ABSTRACT
Previous research has shown that a subpopulation of cells within cultured human dermal fibroblasts, termed multilineage-differentiating stress enduring (Muse) cells, are preferentially reprogrammed into induced pluripotent stem cells. However, controversy exists over whether these cells are the only cells capable of being reprogrammed from a heterogeneous population of fibroblasts. Similarly, there is little research to suggest such cells may exist in embryonic tissues or other species. To address if such a cell population exists in pigs, we investigated porcine embryonic fibroblast populations (pEFs) and identified heterogeneous expression of several key cell surface markers. Strikingly, we discovered a small population of stage-specific embryonic antigen 1 positive cells (SSEA-1+) in Danish Landrace and Göttingen minipig pEFs, which were absent in the Yucatan pEFs. Furthermore, reprogramming of SSEA-1+ sorted pEFs led to higher reprogramming efficiency. Subsequent transcriptome profiling of the SSEA-1+ vs. the SSEA-1neg cell fraction revealed highly comparable gene signatures. However several genes that were found to be upregulated in the SSEA-1+ cells were similarly expressed in mesenchymal stem cells (MSCs). We therefore termed these cells SSEA-1 Expressing Enhanced Reprogramming (SEER) cells. Interestingly, SEER cells were more effective at differentiating into osteocytes and chondrocytes in vitro. We conclude that SEER cells are more amenable for reprogramming and that the expression of mesenchymal stem cell genes is advantageous in the reprogramming process. This data provides evidence supporting the elite theory and helps to delineate which cell types and specific genes are important for reprogramming in the pig.
Introduction
An improvement in the generation of porcine induced pluripotent stem cells (piPSCs) remains desirable as they provide an important and highly proliferative source of cells for gene targeting and modification studies for the development of disease models and for studying disease mechanisms in vitro. In addition, they could be a useful model for studying the potentials and not least safety of iPSC-based cell therapies and transplantation.Citation1,2 The pig is a large mammal with high genetic homology and many organs with similar size and anatomy to humans. Hence, it could serve as a great model for the development of strategies for transplantation.Citation3
Our understanding of how somatic cells are reprogrammed into pluripotent stem cells has improved over recent years, although certain details remain elusive. The current understanding is that reprogramming is either a 2 or 3 step processCitation4-6 involving an initial stochastic phase, whereby the cell increases proliferation, undergoes metabolic changes, initiates the mesenchymal-epithelial transition, changes its expression of histone marks, and activates both DNA repair and RNA processing. Later events are marked by a maturation and stabilization phase, whereby activation of the core pluripotency circuit is initiated, among several other cellular changes.Citation7 Despite this recent knowledge, a rate-limiting step exists; that is, only a few cells of the starting population are successfully reprogrammed and reasons for this remain unknown. There are currently 2 conflicting theories on nuclear reprogramming of somatic cells into induced pluripotent stem cells (iPSCs), which infer that reprogrammed cells arise from either a selective cell type in the cell population (the Elite theory) or emerge equally from all cell types present in the starting population (the Stochastic theory). In the latter case, the stochastic model proposes that every cell type has the potential to be reprogrammed.Citation8 In this case, it has been argued that any terminally differentiated nucleated cell, such as B cell, T cell, liver or spleen cell can be effectively reprogrammed,Citation7 while the elite model proposes that reprogrammed cells are generated from a permissive subpopulation of cells.Citation9,10 Research in support of the elite theory has revealed that a MSC-like cell population, termed multilineage-differentiating stress enduring (Muse) cells, exist in human dermal fibroblasts and adipose tissue (Muse-AT) and that these are preferentially reprogrammed.Citation11,12 Muse cells are sorted based on co-expression of SSEA-3 and CD105 and possess at least some iPSC characteristics, such as increased expression of pluripotency markers, self-renewal, and multipotential differentiation capabilities.Citation10 A similar cell population has also been reported in skin fibroblasts derived from goat,Citation13 suggesting that the cell type is not confined to a single species. These findings imply that the cellular expression profile of the original cell type might play an important role in the reprogramming process.
It is therefore of interest to determine whether the stochastic or elite reprogramming theory plays a pivotal role in the generation of iPSCs from a not readily reprogrammable species like the pig. The development of bona fide iPSCs in animal models other than mouse and primates has been particularly difficult for reasons that remain unclear. In the case of the pig, potential iPSCs have been generated and share many characteristics with their mouse and primate counterparts,Citation14-20 but are considered to be incompletely reprogrammed,Citation21 as the exogenous transgenes appear unable to be silenced and are critical for cell sustainability in vitro.Citation7 Only one study has shown that piPSCs can contribute to the germline through chimeric offspringCitation22 and, interestingly, these were generated from bone marrow-derived MSCs. This points to the fact that the starting generation of cells and that the epigenetic profile of MSCs may play an important role in producing stable fully reprogrammed iPSCs in this species.
Porcine embryonic fibroblasts (pEFs) are often used as the starting cell population in piPSCs studies.Citation14,15,21,23 These cells are derived from multiple embryonic tissues and are heterogeneous in nature. To date, there has been no research to identify whether a subset of cells, more amenable to reprogramming akin to Muse cells, exist within the pEF population. We hypothesized that selection of these cells may enhance reprogramming. Furthermore, we also investigated whether differences in these cell populations exist between pEFs derived from different pig breeds, as recent research revealed that pEFs from different breeds affected somatic cell nuclear transfer efficiency.Citation24 In this case, it was shown that pEFs derived from the Chinese Laiwu pig resulted in significantly improved reprogramming compared with pEFs derived from other pig breeds.
To identify if cell heterogeneity is present among different cell lines and to address if these differences can be linked to increased reprogramming efficiencies, we screened several pEF cell lines derived from 3 different breeds: the Danish Landrace/Yorkshire x Duroc (termed Danish Landrace herein), the Göttingen minipig, and the Yucatan minipig. Immunocytochemical (ICC) staining indeed revealed cell heterogeneity, both between lines and breeds. Intriguingly, both Danish Landrace and Göttingen minipig pEFs expressed Stage-Specific Cell Antigen-1 (SSEA-1) (a typical marker of mouse embryonic stem cells) in a minor cell population. Subsequent, reprogramming studies of these SSEA-1+ sorted cells revealed that this subpopulation was more amenable to reprogramming. Furthermore, transcriptome profiling of the SSEA-1+ cells using qPCR and a porcine-specific microarray revealed that these cells, although highly similar to the remaining pEFs (SSEA-1neg), had an expression profile of OCT4, SOX2 and NANOG that resemble the expression profile of multipotent porcine skin-derived progenitor (SKP)Citation25 cells and had a distinct gene signature sharing closer expression to mesenchymal tissues and cells. These cells were negative for both SSEA-3 and CD105 and therefore different from human Muse and Muse-AT cells. To reflect the relationship to MSC and their increased capability to be reprogrammed into iPSC we termed this new subclass of embryonic progenitors SSEA-1 Expressing Enhanced Reprogramming (SEER) cells.
Results
Minor populations and breed differences are associated with SSEA-1 and CD105 expression in porcine embryonic fibroblasts
An immunohistochemical screening of several pEFs was performed to determine whether subpopulations of cells existed that might have more pluripotent or multipotent characteristics. We selected several pluripotent-associated cell surface markers, including stage-specific embryonic antigen 1, 3, and 4 (SSEA-1, SSEA-3, SSEA-4), tumor rejection antigen-1–60 and 1–81 (TRA-1–60, TRA-1–81), as well as the MSC marker, endoglin (CD105), and evaluated these in 9 pEFs derived from 3 different breed backgrounds, Danish Landrace (cell lines D1, D2, D3), Göttingen minipig (cell lines G1, G2, G3) and Yucatan minipig (cell lines Y1, Y2, Y3). Our results revealed that all the 9 cell lines were positive for Vimentin and negative for SSEA-3, TRA-1–60 and TRA1–81 ( and ). A minor population however of SSEA-1+ cells was detectable in all lines obtained from Danish Landrace and Göttingen pEFs, but completely absent in Yucatan pEFs ( and ). The level of SSEA-1 expression did not significantly differ between Danish Landrace and Göttingen pEFs. Two out of the 9 lines also contained a small fraction of SSEA-4+ cells (D1 and Y2) ( and ). The MSC marker, CD105 was expressed in a small number of cells in the 3 Yucatan pEF lines, but not in the other 2 breed's pEFs ( and ). Together, this revealed the pEFs were heterogeneous and contained small subpopulations of cells, which differed slightly between the breeds.
Figure 1. Expression of cell surface makers in porcine embryonic fibroblasts (pEFs) from different breeds. (A) Chromogen immunocytochemistry staining (ICC) representative of pEFs from each breed. Scale bar represents 100 μm. Arrows mark positively labeled cells. (B) Representative immunocytochemical staining of SSEA-4 positive (+) cells in Danish Landrace and Yucatan pEFs. Scale bar represents 100 μm. (C) Mean cell populations (%) of SSEA-1, SSEA-4 and CD105 fibroblasts for pEFs from all breeds.
