A fundamental biological question is how organ size is regulated during development. Genetic screens in flies have identified components of the Hippo signaling pathway as key regulators of tissue growth. Activation of the “core” Hippo pathway, consisting of kinases (Tao, Hippo and Warts) and scaffold proteins (Salvador and Mats), results in phosphorylation of the transcriptional co-factor Yorkie, preventing its nuclear entry and thereby suppressing cell proliferation and tissue growth.Citation1
How is the activity of the “core” Hippo pathway regulated? One input comes from Fat and Dachsous, 2 protocadherins that function as tumor suppressers; loss of either leads to hyperplastic overgrowth in epithelial tissue. Fat and Dachsous bind in a preferentially heterophilic fashion, and act as a receptor-ligand pair. Dachsous promotes phosphorylation of the intracellular domain (ICD) of Fat by Casein kinase I ϵ, known as Discs over-grown (Dco) in flies, and thereby promotes Fat's ability to restrict growth.Citation2,3 Fat is believed to suppress growth by preventing the accumulation of the unconventional myosin Dachs at the apical junctional region (AJR).Citation4 The available evidence indicates that Dachs stimulates growth by promoting the degradation and/or conformational inactivation of Warts. Thus, Fat signaling provides a unique and complex system in which to study upstream regulation of this highly conserved growth control pathway.
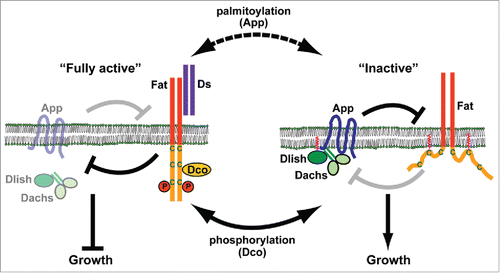
Our recent findings have revealed 2 additional regulators of Fat signaling, Approximated (App) and Dachs Ligand with SH3s (Dlish), that play key roles linking Fat's ICD to Dachs levels and localization ().Citation5,6 app mutants were originally discovered by their dachs-like phenotype, in particular proximo-distal patterning defects in the appendages. App encodes a transmembrane protein of the DHHC palmitoyltransferase family, which catalyze addition of palmitoyl fatty acids to cysteine residues in substrate proteins and often thereby regulate their association with cell membranes.Citation7 The DHHC motif is highly conserved and acts as the core catalytic domain. Consistent with the idea that App functions as a palmitoyltransferase, single amino acid substitutions in the DHHC motif cause hypomorphic phenotypes. App localizes at the AJR with other Fat signaling components including Fat, Dachsous and Dachs.Citation7
Figure 1. Post-translational modifications that regulate Fat activity. Dco suppresses growth by phosphorylating Fat's intracellular domain, thereby activating Fat and promoting degradation of Dachs at the AJR. Conversely, App promotes growth by palmitoylating Fat's intracellular domain and suppressing Fat activity. App also recruits the Dlish-Dachs complex to the AJR by palmitoylating Dlish and forming a complex with these proteins.
Dlish was identified as a Dachs interacting protein from yeast 2-hybrid screening.Citation6 Similar to app, loss of dlish leads to proximo-distal patterning defects. Also similar to App, Dlish is concentrated in the AJR, although Dlish is not a transmembrane protein. In the absence of app or dlish, tissues are undergrown and Dachs fails to localize at the AJR. Thus, both App and Dlish appear to promote growth by supporting localization of Dachs at the AJR.Citation5,6
What is App's target for palmitoylation? Since Dachs is mislocalized in app mutants, a likely target might be Dachs. However, Dachs is not detectably palmitoylated and mutation of potential target cysteine residues has no effect on Dachs function. Instead, we discovered that 2 other Fat signaling components are palmitoylated by App. One target is Dlish, which is mislocalized in the absence of App and is palmitoylated in an App-dependent manner.Citation6 Since Dlish binds Dachs, palmitoylation of Dlish could help target Dachs to the plasma membrane. Both Dachs and Dlish also bind App, providing another potential mechanism for tethering the complex to the AJR.
