Aging and cancer are currently a main concern of modern society. Although they seem to be primarily different diseases, they both share a common characteristic - the activation of a cellular phenotype called senescence. Senescence is a cellular response to a stress signal. It was initially thought to be an end-point to a stress situation, where the cells lost their proliferative capacity. However, the evidence for a non-cell autonomous function for senescent cells has increasingly grown in the last decade. In fact, senescent cells secrete a variety of inflammatory proteins, matrix-degrading enzymes and growth factors named senescence-associated secretory phenotype (SASP), which alter the behavior of neighboring cells.Citation1
Integrins are cell surface adhesion receptors formed by an “α“ and a “β” subunit. They identify changes in the extracellular space and mediate intracellular signaling, but they are also capable of transmitting signaling from their cytoplasmic tail to the extracellular space, providing bi-directional signaling. However, integrins can also have ligand independent activity, inducing apoptosis, tumor progression and anchorage-independent growth.
Integrins mediate important downstream signaling inducing changes in the cellular phenotype such as migration, adhesion and proliferation. As integrins are such important regulators of cellular processes, they play key roles in different physiologic and pathological conditions, including development, cancer and aging.Citation2
In our study, we found that the integrin subunit β3 (β3 or ITGB3) plays a key role in regulating senescence ().Citation3 By performing an unbiased quantitative proteomic screen (SILAC), we found the ECM-receptor interaction and focal adhesion (FA) pathways highly upregulated during senescence. These findings are not entirely surprising, as senescence is characterized by a change in cellular morphology and integrin β1 has been previously found to regulate senescence during would healing.Citation4 The SILAC screen was performed in the absence of the epigenetic regulator, the chromobox polycomb protein 7, CBX7. Therefore, we reasoned that genes encoding for proteins involved in cellular adhesion could be regulated by CBX7. In fact, we found the ITGB3 locus regulated not only by CBX7, but also by other members of the Polycomb Repressive Complex 1 (PRC1). Most studies investigating integrin signaling have focused on their cellular and biologic functions. Our study provides novel insights into an additional layer of integrin regulation, its epigenetic regulation. Most integrin-targeted therapies aim to disrupt the integrin-ECM binding property, although there is increasing evidence for integrin ligand-independent functions.Citation2 Thus, understanding the mechanisms implicated in integrin gene regulation is important.
Our data shows that integrin β subunits are significantly deregulated during oncogene-induced senescence (OIS) stimulated by the expression of H-RasG12V. ITGB3 is the subunit most upregulated in OIS and the genetic manipulation of ITGB3 overcomes senescence. However, treatment of senescent cells with the RGD-mimetic cyclic peptide, cilengitide, which affects αvβ3 ligand binding, could not reverse the proliferation arrest during senescence, but did alter the SASP release and composition. Therefore, genetic ablation and ligand-binding inhibition of ITGB3 have different outcomes during OIS.
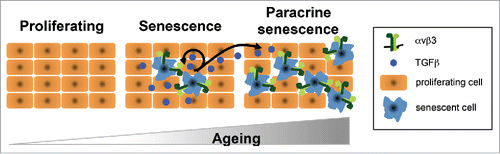
Recent studies have highlighted the importance of TGFβ as a component of the SASP, with the Narita laboratory recently identifying NOTCH as a temporal regulator of a heterogeneous secretome.Citation5 Our study shows that β3 expression induces senescence by activating TGFβ. In fact, genetic and pharmacological manipulation of key components of the TGFβ pathway overcomes the senescence phenotype induced by β3. Furthermore, the TGFβ-enriched SASP induced by β3 has both cell autonomous and non-cell autonomous functions ().
As cellular senescence is a hallmark of aging, we set to investigate if ITGB3 expression levels were altered during aging.Citation6 We show that ITGB3 levels are dynamically regulated throughout aging in a subset of tissues in mice and in human fibroblasts. Furthermore, in accordance with our previous results, genetic manipulation of ITGB3 ablated the aging phenotype in fibroblasts, while pharmacological inhibition had no effect. However, further experiments and animal models would be needed to determine a role for β3 in aging.
In summary, our study contributes to clarify the implication and signaling mechanisms of integrins during the activation of senescence and identifies cellular adhesion as a feature of cellular senescence.
Figure 1. Integrin β3 (αvβ3) regulates senescence and is highly expressed during aging. The levels of αvβ3 (dark/light green hooks) increase progressively upon the induction of cellular senescence and aging. In fact, there are very low levels of expression of αvβ3 in proliferating cells (left panel). However, once senescence is established, αvβ3 expression increases inducing the release of TGFβ (middle panel), which is capable of activating senescence in an autocrine (middle panel) and paracrine fashion (right panel).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
AO's laboratory is supported by the BBSRC (BB/P000223/1). MB is funded by the MRC (MR/K501372/1)
References
- Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 2014; 15:482-96; PMID:24954210; http://dx.doi.org/10.1038/nrm3823
- Seguin L, Desgrosellier JS, Weis SM, Cheresh DA. Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol 2015; 25:234-40; PMID:25572304; http://dx.doi.org/10.1016/j.tcb.2014.12.006
- Rapisarda V, Borghesan M, Miguela V, Encheva V, Snijders AP, Lujambio A, O'Loghlen A. Integrin Beta 3 regulates cellular senescence by activating the TGF-beta pathway. Cell Rep 2017; 18:2480-93; PMID:28273461; http://dx.doi.org/10.1016/j.celrep.2017.02.012
- Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 2010; 12:676-85; PMID:20526329; http://dx.doi.org/10.1038/ncb2070
- Hoare M, Ito Y, Kang TW, Weekes MP, Matheson NJ, Patten DA, Shetty S, Parry AJ, Menon S, Salama R, et al. NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat Cell Biol 2016; 18:979-92; PMID:27525720; http://dx.doi.org/10.1038/ncb3397
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013; 153:1194-217; PMID:23746838; http://dx.doi.org/10.1016/j.cell.2013.05.039