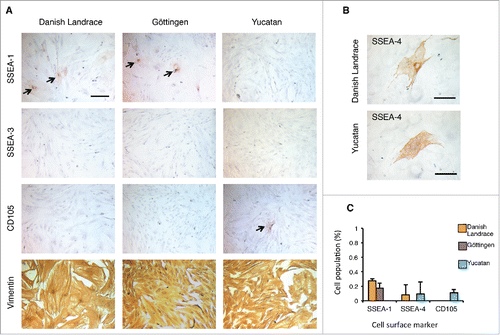
Table 2. Porcine-specific primers used in quantitative PCR to detect endogenous gene expression in the putative piPSCs and to validate microarray analyses.
Table 1. Immunocytochemical analysis and quantification of cell surface markers in porcine embryonic fibroblasts from different cell lines and breeds.
Enhanced reprogramming could be determined in single Danish Landrace and Göttingen pEF lines
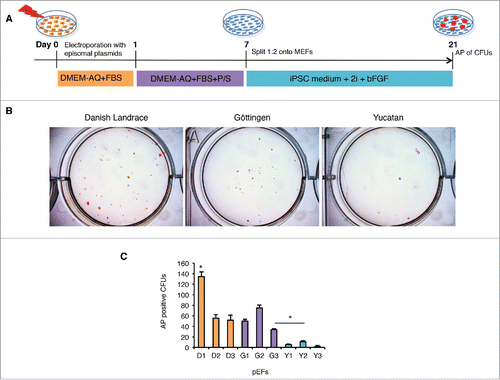
We then performed induced pluripotent reprogramming on the 9 pEFs to determine whether differences in reprogramming rates existed between either breeds and/or cell lines. To this end, we performed a non-integrative episomal vector based reprogramming as described previouslyCitation26 and then evaluated the reprogramming efficiency. A total of 105 pEFs from each of the 9 pEFs were electroporated at early passage (P3) with 3 episomal plasmids encoding human OCT4, SOX2, KLF4, L-MYC, LIN28 and shp53.Citation26 Cells were then cultured in fibroblast growth medium for 7 days, and passaged onto mitomycin C-treated mouse embryonic fibroblasts (MEFs) in basic fibroblast growth factor (bFGF)-dependent iPSC medium supplemented with MEK/ERK (PD0325901) and GSK-3β (CHIR99021) inhibitors (2i).Citation14,17 Colonies with ESC-like morphology were visible as early as 13 d post transfection (dpt). Reprogramming efficacies were calculated as the percentage of alkaline phosphatase (AP) positive colony forming units (CFUs) with ESC-like colony morphology from total cells submitted to reprogramming at 21 d post reprogramming see . The reprogramming efficiencies differed between breeds and cell lines (). Both the Danish Landrace and Göttingen pEFs were reprogrammed at a higher rate compared with the Yucatan lines (). Looking at individual pEFs, the D1 pEFs were reprogrammed at a significantly higher rate than any other cell line (). Interestingly, the 2 lines that reprogrammed most efficiently were the D1 and G2 pEFs (). Two pEFs were selected for further analyses based on their contrasting reprogramming efficiencies; D1 for their high reprogramming efficiency and Y2 for low reprogramming efficiency, Y2 was chosen over Y3 to have sufficient reprogrammed CFUs for characterization. The Danish Landrace and Yucatan pEFS shared expression of SSEA-4+ but differed in SSEA-1+ and CD105+ expression (D1: SSEA-1+, SSEA-4+, CD105-; Y2: SSEA-1neg, SSEA-4+, CD105+). To this end, 3 piPSC-like cell clones from these selected pEFs were picked following reprogramming and evaluated further for pluripotency characteristics; Danish Landrace iPSC-like (D-piPSC 1, 2, 3) and Yucatan iPSC-like (Y-piPSC 1, 2, 3).
Figure 2. Reprogramming of porcine embryonic fibroblasts (pEFs) from different breeds reveals differences between lines and breeds. (A) Schematic of the episomal plasmid-based reprogramming protocol. (B) Alkaline phosphatase (AP) positive (+) colony forming units with ESC like morphology (CFUs) in 3 representative pEF lines in the different breeds. (C) Total mean number of AP+ CFUs for each cell line. Data was collected from 3 independent episomal plasmid-based reprogramming and averaged (n = 3) as mean ± standard deviation and tested for significance using the students t-test (p < 0.05). Asterix and bar denotes significance was obtained from Y2 when compared with G3. Likewise, all D and G cell lines were found to be significantly different from the Y lines.
Characterization of the Danish Landrace-derived piPSC-like lines reveal enhanced expression of pluripotency markers and increased capacity for in vitro differentiation
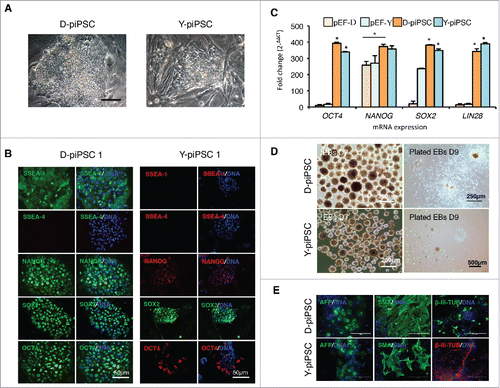
The selected iPSC-like clones were then evaluated according to colony morphology, pluripotency markers and capacity to form embryoid bodies. Upon expansion, the 3 D-piPSC-like clones exhibited human ESC-like morphology and grew as flat and compact colonies with clear boundaries, prominent nucleoli and a high nuclei-to-cytoplasm ratio (). In contrast, the majority of the Y-piPSC-like colonies were composed of a heterogenous cell population, with many smaller colonies exhibiting a neural-like morphology (). To investigate the expression of pluripotent markers we performed fluorescent immunocytochemistry on the D-piPSC-like clone 1 and Y-piPSC-like clone 1 at P8. The D-piPSC-like cells stained positive for SSEA-1, NANOG, SOX2, and OCT4, but were negative when labeled with anti-SSEA-4 (), anti-SSEA-3, anti-TRA-1–60, and anti-TRA-1–81 (data not shown). In contrast, the Y-piPSC-like cells strongly expressed SOX2, whereas only a subset of cells within the colonies stained positive for NANOG and OCT4 (). Moreover, the cell surface makers SSEA-4 (), SSEA-3, TRA-1–60 and TRA-1–81 (data not shown) were not detected in Y-piPSC-like cells. This indicated that the D-piPSC-like clones expressed several porcine pluripotent markers, whereas the Y-piPSC-like cells failed to express a typical pluripotent expression profile. We then evaluated the expression level of pluripotent genes including endogenous NANOG, OCT4, SOX2 and LIN28 in D-piPSC-like clones 1–3 and Y-piPSC-like clones 1–3 by quantitative PCR (qPCR). Expression of the pluripotency genes was in general significantly higher in the iPSC-like cells compared with the pEFs, with the exception of NANOG which was only significantly higher in the D-piPSC-like cells compared with the Danish Landrace pEFs (). There was no statistical difference in the expression level of these transcription factors except between the D-piPSC-like cells and Y-piPSC-like cells (). Thus the difference in expression levels of NANOG and OCT4 expression observed by immunocytochemistry between the D-piPSC and Y-piPSC-like cells must clearly be linked to altered differences of exogenous expression from the plasmids, since the qPCR detected only endogenous porcine gene expression.
Figure 3. Characterization of pluripotency in the Danish Landrace (D)–piPSC-like and Yucatan (Y)-piPSC-like clones. (A) Morphology of representative D-piPSC-like and Y-piPSC-like colonies. Scale bar represents 100 µm. (B) Expression of pluripotent markers in D-piPSC-like and Y-piPSC-like cells. (C) Quantitative real-time PCR for the endogenous pluripotency markers NANOG, OCT4, SOX2 and LIN28 in D-piPSCs and Y-piPSCs. Expression values were normalized to RPL4 and TCF3, and fold change was calculated using 2−ΔΔCT. Data is shown as mean and error bars denote the standard deviation. Asterisk indicates statistically significant differences (p < 0.05) in different cell lines within each gene analyzed. Error bar indicates D-piPSC is significantly different from pEF-D. (D) Embryoid bodies from the D-piPSCs and Y-piPSCs differed slightly size but most importantly in their ability to plate onto plastic substrates in vitro. (E) In vitro differentiation of D-iPSCs and Y-piPSCs reveal both cells can differentiate into all 3 germ lineages. Scale bar represents 200 µm.
To examine the in vitro differentiation capacity of the D-piPSC-like and Y-piPSC-like clones, embryoid body (EB) formation was performed. The D-piPSC-like clone 1 and Y-piPSC-like clone 1 was grown in suspension culture for 7 d and then plated onto gelatin-coated plates in fibroblast medium for a further 14 d to induce differentiation. The D-piPSCs formed large EBs and when transferred onto gelatin-coated dishes, attached to the substrate and started to differentiate as early as one day following plating (). In contrast, the Y-piPSC-like cells formed a comparable amount of EBs in suspension culture, but the EBs were much smaller in size and more heterogeneous compared with the D-piPSCs derived EBs (). Furthermore, 2 d after plating onto gelatin-coated culture plates approximately 90% of the Y-piPSC-derived EBs failed to attach (). The differentiated cells were then investigated for lineage-specific marker expression using fluorescent immunocytochemistry. We were surprised to find that both the D-piPSC-like cells and Y-piPSC-like cells stained positive for both α-feta protein (AFP) (endoderm), smooth muscle actin (SMA) (mesoderm) and β-III tubulin (ectoderm) (), which demonstrated their capability of differentiating into all 3 embryonic germ layers. However, only a minority of the differentiated Y-piPSC-like cells expressed these markers. These results demonstrated that D-piPSC-like cells were more efficient to differentiate into ectodermal, mesodermal, and endodermal cells, whereas the in vitro differentiation capability of Y-piPSC-like cells was limited.
