cancer progression. Prostate 2016; 76:140-50; PMID:26437743; http://dx.doi.org/10.1002/pros.23104
- Danza G, Di Serio C, Ambrosio MR, Sturli N, Lonetto G, Rosati F, Rocca BJ, Ventimiglia G, del Vecchio MT, Prudovsky I, et al. Notch3 is activated by chronic hypoxia and contributes to the progression of human prostate cancer. Int J Cancer 2013; 133:2577-86; PMID:23729168; http://dx.doi.org/10.1002/ijc.28293
- Kwon OJ, Valdez JM, Zhang L, Zhang B, Wei X, Su Q, Ittmann MM, Creighton CJ, Xin L. Increased Notch signalling inhibits anoikis and stimulates proliferation of prostate luminal epithelial cells. Nat Commun 2014; 5:4416; PMID:25048699; http://dx.doi.org/10.1038/ncomms5416
- Soylu H, Acar N, Ozbey O, Unal B, Koksal IT, Bassorgun I, Ciftcioglu A, Ustunel I. Characterization of Notch signalling pathway members in normal prostate, prostatic intraepithelial neoplasia (PIN) and prostatic adenocarcinoma. Pathol Oncol Res 2016; 22:87-94; PMID:26341090; http://dx.doi.org/10.1007/s12253-015-9983-y
- Kwon OJ, Zhang L, Wang J, Su Q, Feng Q, Zhang XH, Mani SA, Paulter R, Creighton CJ, Ittmann MM, et al. Notch promotes tumor metastasis in a prostate-specific Pten-null mouse model. J Clin Invest 2016; 126:2626-41; PMID:27294523; http://dx.doi.org/10.1172/JCI84637
- Santio NM, Landor SK, Vahtera L, Yla-Pelto J, Paloniemi E, Imanishi SY, Corthals G, Varjosalo M, Manoharan GB, Uri A, et al. Phosphorylation of Notch1 by Pim kinases promotes oncogenic signaling in breast and prostate cancer cells. Oncotarget 2016; 7:43220-38; PMID:27281612; http://dx.doi.org/10.18632/oncotarget.9215
- Revandkar A, Perciato ML, Toso A, Alajati A, Chen J, Gerber H, Dimitrov M, Rinaldi A, Delaleu N, Pasquini E, et al. Inhibition of Notch pathway arrests PTEN-deficient advanced prostate cancer by triggering p27-driven cellular senescence. Nat Commun 2016; 7:13719; PMID:27941799; http://dx.doi.org/10.1038/ncomms13719
- Lefort K, Ostano P, Mello-Grand M, Calpini V, Scatolini M, Farsetti A, Dotto GP, Chiorino G. Dual tumor suppressing and promoting function of Notch1 signaling in human prostate cancer. Oncotarget 2016; 7:48011-26; PMID:27384993; http://dx.doi.org/10.18632/oncotarget.10333
- Wang Z, Li Y, Kong D, Banerjee S, Ahmad A, Azmi AS, Ali S, Abbruzzese JL, Gallick GE, Sarkar FH. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res 2009; 69:2400-7; PMID:19276344; http://dx.doi.org/10.1158/0008-5472.CAN-08-4312
- Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol 2014; 16:488-94; PMID:24875735; http://dx.doi.org/10.1038/ncb2976
- Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell 2016; 166:21-45; PMID:27368099; http://dx.doi.org/10.1016/j.cell.2016.06.028
- De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 2013; 13:97-110; PMID:23344542; http://dx.doi.org/10.1038/nrc3447
- Chaffer CL, San Juan BP, Lim E, Weinberg RA. EMT, cell plasticity and metastasis. Cancer Metastasis Rev 2016; 35:645-54; PMID:27878502; http://dx.doi.org/10.1007/s10555-016-9648-7
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014; 15:178-96; PMID:24556840; http://dx.doi.org/10.1038/nrm3758
- Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med 2013; 19:1438-49; PMID:24202396; http://dx.doi.org/10.1038/nm.3336
- Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC, et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev 2004; 18:99-115; PMID:14701881; http://dx.doi.org/10.1101/gad.276304
- Saad S, Stanners SR, Yong R, Tang O, Pollock CA. Notch mediated epithelial to mesenchymal transformation is associated with increased expression of the Snail transcription factor. Int J Biochem Cell Biol 2010; 42:1115-22; PMID:20348013; http://dx.doi.org/10.1016/j.biocel.2010.03.016
- Chanrion M, Kuperstein I, Barriere C, El Marjou F, Cohen D, Vignjevic D, Stimmer L, Paul-Gilloteaux P, Bieche I, Tavares Sdos R, et al. Concomitant Notch activation and p53 deletion trigger epithelial-to-mesenchymal transition and metastasis in mouse gut. Nat Commun 2014; 5:5005; PMID:25295490; http://dx.doi.org/10.1038/ncomms6005
