ABSTRACT
A metastatic melanoma obtained from the right chest wall of a patient was previously established orthotopically in the right chest wall of nude mice as a patient-derived orthotopic xenograft (PDOX) model. We previously showed that the combination of tumor-targeting Salmonella typhimurium A1-R (S. typhimurium A1-R) and chemotherapy was highly effective against the melanoma PDOX. In the present study, we investigated the mechanism of the high efficacy of this combination. Two weeks after implantation, 40 PDOX mouse models were randomized into 4 groups of 10 mice each: untreated control (n = 10); treated with S. typhimurium A1-R (5 × 107 CFU/100 μl, i.v., once a week for 2 weeks, n = 10); treated with temozolomide (TEM) (25 mg/kg, p.o. for 14 consecutive days) combined with S. typhimurium A1-R (5 × 107 CFU/100 μl, i.v., once a week for 2 weeks, n = 10); treated with vemurafenib (VEM) (30 mg/kg, p.o., for 14 consecutive days) combined with S. typhimurium A1-R (5 × 107 CFU/100 μl, i.v., once a week for 2 weeks) (n = 10). On day 14 from initiation, all treatments significantly inhibited tumor growth compared with untreated control (S. typhimurium A1-R: p < 0.01; TEM combined with S. typhimurium A1-R: p < 0.01; VEM combined with S. typhimurium A1-R: p < 0.01). Combination therapy with S. typhimurium A1-R was significantly more effective on tumor growth than S. typhimurium A1-R alone (with TEM: p < 0.01; with VEM: p < 0.01). Combination therapy significantly increased S. typhimurium A1-R tumor targeting alone (S. typhimurium A1-R + TEM: p < 0.01, S. typhimurium A1-R + VEM: p < 0.01), relative to S. typhimurium A1-R alone, respectively. In conclusion, chemotherapy drugs promoted targeting of S. typhimurium A1-R of melanoma, thereby enhancing efficacy against the melanoma PDOX.
Introduction
The tumor-targeting Salmonella typhimurium A1-R (S. typhimurium A1-R), developed by our laboratory,Citation1 is auxotrophic for Leu-Arg, which prevents it from mounting a continuous infection in normal tissues. S. typhimurium A1-R was effective against primary and metastatic tumors as monotherapy in nude mouse models of major cancers, including prostate,Citation2,3 breast,Citation4-6 lung,Citation7,8 pancreatic,Citation9-13 ovarianCitation14,15 stomach,Citation16 and cervical cancerCitation17 In addition, S. typhimurium A1-R was effective against patient-derived orthotopic models (PDOX) of pancreatic cancer,Citation9,13 sarcomaCitation18-20 and melanoma.Citation20,21
S. typhimurium A1-R has been shown to directly kill cancer cells in vitro.Citation2
We have previously developed a patient-derived orthotopic xenograft (PDOX) nude-mouse model of melanoma with a BRAF-V600E mutation.Citation22-24 S. typhimurium A1-R combined with temozolomide (TEM) was significantly more effective on tumor growth than either S. typhimurium A1-R alone or TEM alone. TEM combined with S. typhimurium A1-R could regress the melanoma in the PDOX model.Citation22 We also previously demonstrated that S. typhimurium A1-R was significantly more effective than VEM alone, in the melanoma PDOX model.Citation24
In the present study, we demonstrate a mechanism of how chemotherapy enhances the anti-melanoma efficacy of S. typhimurium A1-R.
Results and discussion
All treatments significantly inhibited tumor growth compared with untreated control (S. typhimurium A1-R: p < 0.01; TEM combined with S. typhimurium A1-R: p < 0.01; VEM combined with S. typhimurium A1-R: p < 0.01; on day 14 after initiation. Combination therapy with S. typhimurium A1-R was significantly more effective than S. typhimurium A1-R alone with TEM: p < 0.01; with VEM: p < 0.01 ().
Figure 1. Representative photographs of mice from each treatment group. A. Untreated control. B. Treated with S. typhimurium A1-R. C. Treated with TEM and S. typhimurium A1-R. D. Treated with VEM and S. typhimurium A1-R. Scale bar: 5 mm.