Reprogramming of SSEA-1+ sorted cells using 2 different methods reveals a cell population with enhanced reprogramming capability
We considered whether the increased pluripotency characteristics in the D-piPSC-like cells might relate to expression differences in the original pEF populations, and in particular whether the minor SSEA-1 subpopulation played any role in the reprogramming process. To test this hypothesis, we performed live fluorescence activated cell sorting (FACS) on the Danish Landrace pEFs (D1) (P3) using a PE-labeled antibody directed against SSEA-1. Three cell populations, SSEA-1+, SSEA-1neg, and non-sorted pEFs were then reprogrammed and the reprogramming efficiency determined (). For FACS, specific gating of the forward scatter/side scatter plot of the labeled cells resulted in selection of 1.95% of the SSEA-1+ cell population (), the remaining cells were considered SSEA-1neg. The 3 cell populations, including also a non-sorted population (each 105 cells) were then subjected to doxycycline-inducible lentiviral transduction using a virus carrying porcine OCT4, SOX2, cMYC and KLF4 (pOSMK) and cultured for 21 d (). We observed enrichment in the number of AP+ CFUs with ESC-like morphology in the SSEA-1+ population 21 dpt (). In contrast, the SSEA-1neg cell fraction resulted in the formation of only a few CFUs with ESC-like morphology (). This suggested that a correlation between SSEA-1+ expression in pEFs and enhanced reprogramming, which we validated further with alternative techniques to ensure that this is not an experimental artifact.
Figure 4. Reprogramming of SSEA-1 positive cells reveals enhanced reprogramming capabilites. (A) Schematic of reprogramming SSEA-1+, SSEA-1neg and unsorted D1 pEFs using lentiviral transfection. (B) Flow cytometry analysis of SSEA-1+ cell fraction in D1 pEFs. (C) Representative Alkaline Phosphatase positive (AP+) colony forming unit with ESC-like morphology (CFU) derived from D1 SSEA-1+ cells 21 d post transduction. (D) Number of AP+ CFUs derived from the 3 cell populations. (E) Schematic of magnetic cell sorting (MACS) targeting SSEA-1+ cell fraction and subsequent episomal plasmid-based reprogramming of SSEA-1+ and SSEA-1neg fractions. (F) Total mean number of AP+ CFUs derived from MACS sorted SSEA-1+ and SSEA-1neg fractions. (G) Representative image of AP+ CFUs from MACS sorted SSEA-1+ and SSEA-1neg fractions.
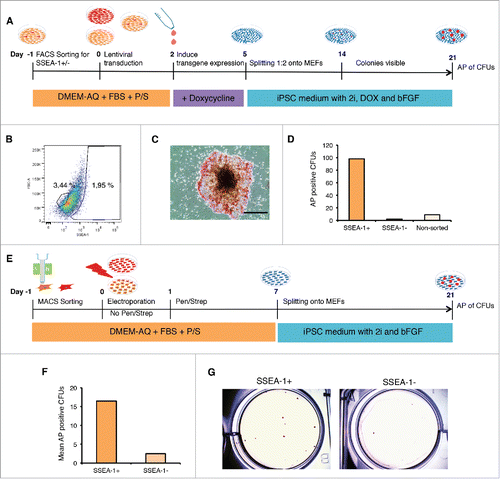
To validate these results, D1 pEFs (P4) were separated using magnetic activated cell sorting (MACS) into SSEA-1+ and SSEA-1neg fractions, and subsequently reprogrammed using episomal vectors as used earlier and again cultured for 21 d (). This experiment was repeated twice. The percentage of cells isolated was 0.37%, which helped to confirm the low expression levels determined by use of immunocytochemistry. The CFUs were stained at 21dpt with AP to assess reprogramming efficiency. Again, the number of AP positive CFUs with ESC-like colony morphology was higher in the SSEA-1+ cell population compared with the SSEA-1neg fraction (). Interestingly, however, the number of CFUs was lower than using the FACS/viral method. Together these 2 independent approaches revealed that the SSEA-1+ cell population has an increased preference to be reprogrammed than the SSEA-1neg fraction.
Transcriptome analysis of the SSEA-1+ cell population reveals a subclass of progenitors with a more mesenchymal stromal cell-like identity
To further characterize the SSEA-1+ pEFs, we performed transcriptome profiling using an Affymetrix porcine microarray. Five independent MACS of D1 pEFs were performed to produce an adequate number of biologic replicates for analysis. The data set supporting the results of this article is in the GEO database repository (unique identifier and hyperlink to data set(s) in http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc = GSE72025). Normalized RMA data was analyzed and revealed that the SSEA-1+ and SSEA-1neg populations were very similar in expression (). Further analyses revealed a relatively small number of differentially regulated genes (149 genes) when using a cut-off of 1.2-fold change (). Of these, 83 genes were upregulated in the SSEA-1+ pEFs and 63 genes were downregulated. Several of these are listed in . Among the 83 upregulated genes in SSEA-1+ pEFs, only 25 (30%) were annotated (see Fig. S3), while 22 had only basic annotation (predictive genes) and the remaining 36 were not annotated. We also found that only 20 (32%) of the 63 downregulated genes were assigned gene names. The upregulated SSEA-1+ genes were found to be involved in several biological categories, with physiological, cellular, and developmental processes being most represented (). In the case of the downregulated SSEA-1+ genes, the most represented biological categories were physiological processes, cellular processes, and biological processes (). A gene condition tree heat map revealed several interactions in the upregulated SSEA-1+ genes (). Most of these interactions were at the gene level. A large number of genes were also depicted to have physical interactions and co-expression indicating interconnecting networks within this gene subset.
Figure 5. This data are based on 5 biologic replicates (A) The significantly differentially expressed Genes from the Volcano Plot built by comparing “SSEA-1+ vs SSEA-1-neg,” using Multiple Testing Correction: Benjamini and Hochberg False Discovery Rate. Differentially expressed genes were defined by Fold Difference: 1.2 and a P-value Cutoff: 0.05. (B) A gene condition tree heat map clustering of all genes was built by comparing “SSEA-1+ vs SSEA-1-neg.” Similarity Measurement, Distance. The clustering algorithm was performed using average linkage. (C) Gene condition tree heat map clustering of all genes built by comparing “SSEA-1+ vs SSEA-1-neg.” Discarded genes with no gene symbol annotation from the starting conditions. Rows are centered; unit variance scaling is applied to rows. Both rows and columns are clustered using correlation distance and average linkage.
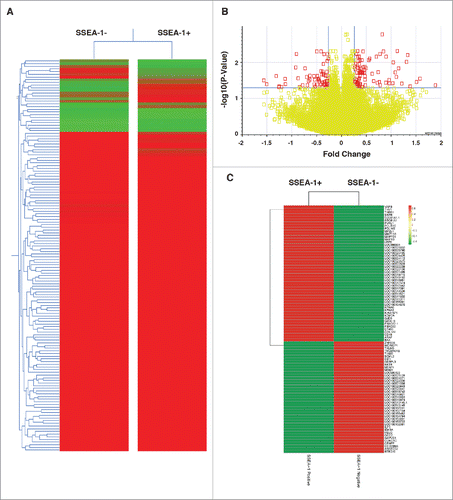
Figure 6. (A) Biological processes enrichment categories graph gene ontology in SSEA-1+ and SSEA-1neg upregulated genes. (B) Functional association interaction network of upregulated genes in SSEA-1+ vs SSEA-1neg, using protein and genetic interactions, pathways, co-expression, co-localization, protein domain and physical interaction data (Cytoscape-GeneMANIA, University of Toronto, Donnelly Center for Cellular and Biomolecular Research, www.genemania.org). (C) GeneMANIA functional association Cytoscape diagram (University of Toronto, Donnelly Center for Cellular and Biomolecular Research, www.genemania.org), association data of upregulated gene interaction with SSEA-1+ vs SSEA-1neg porcine embryonic fibroblasts (pEFS), which includes protein and genetic interaction pathways, co-expression, co-localization and protein domain similarity.