Surprisingly, the second palmitoylation target of App is the ICD of Fat.Citation5 In some other transmembrane proteins, palmitoylation has been shown to inactivate the intracellular domain, probably by increasing its interaction with the inner face of the plasma membrane. Consistent with this idea, genetic evidence suggests that App antagonizes Fat's ability to restrict growth. Furthermore, mutation of 2 target cysteines in Fat's ICD blocks its palmitoylation and mimics “app”-like undergrowth.Citation5 Thus App palmitoyltransferase activity functions in at least 2 ways: by inhibiting Fat activity and by recruiting Dlish to the plasma membrane.
Intriguingly, the sites of App-dependent palmitoylation in Fat's ICD are near the sites phosphorylated by Dco, raising the possibility that these modifications interact. Genetically, dco and app mutations function antagonistically, and remarkably the lethality of dco can be strongly suppressed by simultaneous loss of app palmitoyltransferase activity.Citation5 As palmitoylation represses Fat and phosphorylation activates it, then are these 2 post-translational modifications mutually exclusive? Interestingly, the available evidence suggests that they are not, and that as a result Fat might exist in multiple activity states, resulting in varying Dachs levels at the AJR.
Overall, these studies reveal a complex but robust network of protein interactions and post-translational modifications that together control the activity of an important growth regulator, Dachs. It is still unclear exactly how Fat reduces Dachs accumulation and activity at the AJR, but both App and Dlish form a complex not only with Dachs, but also with Fat's ICD. An additional level of control is exerted at the level of Dachs stability: recruitment of Dachs to the AJR paradoxically not only activates Dachs, but also promotes its degradation, and this degradation is stimulated by Fat. An intriguing model is that, depending on the relative activities of App and Dco, different protein complexes promote either Dachs activation or degradation. Additionally, an important question for future studies is how App and Dco themselves are regulated.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Pan D. The Hippo signaling pathway in development and cancer. Dev Cell 2010; 19:491-505; PMID:20951342; http://dx.doi.org/10.1016/j.devcel.2010.09.011
- Sopko R, Silva E, Clayton L, Gardano L, Barrios-Rodiles M, Wrana J, Varelas X, Arbouzova N, Shaw S, Saburi S, et al. Phosphorylation of the tumor suppressor fat is regulated by its ligand dachsous and the kinase disc overgrown. Curr Biol 2009; 19:1112-7; PMID:19540118; http://dx.doi.org/10.1016/j.cub.2009.05.049
- Feng Y, Irvine K. Processing and phosphorylation of the Fat receptor. Proc Natl Acad Sci USA 2009; 106: 11989-94; PMID:19574458; http://dx.doi.org/10.1073/pnas.0811540106
- Mao Y, Rauskolb C, Cho E, Hu W, Hayter H, Minihan G, Katz F, Irvine K. Dachs: an unconventional myosin that functions downstream of Fat to regulate growth, affinity and gene expression in Drosophila. Development 2006; 133: 2539-51; PMID:16735478; http://dx.doi.org/10.1242/dev.02427
- Matakatsu H, Blair S, Fehon R. The palmitoyltransferase Approximated promotes growth via the Hippo pathway by palmitoylation of Fat. J Cell Biol 2017; 216:265-77; PMID:28031421; https://doi.org/10.1083/jcb.201609094
- Zhang Y, Wang X, Matakatsu H, Fehon R, Blair S. The novel SH3 domain protein Dlish/CG10933 mediates fat signaling in Drosophila by binding and regulating Dachs. Elife 2016; 5:e16624; PMID:27692068; http://dx.doi.org/10.7554/eLife.16624.001
- Matakatsu H, Blair S. The DHHC palmitoyltransferase approximated regulates Fat signaling and Dachs localization and activity. Curr Biol 2008; 18:1390-5; PMID:18804377; http://dx.doi.org/10.1016/j.cub.2008.07.067