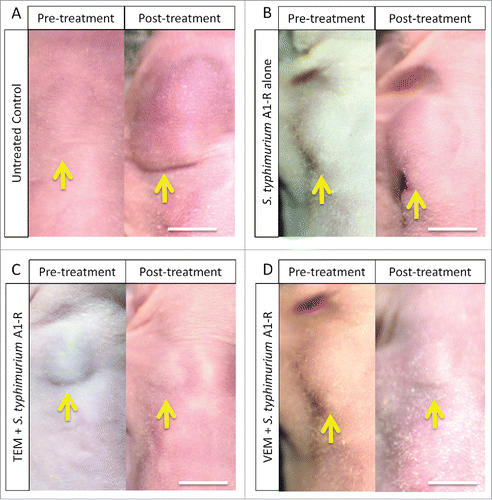
Figure 2. Relative tumor volume in the various treatment groups. Bar graph shows relative tumor volume at post-treatment point relative to the initial pre-treatment tumor volume. Error bars: ± SD.
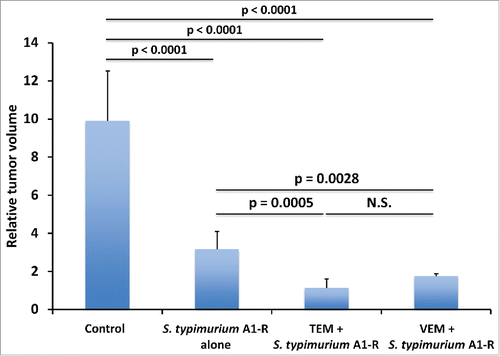
Figure 3. Fluorescence imaging of S. typhimurium A1-R-GFP targeting alone and in combination with chemotherapy in the melanoma PDOX. Confocal imaging with the FV1000. Scale bars: 12.5 μm.
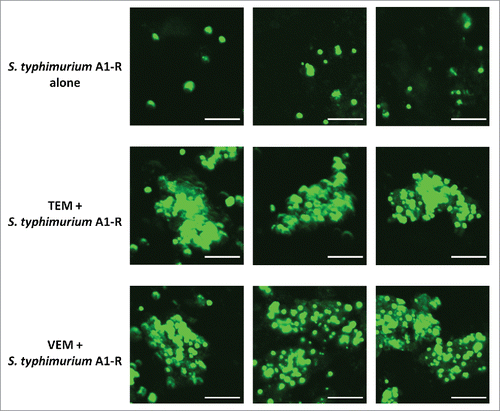
Figure 4. Quantitative tumor targeting by S. typhimurium A1-R-GFP alone and in combination with chemotherapy on the melanoma PDOX model. Bar graphs show S. typhimurium A1-R-GFP fluorescent area (μmCitation2) for each treatment group. Error bars: ± SD.
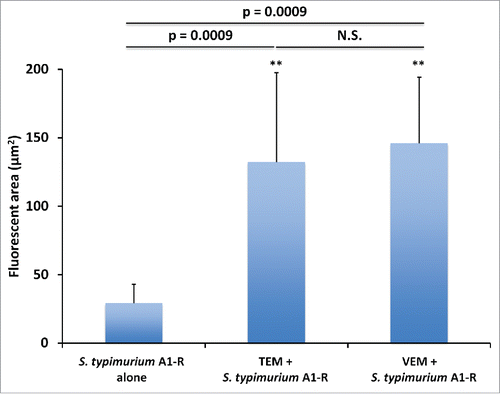
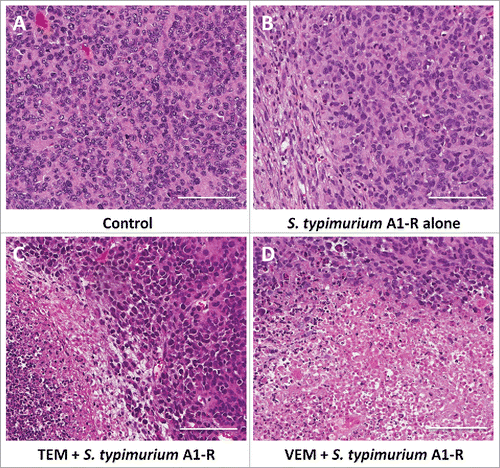
Figure 5. Histological analysis. Hematoxylin and eosin (H&E) stained slides from tumor tissue of each treatment group. A. Untreated control. B. S. typhimurium A1-R alone. C. S. typhimurium A1-R and TEM. D. S. typhimurium A1-R and VEM. Scale bars: 100 μm.