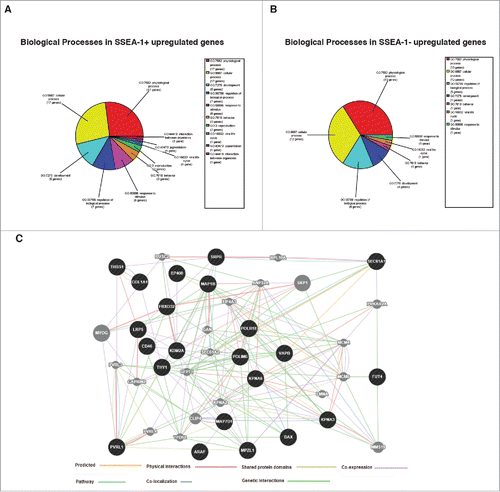
In the SSEA-1+ upregulated gene set, we found several genes that are also expressed in human MSCs (THY1 (aka CD90), MAP1B, LRP5, THBS1, GATA-6)Citation27-29 and specifically in BM-MSCs (CD46, MMP-13).Citation30,31 We also found 2 genes, which were expressed in either mesoderm (GJD3, aka Cd36)Citation32 or mesendoderm (ARAF).Citation33 In addition, we found genes that have been shown to be important in the epithelial-mesenchymal transition (PVRL1, THBS1, MMP-13).Citation34-36 Given the background of these genes, we proposed that the SSEA-1+ cell population may have a slightly different identity expressing higher levels of genes found in mesenchymal stem cells and mesoderm/mesendoderm.
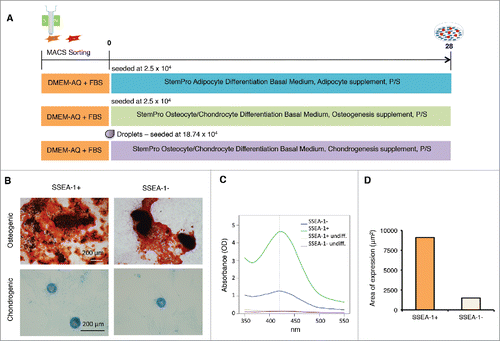
To explore whether the SSEA-1+ cells behaved differently from the SSEA-1neg population of fibroblasts, we decided to test their mesodermal differentiation capability compared with the SSEA-1neg population and performed differentiation into the 3 cell lineages, typically used to confirm MSC identity (i.e osteogenic, adipogenic, and chondrogenic lineages). We performed MACS to separate SSEA1+ cells from SSEA-1neg cells and differentiated both populations into the 3 lineages (). In the case of osteogenic differentiation, both cell fractions developed widespread mineralization and formed dense nodules in areas of high cell density (). This was obvious from visual inspection and quantification, which demonstrated a large difference from SSEA-1+ to SSEA-1neg populations. At 420 nm, optical density of SSEA-1+ derived nodules was 4.64 while SSEA-1neg derived nodules reached only 1.28 (). Stained mineralization was virtually absent in undifferentiated controls (). Similar to osteogenic differentiation, chondrogenic differentiation was more profound in SSEA-1+ cultures. Quantitative analysis of micrographs revealed a count of 13 matrix deposition spheroids in SSEA1+ vs 6 counts in SSEA-1neg. For the SSEA-1+ culture, the total stained area reached 9121.5 μm2 while the area was 1499.0 μm2 for the SSEA-1neg culture (). The average size of spheroids was 701.7 μm2 in SSEA-1+, and 249.9 μm2 for SSEA-1neg. In the case of adipogenic differentiation, neither cell population could differentiate into adipocytes or revealed cells with discernible neutral lipid droplets (data not shown). To sum up, the differentiation potential of the SSEA-1+ cells into osteocytes and chondrocytes was enhanced compared with the SSEA-1neg fraction. To give these SSEA-1+ cells an identity, we termed them SSEA-1 Expressing Enhanced Reprogramming (SEER) cells since they expressed higher levels of many mesenchymal-related genes and had a better propensity to form mesenchymal tissues in vitro.
Figure 7. Differentiation of magnetic sorted SSEA-1+ and SSEA-1neg D1 porcine embryonic fibroblasts (pEFSs) reveals enhanced differentiation into osteocytes and chondrocytes for SSEA-1+ populations. (A) Schematic representation of differentiation into mesenchymal cell lineages. (B) Both SSEA-1+ and SSEA-1neg cell fractions could undergo osteogenic and chrondrogenic differentiation following differentiation for 28 d. (C) Quantification of osteogenic differentiation was performed by reading absorbance levels of Alizarin red and revealed a higher propensity of differentiation in the SSEA-1+ cell fraction compared with the SSEA-1neg cell fraction. (D) Quantitative analyses of Alcian Blue revealed SSEA-1+ cells differentiated more proficiently than SSEA-1neg cells.
Discussion
In the present study, we identified a small subset of SSEA-1+ cells within porcine embryonic fibroblasts (pEFs) which possess enhanced reprogramming efficiency compared with SSEA-1neg cells. These cells expressed mesenchymal-related genes and were termed SSEA-1 Expressing Enhanced Reprogramming (SEER) cells.
Initially, we determined cell line and breed differences in the expression of pluripotent cell surface markers and one of the many MSC markers, CD105. Of particular interest was that the Yucatan pEFs lacked SSEA-1 expression, but also were the only breed that had a minor population of CD105+ cells. These breed differences may not be related to breed differences per se, but might reflect potential differences related to timing of development of different breed embryos, since gestational lengths are slightly shorter in Yucatan minipigs compared with domestic and other miniature pig breeds (111 vs 114 days).Citation37 In addition, birth weights differ between breeds, as does the development of the musculoskeletal system.Citation38 As an effect of breed and these other potential differences, we found that the reprogramming efficiency was lowest in the Yucatan breed. In contrast, the highest reprogramming efficiency was observed in Danish Landrace and Gottingen cell lines, which both shared a common minor population of SSEA-1+ cells.
Reprogramming and characterization of pEFs into piPSCs from cell lines that were “better” at reprogramming (i.e. Danish Landrace D1) or “worse” at reprogramming (i.e., Yucatan Y1) revealed differences regarding the expression of pluripotency markers and the capacity for in in vitro differentiation in the resultant piPSC-like cells. The Danish Landrace pEF cell line that produced more colonies also produced piPSC-like cells that exhibited more pluripotency characteristics. We assumed therefore that there might be inherent cellular characteristics of cells within these 2 cell lines that resulted in a more complete and less complete reprogramming. We considered that the elite population would unlikely be the SSEA-4+ cells since it was equally expressed in both D1 and Y1. The more likely candidate was SSEA-1, since SSEA-1+ expressing pEFs had higher reprogramming efficiencies and was absent in the difficult to reprogram Yucatan pEFs. Identification of SSEA-1 as a potential marker of enhanced reprogramming is a novel finding, but may be species-specific for the pig.
The SEER cells have a clearly different expression profile to the reported Muse cells or Muse-AT cells discovered in adult dermal fibroblasts and adipose tissue, respectively, as they lacked SSEA-3+/CD105+ expression. This was not that unexpected since they arise from a more primitive population of cells from the developing embryo. These SSEA-1+ cells were found to have some similarities to mesenchymal-like cells. Transcriptome profiling revealed that SSEA-1+ cells are very similar to the SSEA-1 negative cell population but differed in a small subset of genes, many of which could be linked to mesoderm-, MSC-, and BM-MSC-specific expression and the epithelial-mesenchymal transition. Furthermore the expression profile of OCT4, SOX2 and NANOG in the SEER cells () is highly similar to porcine skin-derived progenitors.Citation25 This is in accordance with the hypothesis by Zhao and colleagues which suggests that this expression profile may be indicative of cell multipotency. Thus, it appears at least in the pig, that OCT4, SOX2 and NANOG are not exclusive markers of pluripotency, as observed in mice.Citation39 This also means that the expression profile of bona fide piPSC remains controversial, since exclusive genes marking pluripotent stem cells are absent.
The SEER cells, i.e., the SSEA-1+ subpopulations, as well as the SSEA-1neg subpopulations displayed the potential to differentiate into more than one terminal phenotype; however, the SSEA-1+ subpopulations demonstrated enhanced differentiation capabilities. The increased propensity to differentiate into osteocytes and chondrocytes also support that they had closer shared properties with MSCs than the SSEA-1neg cells, but do not share the capacity to self-renew with stem cells. Surprisingly, no observable degree of adipogenic differentiation occurred in either of the cell populations. In our hands, human MSCs readily display lipid accumulation after short periods,Citation40 while this is difficult to attain with porcine MSCs even with prolonged induction periods (personal observation). Whether this is related to species variation or a unique attribute of SEER cells remains uncertain.
Human dermal fibroblasts have also previously been shown to have some mesenchymal properties. Human skin fibroblasts express several MSC markers including CD44, CD73 and CD105, and can differentiate into adipocytes, osteoblasts and chondrocytes,Citation41 or differentiate into chondrocytes alone.Citation42 This is not surprising since cartilage and bone develops within the embryonic mesenchyme. However, there has been little investigation in earlier sources of fibroblasts, such as fetal or embryonic fibroblasts and whether subpopulations may exist in such cell cultures, which have greater propensity over others. One investigation has shown that MSCs have enhanced reprogramming capability over other cell types such as tail tip fibroblasts and osteo-progenitor cellsCitation43 which suggests they are more amenable to reprogramming than other cell types, but reasons for this are unknown.