- Zhou J, Jain S, Azad AK, Xu X, Yu HC, Xu Z, Godbout R, Fu Y. Notch and TGFbeta form a positive regulatory loop and regulate EMT in epithelial ovarian cancer cells. Cell Signal 2016; 28:838-49; PMID:27075926; http://dx.doi.org/10.1016/j.cellsig.2016.03.016
- Fender AW, Nutter JM, Fitzgerald TL, Bertrand FE, Sigounas G. Notch-1 promotes stemness and epithelial to mesenchymal transition in colorectal cancer. J Cell Biochem 2015; 116:2517-27; PMID:25914224; http://dx.doi.org/10.1002/jcb.25196
- Zhao ZL, Ma SR, Wang WM, Huang CF, Yu GT, Wu TF, Bu LL, Wang YF, Zhao YF, Zhang WF, et al. Notch signaling induces epithelial-mesenchymal transition to promote invasion and metastasis in adenoid cystic carcinoma. Am J Transl Res 2015; 7:162-74; PMID:25755838
- Sethi S, Macoska J, Chen W, Sarkar FH. Molecular signature of epithelial-mesenchymal transition (EMT) in human prostate cancer bone metastasis. Am J Transl Res 2010; 3:90-9; PMID:21139809
- Du B, Shim JS. Targeting epithelial-mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules 2016; 21(7); PMID:27455225; http://dx.doi.org/10.3390/molecules21070965
- Mitra A, Mishra L, Li S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget 2015; 6:10697-711; PMID:25986923; http://dx.doi.org/10.18632/oncotarget.4037
- Ye QF, Zhang YC, Peng XQ, Long Z, Ming YZ, He LY. siRNA-mediated silencing of Notch-1 enhances docetaxel induced mitotic arrest and apoptosis in prostate cancer cells. Asian Pac J Cancer Prev 2012; 13:2485-9; PMID:22938409; http://dx.doi.org/10.7314/APJCP.2012.13.6.2485
- Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, Castillo-Martin M, Quinn SA, Rodriguez-Barrueco R, Bonal DM, Charytonowicz E, Gladoun N, de la Iglesia-Vicente J, et al. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell 2012; 22:373-88; PMID:22975379; http://dx.doi.org/10.1016/j.ccr.2012.07.016
- Cui D, Dai J, Keller JM, Mizokami A, Xia S, Keller ET. Notch pathway inhibition using PF-03084014, a gamma-secretase inhibitor (GSI), enhances the antitumor effect of docetaxel in prostate cancer. Clin Cancer Res 2015; 21:4619-29; PMID:26202948; http://dx.doi.org/10.1158/1078-0432.CCR-15-0242
- Stoyanova T, Riedinger M, Lin S, Faltermeier CM, Smith BA, Zhang KX, Going CC, Goldstein AS, Lee JK, Drake JM, et al. Activation of Notch1 synergizes with multiple pathways in promoting castration-resistant prostate cancer. Proc Natl Acad Sci U S A 2016; 113:E6457-E66; PMID:27694579; http://dx.doi.org/10.1073/pnas.1614529113
- Sun B, Zhang D, Zhao N, Zhao X. Epithelial-to-endothelial transition and cancer stem cells: Two cornerstones of vasculogenic mimicry in malignant tumors. Oncotarget 2016; PMID:27034014; http://dx.doi.org/10.18632/oncotarget.8461
- Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell 2008; 132:681-96; PMID:18295583; http://dx.doi.org/10.1016/j.cell.2008.01.036
- Gu G, Yuan J, Wills M, Kasper S. Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res 2007; 67:4807-15; PMID:17510410; http://dx.doi.org/10.1158/0008-5472.CAN-06-4608
- Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells - what challenges do they pose? Nat Rev Drug Discov 2014; 13:497-512; PMID:24981363; http://dx.doi.org/10.1038/nrd4253
- Beck B, Blanpain C. Unravelling cancer stem cell potential. Nat Rev Cancer 2013; 13:727-38; PMID:24060864; http://dx.doi.org/10.1038/nrc3597
- Shou J, Ross S, Koeppen H, de Sauvage FJ, Gao WQ. Dynamics of notch expression during murine prostate development and tumorigenesis. Cancer Res 2001; 61:7291-7; PMID:11585768
- Whelan JT, Kellogg A, Shewchuk BM, Hewan-Lowe K, Bertrand FE. Notch-1 signaling is lost in prostate adenocarcinoma and promotes PTEN gene expression. J Cell Biochem 2009; 107:992-1001; PMID:19479935; http://dx.doi.org/10.1002/jcb.22199
- Nowell CS, Radtke F. Notch as a tumour suppressor. Nat Rev Cancer 2017; 17:145-59; PMID:28154375; http://dx.doi.org/10.1038/nrc.2016.145
- Bray SJ. Notch signalling in context. Nat Rev Mol Cell Biol 2016; 17:722-35; PMID:27507209; http://dx.doi.org/10.1038/nrm.2016.94
- Wang L, Hou Y, Yin X, Su J, Zhao Z, Ye X, Zhou X, Zhou L, Wang Z. Rottlerin inhibits cell growth and invasion via down-regulation of Cdc20 in glioma cells. Oncotarget 2016; 7:69770-82; PMID:27626499; http://dx.doi.org/10.18632/oncotarget.11974