Confocal microscopy showed that S. typhimurium A1-R could directly target the melanoma PDOX. The targeting ability for melanoma of S. typhimurium A1-R was evaluated by the S. typhimurium A1-R-GFP fluorescent area (). Both VEM and TEM significantly increased the S. typhimurium A1-R-GFP fluorescent area compared with S. typhimurium A1-R alone (TEM: p < 0.01; VEM: p < 0.01) ().
The histology of the original patient tumor and the untreated PDOX tumor were similar, containing the same types of cells. VEM and TEM caused extensive necrosis in the tumor when each was combined with S. typhimurium A1-R and much more extensive than S. typhimurium A1-R alone ().Citation22-24
Salmonella typhimurium (VNP20009) has been previously used for effective therapy of a melanoma.Citation25-31 VNP20009 was attenuated by a lipid A-mutation (msbB), purine auxotrophy (purI) and amino acid auxotrophy. The tumor-targeting S. typhimurium A1-R, developed by our laboratory,Citation32 is auxotrophic only for Leu-Arg and is less attenuated.
We previously reported that chemotherapy combined with S. typhimurium A1-R was effective for the BRAF-V600E mutant melanoma PDOX.Citation22,24 In the present study, we showed that both VEM and TEM promoted S. typhimurium A1-R tumor targeting with elevated accumulation of S. typhimurium A1-R that led to extensive tumor necrosis. This is the first report to elucidate the mechanism by which the combination of chemotherapy with S. typhimurium A1-R is extremely effective.
Despite progress in melanoma therapy, there is still no cure for stage III and IV disease due to drug resistance, tumor heterogeneity and an immunosuppressive tumor environment.Citation33-37 In addition, the presence of melanin appears to interfere with chemotherapy and radiotherapy of this recalcitrant disease.Citation36 The present results suggests that S. typhimurium A1-R could potentiate chemotherapy for melanoma. Clinical trials are warranted for this strategy.
The present report demonstrates the enhancement of tumor-targeting of S. typhimurium A1-R by chemotherapy drugs. We have previously demonstrated that S. typhimurium A1-R can decoy quiescent cancer cells in tumors to cycle and become more sensitive to chemotherapy. Therefore, chemotherapy enhances bacterial targeting and bacterial targeting enhances chemotherapy, a very powerful mutual effect for cancer therapy.
Previously-developed concepts and strategies of highly-selective tumor targeting can take advantage of molecular targeting tumors, including tissue-selective therapy with focuses on unique differences between normal and tumor tissues.Citation38-43
Conclusions
The combination of chemotherapy with S. typhimurium A1-R was highly effective on a chemotherapy-resistant melanoma in a PDOX mouse model. In future experiments, the powerful combination of chemotherapy and S. typhimurium A1-R, described in the present report, will be used to determine increased survival of melanoma PDOX models. This treatment strategy has important future clinical potential, which possibly can be realized in the near future.
Materials and methods
Mice
Athymic nu/nu nude mice (AntiCancer Inc., San Diego, CA), 4–6 weeks old, were used in this study. Animals were housed in a barrier facility on a high efficacy particulate arrestance (HEPA)-filtered rack under standard conditions of 12-hour light/dark cycles. The animals were fed an autoclaved laboratory rodent diet. All mouse surgical procedures and imaging were performed with the animals anesthetized by subcutaneous injection of a ketamine mixture (0.02 ml solution of 20 mg/kg ketamine, 15.2 mg/kg xylazine, and 0.48 mg/kg acepromazine maleate). The response of animals during surgery was monitored to ensure adequate depth of anesthesia. The animals were observed on a daily basis and humanely killed by CO2 inhalation if they met the following humane end point criteria: severe tumor burden (more than 20 mm in diameter), prostration, significant body weight loss, difficulty breathing, rotational motion and body temperature drop. All animal studies were conducted in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Animals under Assurance Number A3873–1.