Here, we show that although some cell heterogeneity exists in pEFs, transcriptional profiling revealed very few differences in gene expression. These differences have implications for the reprogramming capability of the cells. Interestingly the SEER cells, which express higher levels of mesenchymal-like genes and mesoderm/mesendoderm genes, have a higher propensity to reprogram than the remaining cell population, and seem to have enhanced epithelial-mesenchymal transition potential, which is an important event in the reprogramming process. Therefore, we can speculate that the expression of these genes gives these cells an added advantage. It may mean that in the future, cell populations expressing more mesenchymal genes might be a more amenable source of cells for reprogramming and may be isolated by simple means as e.g. MACS.
In summary, our research supports a theory that lies in the middle of both the stochastic and elite theory. That is, the mesenchymal characteristics of certain cell populations in a heterogeneous cell population will lead to a higher reprogramming efficiency. This population may not be exclusively reprogrammed, as seen in our case. Refining this molecular signature could be advantageous in determining the optimal cell needed for reprogramming and for producing bona fide piPSC. These results also help to highlight the importance in screening pEF populations for SSEA-1 before reprogramming, to enhance the quality of piPSCs produced. To conclude, we have identified a SEER cell population in pEFs that differ slightly in their RNA expression profile to the remaining pEFs. Future research on reprogramming mechanisms will help to unfold the rate-limiting step that is important in selecting which cells pass through the stochastic reprogramming phase to the maturation and stabilization phase.
Materials and methods
Porcine embryonic fibroblast cell culture
Porcine embryonic fibroblasts were derived from 24-day-old fetuses following removal of the head by using standard procedures. Citation21 In this study, 9 porcine embryonic fibroblast lines at early passages were selected from 3 different breed backgrounds (Danish Landrace 1, 2, 4 (D1, D2, D3); Göttingen minipig 1, 2, 3 (G1, G2, G3) and Yucatan minipig lines 1, 2, 3 (Y1, Y2, Y3)). These cells were cultured in fibroblast growth medium, consisting of Dulbecco's Modified Eagle's Medium (DMEM) (Sigma-Aldrich, # D5546) supplemented with 1% penicillin/streptomycin (pen/strep) (Sigma-Aldrich, # P4333) and 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, #10082147), on 0.1% gelatin coated cell culture dishes. Cells were maintained in 5% CO2 in a humidified environment at 38.5˚C. Cells were allowed to expand to 80–90% confluency before passaging with 0.25% Trypsin-EDTA (Sigma-Aldrich, # T4049 ).
Immunocytochemistry
Cells were transferred onto 0.1% gelatin-coated 3.5 cm2 dishes (Thermo/Nunc, # 150628) for chromogen immunocytochemistry. Porcine embryonic fibroblasts from 9 cell lines and control cell lines (human ESCs and mouse ESCs) were fixed in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for 15 min at room temperature (rt). Fixed cells were washed 3 times in PBS and either stored in PBS at 4˚C or directly used after. Endogenous peroxidase blocking was performed by incubating cells in 3% hydrogen peroxide (H2O2) in water for 10 min at rt using gentle rotation. Cells were washed 2 times with PBS and then pretreated by boiling citrate buffer (0.01 M, PH 6.0) for 15min. Blocking was performed in 10% normal goat serum (Sigma-Aldrich, # G9023) and in 1% BSA/PBS for 30 min at rt using gentle rotation. Cells were briefly washed in PBS and incubated in primary antibodies overnight at 4˚C with gentle rotation. Primary antibodies were diluted in 1% BSA/PBS at dilutions as stated in Table S1. Isotype antibodies were diluted in 1% BSA/PBS and diluted as stated in Table S2. Biotinylated secondary antibodies (DAKO, #E0433) were diluted in1% BSA/PBS at 1:250. Cells were washed twice with PBS and incubated in diluted secondary antibodies for 30 min at rt with gentle rotation before incubation in streptavidin/horseradish peroxidase (HRP) (DAKO, K5003) at 1:500 for 30 min at rt. Cells were labeled with diaminobenzidine (DAB) (DAKO, #K3408) for 20–30 min according to manufacturer's recommendations, and cell nuclei were counterstained with Mayer's hematoxylin for 30 sec. Cells were stored in MilliQ H2O at 4˚C. Images were taken using an inverted Leica DM IL microscope (Leica Microsystems) and a minimum of 500 cells were scored for assessment of cell surface maker expression. A human ESC line (H1/WA01) (purchased from WiCell) was used as a positive control for SSEA-3, SSEA-4, TRA-1–60, and TRA-1–81. A mouse ESC line derived in Hungary by Biotalentum Ltd. was used as positive controls for SSEA-1.
For fluorescence immunocytochemistry, cells (D1 pEFs, D-piPSCs and Y-piPSCs at P8) were cultured on Lab-Tek chamber slides (Thermo/Nunc, # 154461) or 6-well plates (differentiated D-piPSCs and Y-piPSCs) and fixed in 4% PFA for 15 min at rt. They were permeabilized in 0.1% Triton-X 100 (Sigma-Aldrich, # T9284) in PBS for 30 min at rt. Cells were rinsed twice in PBS and incubated in blocking buffer [5% normal donkey serum (Sigma-Aldrich, # D9663) in 0.25% bovine serum albumin (BSA)/PBS] for 1 h at rt. Primary antibodies were diluted in 0.25% BSA/PBS and cells were cultured overnight at 4˚C with gentle rotation. Primary antibodies and their dilutions are described in Table S1. Isotype antibodies were diluted in 0.25% BSA/PBS, which are listed in Table S2. Secondary antibody negative controls were performed for NANOG and OCT4. The human ESC line (H1/WA01) was used as a positive control for SSEA-3, SSEA-4, TRA-1–60, and TRA-1–81 and the mouse ESC line derived in Hungary by Biotalentum Ltd. was used as positive control for SSEA-1, OCT4 and NANOG. Cells were washed 2 times with PBS and incubated in either Alexa Fluor 488-conjugated or Alexa Fluor 594-conjugated secondary antibodies (Invitrogen/Thermo), which were diluted at 1:200 in 0.25% BSA/PBS. DNA labeling was performed by incubating the cells in 5µg/ml Hoechst (Sigma-Aldrich, # 33342) for 10 min. Fluorescent mounting medium (DAKO, # S3023 ) was used to mount the glass slides. Images were captured using a DMRB fluorescent microscope (Leica Microsystems).
Reprogramming porcine embryonic fibroblast cell lines using episomal plasmids
Three episomal plasmids, pCXLE-hOCT3/4-shp53, pCXLE-hSK, and pCXLE-hUL (Addgene, plasmids #27077, #27078, and #27080) were purified using the Purelink™ HQ Mini Plasmid purification kit (Invitrogen, # K210001). Passage 3 pEFs were trypsinized by Trypsin-EDTA (Sigma-Aldrich, # T4049) and 105 cells were electroporated with 1μg of each of the episomal plasmids (1:1:1) using a Neon™ electroporation device (Life Technologies) with a single pulse at 1300 V for 30 ms. Cells were then plated in DMEM containing 10% FBS without antibiotics. The medium was replaced with the addition of Pen/Strep on day 2. On day 7, cells were trypsinized with 0.25% Trypsin-EDTA, passaged onto mitomycin C–treated (Sigma-Aldrich, # Y0000378) CF1 mouse embryonic fibroblasts (MEFs) (Millipore) and cultured in DMEM/F12 medium (Sigma-Aldrich, # D6421) supplemented with 20% KnockOut Serum Replacement (Invitrogen/Thermo, # 10828028), 1 x pen/strep, 1 x nonessential amino acids (Sigma-Aldrich), 100 µM β-mercaptoethanol (Gibco/Life Technologies, # 21985023), 20 ng/ml human recombinant basic fibroblast growth factor (bFGF) (Prospec, # CYT-218), and 2 kinase inhibitors; 1µM PD0325901 (Sigma-Aldrich, # PZ0181), which inhibits the MEK signal pathway, and 3µM CHIR99021 (Sigma-Aldrich, # SML1046), a GSK-3β inhibitor (iPSC 2i medium). The cells were cultured in reduced oxygen (5% O2, 5% CO2 in N2) at 38.5˚C. The culture medium was changed daily. On day 21, alkaline AP activity in reprogrammed colonies was analyzed. Reprogramming efficiency was calculated as the percentage of colony forming units (CFUs) with ESC-like morphology (stained positive for AP) from total reprogrammed cells. Colonies with ESC-like morphology were manually picked using a 0.5ml syringe (Terumo) and transferred onto new MEFs as one colony per well in a 24-well plates (Thermo/Nunc, # 142475). After 4 d culture, these colonies were dissociated with TrypLE Select (Gibco/Life Technologies) and transferred into single wells of 6-well plates (Thermo/Nunc, # 140675). Colonies derived from the D1 and Y2 pEF background were designated as D-piPSC-like and Y2-piPSC-like clones. Three clonal lines were established and used in further analyses. The piPSCs were routinely passaged every 4–5 d with TrypLE Select and the culture medium was changed daily.
Alkaline phosphatase (AP) assay
This assay was performed using a Liquid Permanent Red (LPR) substrate-chromogen system (Dako). The resulting colonies were fixed in 4% PFA for 15 min at rt. One drop of LPR Chromogen (20µl) was added into 3 ml of LPR Substrate Buffer and mixed well immediately before use. The resulting colonies were incubated in fresh Substrate Buffer-Chromogen reagent for 20 min in the dark at rt. Stained cells were washed twice with MilliQ H2O and stored in MilliQ H2O at 4˚C. Images were taken using the EVOS™ XL Core digital inverted microscope (Life Technologies).
Fluorescence-activated cell sorting (FACS)
Danish Landrace (D1) pEFs at passage 3 (P3) were dissociated with Accutase (Thermo Fischer Scientific, # A1110501) for 10–15 min. Single cell suspension was washed once in PBS to remove traces of Accutase, followed by a wash in PBS containing 2% BSA. Cells were labeled with PE anti-SSEA-1 (Nordic BioSite, # 301906) according to manufacturer's instructions in PBS containing 2% BSA with fluorochrome-conjugated antibodies for 60 min at rt, followed by washing twice in PBS containing 2% BSA. Prior to analysis, cells were strained through a 70 µm cell strainer (BD Biosciences). Cells were sorted on a FACSAria III (BD Biosciences) with a 100µm nozzle at approximately 20 PSI (Area scaling 0,39, FSC 170 V, SSC 316 V) and collected in fibroblast growth medium. Sorted cell fractions were centrifuged and re-suspended in fresh fibroblast growth medium for plating and subsequently plated on 0.1% gelatin coated cell culture dishes for recovery.
Magnetic-activated cell sorting (MACS)
Danish Landrace (D1) pEFs at passage 4 (P4) were trypsinized by 0.25% Trypsin-EDTA solution and strained through a 0.4 µm mesh (Miltenyi Biotech) to obtain single-cell suspension. A total of 2.7 × 107 cells were counted before centrifugation at 1000 rpm for 5 min, followed by the re-suspension and labeling with monoclonal anti-SSEA-1 (1:50) (Biolegend, # B134566) for 5 min at 4°C. Cells were washed twice by adding 1ml buffer (PBS containing 0.5% BSA and 2mM EDTA) per 107 cells, followed by centrifugation at 1000 rpm for 3 min. Supernatant was removed by pipetting and the cell pellet was re-suspended in 80 µl of buffer per 107 cells. 20 µl of Rat Anti-Mouse IgM MicroBeads (Miltenyi Biotech, # 130–047–301) per 107 total cells was added into the cell suspension and the cell suspension was incubated for 15 min at 4°C. Cells were again centrifuged and resuspended. The cell suspension was loaded onto a MACS MS Column (Miltenyi Biotech), which was placed into the magnetic field of a MACS Separator (Miltenyi Biotech). The flow-through fraction was collected as an SSEA-1 negative fraction (i.e., SSEA-1neg). After removing the MS Column from the separator, the retained cells were eluted as the SSEA-1+ cell fraction. The 2 fractions were subjected to non-integrative plasmid based reprogramming or microarray analysis, as described above.
Lentivirus production
A total of 2.5 × 106 293T cells were seeded in 10 cm dishes and transfected 1 day later with 20 µg doxycycline inducible lentiviral plasmid (FU delta GW backbone with a tetO promoter containing porcine genes OCT4, cMYC, SOX2, KLF4) and rtTA along with packaging plasmids (5 µg pMDG and 15 µg pBRΔ8.91; a kind gift from D. Trono, Lausanne) using the calcium phosphate method.Citation44 The medium was changed the following day. After 24 h, the medium was harvested, centrifuged for 10 min at 4°C at 2500 g, and filtered through a 0.45 μm PVDF membrane filter (Millipore). To concentrate the lentiviral particles, filtrated medium was ultracentrifuged in a Beckmann ultracentrifuge and a SW32 rotor to reduce the volume 700–1000 times. The titers of the viral preparations were assessed using quantitative PCR as described previously.Citation44 Briefly, cells were transduced with dilution series of the concentrated vector stocks. Cells were then harvested and lysed before the lysates were subjected to quantitative PCR (qPCR) to determine the amount of integrated viral DNA. The titer was then calculated by comparing the viral DNA content with a vector with known titer (as assessed by GFP expression in transduced cells).
Reprogramming porcine embryonic fibroblast cell lines using lentivirus
24h before the lentiviral transduction, Danish Landrace pEFs (P3) SSEA-1+ FACS cells, SSEA-1neg FACS cells and unsorted cells were trypsinized using 0.25% Trypsin-EDTA and plated in 0.1% gelatin-coated 6-well plates at a density of 20,000 cells per well. On the day of transduction, concentrated viruses (pOMSK and rtTA) at 20 multiplicity of infection (MOI), were added into the fibroblast growth medium with 8 µg/ml polybrene (Sigma-Aldrich, # AL-118). The medium was changed 2 d after transduction, with the addition of 4 µg/ml doxycycline (Sigma-Aldrich, # D9891) to induce transgene activation. On day 4 after transduction, cells were harvested with Trypsin-EDTA and transferred onto MEFs and cultured in the presence of iPSC 2i medium. The transduced cells were cultured in 5% O2 in nitrogen. The culture medium was changed every second day. Small colonies could be observed as early as 10 d post transduction. On day 21, the reprogramming efficiency was evaluated by first performing AP staining and then counting the CFUs with ESC-like morphology.
Embryoid body assay
The putative D-piPSC clone 1 and Y-piPSC clone 1 at passage 8 (P8) were dissociated with Accutase and transferred to low cell binding plates (Sigma-Aldrich) for suspension culture in iPSC 2i medium in the absence of bFGF and doxycycline for a period of 7 d. EBs were then transferred onto 0.1% gelatin-coated 6-well plates and cultured in fibroblast growth medium and were allowed to differentiate for 14 d. The differentiated cells were subsequently immunolabeled with anti-β-III tubulin (ectoderm), anti-SMA (mesoderm), and anti-AFP (endoderm). Samples were analyzed using an EVOS™ digital inverted fluorescent microscope (Life Technologies).
Quantitative real-time PCR
The putative D-piPSCs and Y-piPSCs were harvested at P8 and lysed in 350 µl RLT buffer (Qiagen, #) containing β-mercaptoethanol (Sigma-Aldrich, MO). Total RNA was extracted using the RNeasy Micro Kit (Qiagen, # 74004) according to the manufacturer's instructions. First-strand cDNA synthesis was performed using a RevertAid™ First Strand cDNA Synthesis Kit (Fermentas/Thermo Scientific, # K1621) according to the manufacturer's instructions. A total of 1 µg RNA was used as a template for cDNA synthesis. The obtained cDNA from the 2 piPSC lines was used for quantitative real-time PCR (qPCR) reactions. The qPCR was performed in triplicate in 96-well optical reaction plates (Scientific Specialties) using the LightCycler 480 SYBR Green I Master Kit (F-Hoffman La Roche, # 04707516001). Each reaction well contained 5 µl SYBR Green Master Mix, 1 µl forward (10 µM) and 1 µl reverse primers (10 µM) to the target genes, 1 µl dH2O and 2 µl of diluted cDNA in a final reaction volume of 10 µl. Porcine-specific primers used for qPCR are shown in .
Three biological replicates were prepared for each cell line and control cell lines. The housekeeping gene, GAPDH, was used as an internal control to normalize the Ct values of target genes. The relative fold change was calculated using the 2−ΔΔCt method. The 2−ΔΔCt values were used in statistical analysis by students t-tests at a significance level of p ≤ 0.05. Standard deviations were also determined.
Microarray
Sorting of SSEA-1+ and SSEA-1neg fractions was performed on D1 pEFs (P4) using MACS as described above. This was performed 5 times to obtain a suitable number of biologic replicates for analysis. Total RNA was later extracted from the SSEA-1+ and SSEA-1neg populations using the RNAeasy Micro Kit. RNA quality was evaluated on the Agilent Bioanalyzer (Agilent Technologies) before processing. A total of 100ng RNA was reverse transcribed to cDNA and labeled with biotin using the GeneChip 3′ IVT Express Kit (Affymetrix, 902415) according to the manufacturer's procedures. The biotin-labeled cRNA samples were fragmented and hybridized onto a Porcine Genome Array (GeneChip Lot# 4237979) according to the manufacturer. Data was normalized using the rma method.
Bioinformatics and data analysis
The rma normalized values for the SSEA-1+ and SSEA-1neg replicates were used to calculate the mean to evaluate average gene expression and estimation of the fold change. The background corrected data were normalized by per-chip, per-gene and the global median polishing normalization method using GeneSpring bioinformatics software (GeneSpring GX, Agilent). Statistical analysis was performed using the Benjamini and Hochberg False Discovery Rate Multiple Testing Correction. Differentially expressed genes were defined by fold difference of more than 1.2 and a P-value cutoff of p < 0.05 was considered significant. Functional clustering of lists of differentially expressed genes were generated by comparing them against the ontology terms for molecular function, cellular composition and biological processes using Gene Ontology databases (GO). These databases include, the Gene Ontology Consortium (GO, http://geneontology.org/) and the National Human Genome Research Institute (NHGRI). A gene condition tree heat map clustering all SSEA-1+ upregulated genes was performed using the Average Linkage method. Genes with no data was discarded in the starting conditions.