Patient-derived tumor
A 75-year-old female patient was previously diagnosed with a melanoma of the right chest wall. The tumor was resected in the Department of Surgery, University of California, Los Angeles (UCLA). Written informed consent was provided by the patient, and the Institutional Review Board (IRB) of UCLA approved this experiment.Citation22-24
Establishment of PDOX models of melanoma by surgical orthotopic implantation (SOI)
After nude mice were anesthetized with the ketamine solution described above, a 5-mm skin incision was made on the right chest into the chest wall, which was split to make space for the melanoma tissue fragment. A single tumor fragment was implanted orthotopically into the space to establish the PDOX model. The wound was closed with a 6–0 nylon suture (Ethilon, Ethicon, Inc., NJ, USA).Citation22-24
Preparation and administration of S. typhimurium A1-R
GFP-expressing S. typhimurium A1-R bacteria (AntiCancer Inc.) were grown overnight on LB medium (Fisher Sci., Hanover Park, IL, USA) and then diluted 1:10 in LB medium. Bacteria were harvested at late-log phase, washed with PBS, and then diluted in PBS. S. typhimurium A1-R was injected intravenously. A total of 5 × 107 CFU S. typhimurium A1-R in 100 μl PBS was administered to each mouse.Citation2-4
Treatment study design in the PDOX model of melanoma
PDOX mouse models were randomized into 4 groups of 10 mice each: untreated control (n = 10); treated with S. typhimurium A1-R (5 × 107 CFU/100 μl, i.v., qw × 2, n = 10); treated with TEM (25 mg/kg, p.o., qd × 14) combined with S. typhimurium A1-R (5 × 107 CFU/100 μl, i.v., qw × 2, n = 10); treated with VEM (30 mg/kg, p.o., qd × 14) combined with S. typhimurium A1-R (5 × 107 CFU/100 μl, i.v., qw × 2, n = 10). Tumor length and width were measured twice a week. Tumor volume was calculated with the following formula: Tumor volume (mm3) = length (mm) × width (mm) × width (mm) × 1/2. Data are presented as mean ± SD. The tumor volume ratio is defined at the tumor volume at any given time point relative to the initial tumor volume.
Confocal microscopy
The FV1000 confocal microscope (Olympus, Tokyo, Japan) was used for high-resolution imaging. Fluorescence images were obtained using the 20 × /0.50 UPLAN FLN and 40 × /1.3 oil Olympus UPLAN FLN objectives.Citation44 The tumor fluorescent area was analyzed with UVP software (UVP, Upland, CA).Citation24,45
Histological analysis
Fresh tumor samples were fixed in 10% formalin and embedded in paraffin before sectioning and staining. Tissue sections (5 μm) were deparaffinized in xylene and rehydrated in an ethanol series. Hematoxylin and eosin (H&E) staining was performed according to standard protocols. Histological examination was performed with a BHS System Microscope (Olympus Corporation, Tokyo, Japan). Images were acquired with INFINITY ANALYZE software (Lumenera Corporation, Ottawa, Canada).Citation22-24
Statistical analysis
JMP version 11.0 was used for all statistical analyses. Significant differences for continuous variables were determined using the Mann-Whitney U test. Line graphs expressed average values and error bar showed SD. A probability value of P ≤ 0.05 was considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Hoffman RM, Zhao M. Methods for the development of tumor-targeting bacteria. Expert Opin Drug Discov 2014; 9:741-50; PMID:24949888; https://doi.org/10.1517/17460441.2014.916270
- Zhao M, Yang M, Li XM, Jiang P, Baranov E, Li S, Xu M, Penman S, Hoffman RM. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci USA 2005; 102:755-60; PMID:15644448; https://doi.org/10.1073/pnas.0408422102
- Zhao M, Geller J, Ma H, Yang M, Penman S, Hoffman RM. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc Natl Acad Sci USA 2007; 104:10170-4; PMID:17548809; https://doi.org/10.1073/pnas.0703867104
- Zhao M, Yang M, Ma H, Li X, Tan X, Li S, Yang Z, Hoffman RM. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res 2006; 66:7647-52; PMID:16885365; https://doi.org/10.1158/0008-5472.CAN-06-0716
- Zhang Y, Tome Y, Suetsugu A, Zhang L, Zhang N, Hoffman RM, Zhao M. Determination of the optimal route of administration of Salmonella typhimurium A1-R to target breast cancer in nude mice. Anticancer Res 2012; 32:2501-8; PMID:22753706.
- Zhang Y, Miwa S, Zhang N, Hoffman RM, Zhao M. Tumor-targeting Salmonella typhimurium A1-R arrests growth of breast-cancer brain metastasis. Oncotarget 2015; 6:2615-22; PMID:25575815; https://doi.org/10.18632/oncotarget.2811
- Uchugonova A, Zhao M, Zhang Y, Weinigel M, König K, Hoffman RM. Cancer-cell killing by engineered Salmonella imaged by multiphoton tomography in live mice. Anticancer Res 2012; 32:4331-9; PMID:23060555.
- Liu F, Zhang L, Hoffman RM, Zhao M. Vessel destruction by tumor-targeting Salmonella typhimurium A1-R is enhanced by high tumor vascularity. Cell Cycle 2010; 9:4518-24; PMID:21135579; https://doi.org/10.4161/cc.9.22.13744
- Hiroshima Y, Zhang Y, Murakami T, Maawy AA, Miwa S, Yamamoto M, Yano S, Sato S, Momiyama M, Mori R, et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R in combination with anti-angiogenesis therapy on a pancreatic cancer patient-derived orthotopic xenograph (PDOX) and cell line mouse models. Oncotarget 2014; 5:12346-57; PMID:25402324; https://doi.org/10.18632/oncotarget.2641
- Nagakura C, Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Bouvet M, Hoffman RM. Efficacy of a genetically-modified Salmonella typhimurium in an orthotopic human pancreatic cancer in nude mice. Anticancer Res 2009; 29:1873-8; PMID:19528442.
- Yam C, Zhao M, Hayashi K, Ma H, Kishimoto H, McElroy M, Bouvet M, Hoffman RM. Monotherapy with a tumor-targeting mutant of S. typhimurium inhibits liver metastasis in a mouse model of pancreatic cancer. J Surg Res 2010; 164:248-55; PMID:19766244; https://doi.org/10.1016/j.jss.2009.02.023
- Hiroshima Y, Zhao M, Zhang Y, Maawy A, Hassanein MK, Uehara F, Miwa S, Yano S, Momiyama M, Suetsugu A, et al. Comparison of efficacy of Salmonella typhimurium A1-R and chemotherapy on stem-like and non-stem human pancreatic cancer cells. Cell Cycle 2013; 12:2774-80; PMID:23966167; https://doi.org/10.4161/cc.25872
- Hiroshima Y, Zhao M, Maawy A, Zhang Y, Katz MH, Fleming JB, Uehara F, Miwa S, Yano S, Momiyama M, et al. Efficacy of Salmonella typhimurium A1-R versus chemotherapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX). J Cell Biochem 2014; 115:1254-61; PMID:24435915; https://doi.org/10.1002/jcb.24769
- Matsumoto Y, Miwa S, Zhang Y, Hiroshima Y, Yano S, Uehara F, Yamamoto M, Toneri M, Bouvet M, Matsubara H, et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R on nude mouse models of metastatic and disseminated human ovarian cancer. J Cell Biochem 2014; 115:1996-2003; PMID:24924355; https://doi.org/10.1002/jcb.24871
- Matsumoto Y, Miwa S, Zhang Y, Zhao M, Yano S, Uehara F, Yamamoto M, Hiroshima Y, Toneri M, Bouvet M, et al. Intraperitoneal administration of tumor-targeting Salmonella typhimurium A1-R inhibits disseminated human ovarian cancer and extends survival in nude mice. Oncotarget 2015; 6:11369-77; PMID:25957417; https://doi.org/10.18632/oncotarget.3607
- Yano S, Zhang Y, Zhao M, Hiroshima Y, Miwa S, Uehara F, Kishimoto H, Tazawa H, Bouvet M, Fujiwara T, et al. Tumor-targeting Salmonella typhimurium A1-R decoys quiescent cancer cells to cycle as visualized by FUCCI imaging and become sensitive to chemotherapy. Cell Cycle 2014; 13:3958-63; PMID:25483077; https://doi.org/10.4161/15384101.2014.964115
- Hiroshima Y, Zhang Y, Zhao M, Zhang N, Murakami T, Maawy A, Mii S, Uehara F, Yamamoto M, Miwa S, et al. Tumor-targeting Salmonella typhimurium A1-R in combination with Trastuzumab eradicates HER-2-positive cervical cancer cells in patient-derived mouse models. PLoS One 2015; 10:e0120358; PMID:26047477; https://doi.org/10.1371/journal.pone.0120358