Adipogenic, osteogenic, and chondrogenic differentiation
Adipogenic, osteogenic, and chondrogenic differentiation capacity was evaluated by induction with StemPro differentiation kits (Gibco, Life Technology) which we have validated work well in-house using primary human MSCs (data not shown). In 24-well plates, adipogenic differentiation was initiated at a seeding density of 5 × 104 cells/well in 24-well plates by induction medium (StemPro Adipocyte Differentiation Basal Medium, Adipocyte Supplement, and pen/strep). Similarly, osteogenic differentiation was commenced at a density of 2.5 × 104 cells/well by medium induction (StemPro Osteocyte/Chondrocyte Differentiation Basal Medium, Osteogenesis Supplement, and pen/strep). For chondrogenic differentiation, 18.75 × 104 cells were resuspended in 100 µl medium before seeding in 5 µl droplets. The cells were allowed to adhere for 3 hours before addition of inductive medium (StemPro Osteocyte/Chondrocyte Differentiation Basal Medium, Chondrogenesis Supplement, and pen/strep). Cells were cultured for 28 days, with medium changed every 3–4 d. Control cells were kept in medium containing 2% FBS and pen/strep without other supplements throughout the culture period.
Samples were washed in PBS and fixed using 4% paraformaldehyde in PBS for 20 minutes at 37°C before staining. Adipogenic samples were stained using Oil Red O (Sigma-Aldrich, # O0625), osteogenic samples with Alizarin Red S (Sigma-Aldrich, # A5533), and chondrogenic samples by Alcian Blue 8GX (Sigma-Aldrich, # A5268).
For quantification of Alizarin Red S staining, samples were washed with deionized water before addition of acetic acid [1.5M] for 15 minutes at rt. All contents of the well was scraped loose and transferred to an Eppendorf tube, suspended by pipetting, and left for an additional 15 minutes of incubation. Tubes were heated to 85°C for 10 minutes, then cooled on ice for 5 min before centrifugation at 15.000·g for 15 min. Supernatant was mixed with ammonium hydroxide [1.43M] to a pH of 4.5 before reading absorbance on a FLUOstar Omega microplate reader (BMG Labtech). Peak absorbance was determined at 420 nm.
Chondrogenic differentiation was assessed by obtaining a cross-section of an entire well by means of overlapping micrographs. The images were stitched to a single image using the MosaikJ plug-in for ImageJ (National Institute of Health), converted to 8-bit, thresholded, and analyzed automatically.
Abbreviations
| AFP | = | α feta protein |
| AP | = | alkaline phosphatase |
| bFGF | = | basic fibroblast growth factor |
| CFU | = | colony forming unit |
| DMEM | = | Dulbecco's modified eagle medium |
| dpt | = | days post transfection |
| EB | = | embryoid body |
| ESC | = | embryonic stem cell |
| FACS | = | fluorescent-activated cell sorting |
| FBS | = | fetal bovine serum |
| GO | = | gene ontology |
| iPSC | = | induced pluripotent stem cell |
| LPR | = | liquid permanent red |
| MACS | = | magnetic-activated cell sorting |
| MSC | = | mesenchymal stem cell |
| Muse | = | multilineage-differentiating stress enduring |
| pEF | = | porcine embryonic fibroblast |
| pen/strep | = | penicillin and steptomycin |
| piPSC | = | porcine induced pluripotent stem cell |
| RMA | = | robust multiarray average |
| rt | = | room temperature |
| SSEA-1 | = | stage-specific embryonic antigen-1 |
| SEER | = | SSEA-1 expressing enhanced reprogramming |
| SMA | = | smooth muscle actin |
Disclosure of potential conflicts of interest
In the case of each author, there is no commercial association or conflict of interest with the submitted manuscript.
KCCY_A_1315490_Supplemental.zip
Download Zip (34.8 KB)Acknowledgments
We would like to thank Tina Christoffersen for cell culture support, Anita Pacht for assistance with qRT-PCR. We also thank Ellegaard Göttingen Minipigs A/S for supplying us with Göttingen embryos that were used to produce the porcine embryonic fibroblasts in this study.
Funding
Funding for this research was provided by: The Danish Council for Independent Research, Natural Sciences (FNU), grant number: HEALTH-2007-B-223485; US Department of Agriculture (USDA) grant number: 2011–67015–30688 and personal PhD fellowship to Dong Li sponsored by China Scholarship Council (CSC).
References
- Ezashi T, Telugu BP, Roberts RM. Induced pluripotent stem cells from pigs and other ungulate species: An alternative to embryonic stem cells?. Reprod Domest Anim 2012; 47 Suppl 4:92-7; PMID: 22827356; https://doi.org/10.1111/j.1439-0531.2012.02061.x
- Wu J, Platero-Luengo A, Sakurai M, Sugawara A, Gil MA, Yamauchi T, Suzuki K, Bogliotti YS, Cuello C, Morales Valencia M, et al. Interspecies chimerism with mammalian pluripotent stem cells. Cell 2017; 168:473-486.e15; PMID: 28129541; https://doi.org/10.1016/j.cell.2016.12.036
- Dehoux J-P, Gianello P. The importance of large animal models in transplantation. Front Biosci 2007; 12:4864-80; PMID: 17569616; https://doi.org/10.2741/2434
- Samavarchi-Tehrani P, Golipour A, David L, Sung H-K, Beyer TA, Datti A, Woltjen K, Nagy A, Wrana JL. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell 2010; 7:64-77; PMID: 20621051; https://doi.org/10.1016/j.stem.2010.04.015
- Golipour A, David L, Liu Y, Jayakumaran G, Hirsch CL, Trcka D, Wrana JL. A late transition in somatic cell reprogramming requires regulators distinct from the pluripotency network. Cell Stem Cell 2012; 11:769-82; PMID: 23217423; https://doi.org/10.1016/j.stem.2012.11.008
- Hansson J, Rafiee MR, Reiland S, Polo JM, Gehring J, Okawa S, Huber W, Hochedlinger K, Krijgsveld J. Highly coordinated proteome dynamics during reprogramming of somatic cells to pluripotency. Cell Rep 2012; 2:1579-92; PMID: 23260666; https://doi.org/10.1016/j.celrep.2012.10.014
- Buganim Y, Faddah DA, Jaenisch R. Mechanisms and models of somatic cell reprogramming. Nat Rev Genet 2013; 14:427-39; PMID: 23681063; https://doi.org/10.1038/nrg3473
- Yamanaka S. Elite and stochastic models for induced pluripotent stem cell generation. Nature 2009; 460:49-52; PMID: 19571877; https://doi.org/10.1038/nature08180
- Byrne JA, Nguyen HN, Reijo Pera RA. Enhanced generation of induced pluripotent stem cells from a subpopulation of human fibroblasts. PLoS One 2009; 4:e7118; PMID: 19774082; https://doi.org/10.1371/journal.pone.0007118
- Wakao S, Kitada M, Kuroda Y, Shigemoto T, Matsuse D, Akashi H, Tanimura Y, Tsuchiyama K, Kikuchi T, Goda M, et al. Multilineage-differentiating stress-enduring (Muse) cells are a primary source of induced pluripotent stem cells in human fibroblasts. Proc Natl Acad Sci 2011; 108:9875-80; PMID: 21628574; https://doi.org/10.1073/pnas.1100816108
- Heneidi S, Simerman AA, Keller E, Singh P, Li X, Dumesic DA, Chazenbalk G. Correction: Awakened by cellular stress: Isolation and characterization of a novel population of pluripotent stem cells derived from human adipose tissue. PLoS One 2013; 8:1-14; PMID: 23755141; https://doi.org/10.1371/annotation/190d4d01-a63c-4adc-a123-e519ee40a03e
- Wakao S, Kitada M, Dezawa M. The elite and stochastic model for iPS cell generation: multilineage-differentiating stress enduring (Muse) cells are readily reprogrammable into iPS cells. Cytometry A 2013; 83:18-26; PMID: 22693162; https://doi.org/10.1002/cyto.a.22069
- Yang Z, Liu J, Liu H, Qiu M, Liu Q, Zheng L, Pang M, Quan F, Zhang Y. Isolation and characterization of SSEA3(+) stem cells derived from goat skin fibroblasts. Cell Reprogram 2013; 15:195-205; PMID: 23668861; https://doi.org/10.1089/cell.2013.0013
- Esteban MA, Xu J, Yang J, Peng M, Qin D, Li W, Jiang Z, Chen J, Deng K, Zhong M, et al. Generation of induced pluripotent stem cell lines from Tibetan miniature pig. J Biol Chem 2009; 284:17634-40; PMID: 19376775; https://doi.org/10.1074/jbc.M109.008938