- Murakami T, DeLong J, Eilber FC, Zhao M, Zhang Y, Zhang N, Singh A, Russell T, Deng S, Reynoso J, et al. Tumor-targeting Salmonella typhimurium A1-R in combination with doxorubicin eradicate soft tissue sarcoma in a patient-derived orthotopic xenograft PDOX model. Oncotarget 2016; 7:12783-90; PMID:26859573; https://doi.org/10.18632/oncotarget.7226
- Kiyuna T, Murakami T, Tome Y, Kawaguchi K, Igarashi K, Zhang Y Zhao M, Li Y, Bouvet M, Kanaya F, Singh A, et al. High efficacy of tumor-targeting Salmonella typhimurium A1-R on a doxorubicin- and dactolisib-resistant follicular dendritic-cell sarcoma in a patient-derived orthotopic xenograft nude mouse model. Oncotarget 2016; 7:33046-54; PMID:27105519; https://doi.org/10.18632/oncotarget.8848
- Murakami T, Igarashi K, Kawaguchi K, Kiyuna T, Zhang Y, Zhao M, Hiroshima Y, Nelson SD, Dry SM, Li Y, et al. Tumor-targeting Salmonella typhimurium A1-R regresses an osteosarcoma in a patient-derived xenograph model resistant to a molecular-targeting drug. Oncotarget 2017; 8:8035-42; PMID:28030831; https://doi.org/10.18632/oncotarget.14040
- Yamamoto M, Zhao M, Hiroshima Y, Zhang Y, Shurell E, Eilber FC, Bouvet M, Noda M, Hoffman RM. Efficacy of tumor-targeting Salmonella typhimurium A1-R on a melanoma patient-derived orthotopic xenograft (PDOX) nude-mouse model. PLoS One 2016; 11:e0160882; PMID:27500926; https://doi.org/10.1371/journal.pone.0160882
- Kawaguchi K, Igarashi K, Murakami T, Chmiewloski B, Kiyuna T, Zhao M, Zhang Y, Singh A, Unno M, Nelson S, et al. Tumor-targeting Salmonella typhimurium A1-R combined with Temozolomide regresses malignant melanoma with a BRAF-V600 mutation in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget 2016; 7:85929-36; PMID:27835903.
- Kawaguchi K, Murakami T, Chmielowski B, Igarashi K, Kiyuna T, Unno M, Nelson SD, Russell TA, Dry SM, Li Y, et al. Vemurafenib-resistant BRAF-V600E mutated melanoma is regressed by MEK targeting drug trametinib, but not cobimetinib in a patient-derived orthotopic xenograft (PDOX) mouse model. Oncotarget 2016; 7:71737-43; PMID:27690220; https://doi.org/10.18632/oncotarget.12328
- Kawaguchi K, Igarashi K, Murakami T, Zhao M, Zhang Y, Chmielowski B, Kiyuna T, Nelson SD, Russell TA, Dry SM, et al. Tumor-targeting Salmonella typhimurium A1-R sensitizes melanoma with a BRAF-V600E mutation to vemurafenib in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J Cell Biochem 2017; PMID:28106277; Epub ahead of print. https://doi.org/10.1002/jcb.25886
- Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, Sherry RM, Topalian SL, Yang JC, Stock F, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol 2002; 20:142-52; PMID:11773163; https://doi.org/10.1200/JCO.2002.20.1.142
- Forbes NS. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer 2010; 10:785-94; PMID:20944664; https://doi.org/10.1038/nrc2934
- Pawelek JM, Low KB, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res 1997; 57:4537-44. PMID:9377566.
- Zheng LM, Luo X, Feng M, Li Z, Le T, Ittensohn M, Trailsmith M, Bermudes D, Lin SL, King IC. Tumor amplified protein expression therapy: Salmonella as a tumor-selective protein delivery vector. Oncol Res 2000; 12:127-35; PMID:11216671; https://doi.org/10.3727/096504001108747602
- Platt J, Sodi S, Kelley M, Rockwell S, Bermudes D, Low KB, Pawelek J. Antitumour effects of genetically engineered Salmonella in combination with radiation. Eur J Cancer 2000 Dec; 36(18):2397-402; PMID:11094316; https://doi.org/10.1016/S0959-8049(00)00336-1
- Clairmont C, Lee KC, Pike J, Ittensohn M, Low KB, Pawelek J, Bermudes D, Brecher SM, Margitich D, Turnier J, et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J Infect Dis 2000; 181(6):1996-2002; PMID:10837181; https://doi.org/10.1086/315497
- Luo X, Li Z, Lin S, Le T, Ittensohn M, Bermudes D, Runyab JD, Shen SY, Chen J, King IC, et al. Antitumor effect of VNP20009, an attenuated Salmonella, in murine tumor models. Oncol Res 2001; 12(11-12):501-8; PMID:11939414; https://doi.org/10.3727/096504001108747512
- Zhang Y, Zhang N, Zhao M, Hoffman RM. Comparison of the selective targeting efficacy of Salmonella typhimurium A1-R and VNP20009 on the Lewis lung carcinoma in nude mice. Oncotarget 2015; 6(16):14625-31; PMID:25714030; https://doi.org/10.18632/oncotarget.3342
- Slominski AT, Carlson JA. Melanoma resistance: A bright future for academicians and a challenge for patient advocates. Mayo Clin Proc 2014; 89(4):429-33; PMID:24684870; https://doi.org/10.1016/j.mayocp.2014.02.009
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et al. BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364:2507-16; PMID:21639808; https://doi.org/10.1056/NEJMoa1103782
- Tang H, Wang Y, Chlewicki LK, Zhang Y, Guo J, Liang W, Wang J, Wang X, Fu YX. Facilitating T Cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell 2016; 29:285-96; PMID:26977880; https://doi.org/10.1016/j.ccell.2016.02.004
- Brożyna AA, Jóźwicki W, Roszkowski K, Filipiak J, Slominski AT. Melanin content in melanoma metastases affects the outcome of radiotherapy. Oncotarget 2016; 7:17844-53; PMID:26910282; https://doi.org/10.18632/oncotarget.7528
- Flaherty LE, Othus M, Atkins MB, Tuthill RJ, Thompson JA, Vetto JT, Haluska FG, Pappo AS, Sosman JA, Redman BG, et al. Southwest Oncology Group S0008: A phase III trial of high-dose interferon Alfa-2b versus cisplatin, vinblastine, and dacarbazine, plus interleukin-2 and interferon in patients with high-riskmelanoma—an intergroup study of cancer and leukemia Group B, children's oncology group, eastern cooperative oncology group, and southwest oncology group. J Clin Oncol 2014; 32:3771-8; PMID:25332243; https://doi.org/10.1200/JCO.2013.53.1590
- Blagosklonny MV. Matching targets for selective cancer therapy. Drug Discov Today 2003; 8:1104-7; PMID:14678733; https://doi.org/10.1016/S1359-6446(03)02806-X
- Blagosklonny MV. Teratogens as anti-cancer drugs. Cell Cycle 2005; 4:1518-21; PMID:16258270; https://doi.org/10.4161/cc.4.11.2208
- Blagosklonny MV. Treatment with inhibitors of caspases, that are substrates of drug ransporters, selectively permits chemotherapy-induced apoptosis in multidrug-resistant cells but protects normal cells. Leukemia 2001; 15:936-41; PMID:11417480; https://doi.org/10.1038/sj.leu.2402127
- Blagosklonny MV. Target for cancer therapy: Proliferating cells or stem cells. Leukemia 2006; 20:385-91; PMID:16357832; https://doi.org/10.1038/sj.leu.2404075
- Apontes P, Leontieva OV, Demidenko ZN, Li F, Blagosklonny MV. Exploring long-term protection of normal human fibroblasts and epithelial cells from chemotherapy in cell culture. Oncotarget 2011; 2:222-33; PMID:21447859; https://doi.org/10.18632/oncotarget.248
- Blagosklonny MV. Tissue-selective therapy of cancer. Br J Cancer 2003; 89:1147-51; PMID:14520435; https://doi.org/10.1038/sj.bjc.6601256
- Uchugonova A, Duong J, Zhang N, König K, Hoffman RM. The bulge area is the origin of nestin-expressing pluripotent stem cells of the hair follicle. J Cell Biochem 2011; 112:2046-50; PMID:21465525; https://doi.org/10.1002/jcb.23122
- Kawaguchi K, Murakami T, Suetsugu A, Kiyuna T, Igarashi K, Hiroshima Y, Zhao M, Zhang Y, Bouvet M, Clary BM, et al. High-efficacy targeting of colon-cancer liver metastasis with Salmonella typhimurium A1-R via intra-portal-vein injection in orthotopic nude-mouse models. Oncotarget 2017; 8:19065-73.