- Ezashi T, Telugu BP, Alexenko AP, Sachdev S, Sinha S, Roberts RM. Derivation of induced pluripotent stem cells from pig somatic cells. Proc Natl Acad Sci U S A 2009; 106:10993-8; PMID: 19541600; https://doi.org/10.1073/pnas.0905284106
- Wu Z, Chen J, Ren J, Bao L, Liao J, Cui C, Rao L, Li H, Gu Y, Dai H, et al. Generation of pig induced pluripotent stem cells with a drug-inducible system. J Mol Cell Biol 2009; 1:46-54; PMID: 19502222; https://doi.org/10.1093/jmcb/mjp003
- Telugu BP, Ezashi T, Roberts RM. Porcine induced pluripotent stem cells analogous to naive and primed embryonic stem cells of the mouse. Int J Dev Biol 2010; 54:1703-11; PMID: 21305472; https://doi.org/10.1387/ijdb.103200bt
- West FD, Terlouw SL, Kwon DJ, Mumaw JL, Dhara SK, Hasneen K, Dobrinsky JR, Stice SL. Porcine induced pluripotent stem cells produce chimeric offspring. Stem Cells Dev 2010; 19:1211-20; PMID: 20380514; https://doi.org/10.1089/scd.2009.0458
- Fujishiro SH, Nakano K, Mizukami Y, Azami T, Arai Y, Matsunari H, Ishino R, Nishimura T, Watanabe M, Abe T, et al. Generation of naive-like porcine-induced pluripotent stem cells capable of contributing to embryonic and fetal development. Stem Cells Dev 2013; 22:473-82; PMID: 22889279; https://doi.org/10.1089/scd.2012.0173
- Montserrat N, Bahima EG, Batlle L, Hafner S, Rodrigues AM, Gonzalez F, Belmonte JCI. Generation of pig iPS cells: A model for cell therapy. J Cardiovasc Transl Res 2011; 4:121-30; PMID: 21088946; https://doi.org/10.1007/s12265-010-9233-3
- Hall VJ, Kristensen M, Rasmussen MA, Ujhelly O, Dinnyés A, Hyttel P. Temporal repression of endogenous pluripotency genes during reprogramming of porcine induced pluripotent stem cells. Cell Reprogram 2012; 14:204-16; PMID: 22578162; https://doi.org/10.1089/cell.2011.0089
- West FD, Uhl EW, Liu Y, Stowe H, Lu Y, Yu P, Gallegos-Cardenas A, Pratt SL, Stice SL. Brief report: Chimeric pigs produced from induced pluripotent stem cells demonstrate germline transmission and no evidence of tumor formation in young pigs. Stem Cells 2011; 29:1640-3; PMID: 22039609; https://doi.org/10.1002/stem.713
- Cheng D, Guo Y, Li Z, Liu Y, Gao X, Gao Y, Cheng X, Hu J, Wang H. Porcine induced pluripotent stem cells require LIF and maintain their developmental potential in early stage of embryos. PLoS One 2012; 7:e51778; PMID: 23251622; https://doi.org/10.1371/journal.pone.0051778
- Li Z, Shi J, Liu D, Zhou R, Zeng H, Zhou X, Mai R, Zeng S, Luo L, Yu W, et al. Effects of donor fibroblast cell type and transferred cloned embryo number on the efficiency of pig cloning. Cell Reprogram 2013; 15:35-42; PMID: 23256540; https://doi.org/10.1089/cell.2013.0010
- Zhao M, Isom SC, Lin H, Hao Y, Zhang Y, Zhao J, Whyte JJ, Dobbs KB, Prather RS. Tracing the stemness of porcine Skin-Derived Progenitors (pSKP) Back to specific marker gene expression. Cloning Stem Cells 2009; 11:111-22; PMID: 19226215; https://doi.org/10.1089/clo.2008.0071
- Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods 2011; 8:409-12; PMID: 21460823; https://doi.org/10.1038/nmeth.1591
- Okoye UC, Malbon CC, Wang H-Y. Wnt and Frizzled RNA expression in human mesenchymal and embryonic (H7) stem cells. J Mol Signal 2008; 3:16; PMID: 18822127; https://doi.org/10.1186/1750-2187-3-16
- Montzka K, Lassonczyk N, Tschöke B, Neuss S, Führmann T, Franzen R, Smeets R, Brook GA, Wöltje M. Neural differentiation potential of human bone marrow-derived mesenchymal stromal cells: Misleading marker gene expression. BMC Neurosci 2009; 10:16; PMID: 19257891; https://doi.org/10.1186/1471-2202-10-16
- Hwang JTK, Kelly GM. GATA6 and FOXA2 regulate Wnt6 expression during extraembryonic endoderm formation. Stem Cells Dev 2012; 21:3220-32; PMID: 22607194; https://doi.org/10.1089/scd.2011.0492
- Moscoso I, Rodriguez-Barbosa J-I, Barallobre-Barreiro J, Anon P, Domenech N. Immortalization of bone marrow-derived porcine mesenchymal stem cells and their differentiation into cells expressing cardiac phenotypic markers. J Tissue Eng Regen Med 2012; 6:655-65; http://doi.wiley.com/10.1002/term.469; PMID: 22162515; https://doi.org/10.1002/term.469
- Reinisch A, Etchart N, Thomas D, Hofmann NA, Fruehwirth M, Sinha S, Chan CK, Senarath-Yapa K, Seo E-Y, Wearda T, et al. Epigenetic and in vivo comparison of diverse MSC sources reveals an endochondral signature for human hematopoietic niche formation. Blood 2015; 125:249-60; PMID: 25406351; https://doi.org/10.1182/blood-2014-04-572255
- Berthoud VM, Singh R, Minogue PJ, Ragsdale CW, Beyer EC. Highly restricted pattern of connexin36 expression in chick somite development. Anat Embryol (Berl) 2004; 209:11-8; PMID: 15455226; https://doi.org/10.1007/s00429-004-0416-z
- Liu X, Xiong C, Jia S, Zhang Y, Chen Y-G, Wang Q, Meng A. Araf kinase antagonizes Nodal-Smad2 activity in mesendoderm development by directly phosphorylating the Smad2 linker region. Nat Commun 2013; 4:1728; PMID: 23591895; https://doi.org/10.1038/ncomms2762
- Chen C-H, Chen W-Y, Lin S-F, Wong RJ. Epithelial-mesenchymal transition enhances response to oncolytic herpesviral therapy through nectin-1. Hum Gene Ther 2014; 25:539-51; PMID: 24568312; https://doi.org/10.1089/hum.2013.177
- Jayachandran A, Anaka M, Prithviraj P, Hudson C, McKeown SJ, Lo P-H, Vella LJ, Goding CR, Cebon J, Behren A. Thrombospondin 1 promotes an aggressive phenotype through epithelial-to-mesenchymal transition in human melanoma. Oncotarget 2014; 5:5782-97; PMID: 25051363; https://doi.org/10.18632/oncotarget.2164
- Narayanan R, Ahn S, Cheney MD, Yepuru M, Miller DD, Steiner MS, Dalton JT. Selective androgen receptor modulators (SARMs) negatively regulate triple-negative breast cancer growth and epithelial:Mesenchymal stem cell signaling. PLoS One 2014; 9:e103202; PMID: 25072326; https://doi.org/10.1371/journal.pone.0103202
- Swindle MM, Smith AC, Laber-Laird K, Dungan L. Swine in biomedical research: Management and models. ILAR J 1994; 36:1; https://doi.org/10.1093/ilar.36.1.1
- Swindle MM, Makin A, Herron AJ, Clubb FJ, Frazier KS. Swine as models in biomedical research and toxicology testing. Vet Pathol 2012; 49:344-56; PMID: 21441112; https://doi.org/10.1177/0300985811402846
- Kim JB, Zaehres H, Arauzo-Bravo MJ, Scholer HR. Generation of induced pluripotent stem cells from neural stem cells. Nat Protoc 2009; 4:1464-70; PMID: 19798081; https://doi.org/10.1038/nprot.2009.173
- Juhl M, Tratwal J, Follin B, Søndergaard RH, Kirchhoff M, Ekblond A, Kastrup J, Haack-Sørensen M. Comparison of clinical grade human platelet lysates for cultivation of mesenchymal stromal cells from bone marrow and adipose tissue. Scand J Clin Lab Invest 2016; 76:93-104; PMID: 26878874; https://doi.org/10.3109/00365513.2015.1099723
- Alt E, Yan Y, Gehmert S, Song YH, Altman A, Gehmert S, Vykoukal D, Bai X. Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol Cell 2011; 103:197-208; PMID: 21332447; https://doi.org/10.1042/BC20100117
- French MM, Rose S, Canseco J, Athanasiou KA. Chondrogenic differentiation of adult dermal fibroblasts. Ann Biomed Eng 2004; 32:50-6; PMID: 14964721; https://doi.org/10.1023/B:ABME.0000007790.65773.e0
- Niibe K, Kawamura Y, Araki D, Morikawa S, Miura K, Suzuki S, Shimmura S, Sunabori T, Mabuchi Y, Nagai Y, et al. Purified mesenchymal stem cells are an efficient source for iPS cell induction. PLoS One 2011; 6:e17610; PMID: 21412425; https://doi.org/10.1371/journal.pone.0017610
- Nielsen TT, Jakobsson J, Rosenqvist N, Lundberg C. Incorporating double copies of a chromatin insulator into lentiviral vectors results in less viral integrants. BMC Biotechnol 2009; 9:13; PMID: 19239708; https://doi.org/10.1186/1472-6750-9-13
