ABSTRACT
Plasma cells (PCs) generation occurs in hypoxic conditions in vivo, whereas the relevance of O2 pressure in PC differentiation remains unknown.
Using our in vitro PC differentiation model, we investigated the role of hypoxia in PC generation.
Hypoxia increases the generation of plasmablasts (PBs) starting from memory B cells, by increasing cell cycle and division number. Reactome analysis demonstrated a significant enrichment of genes involved in HIF1α and HIF2α transcription factor network, metabolism and MYC related pathways in hypoxic compared with normoxic PBs. Hypoxia-induced metabolism alteration and MYC pathway are involved in malignant PC pathophysiology. Therefore, the expression of 28 out of the 74 genes overexpressed in hypoxic PBs compared with normoxic ones was found to be associated with an adverse prognosis (event free survival and overall survival) in newly diagnosed multiple myeloma patients.
According to the role of hypoxia in supporting PBs generation through cell cycle induction, c-MYC activation and metabolism alteration, it could be involved in plasma cell tumorigenesis.
Introduction
Plasma cells (PCs) represent the end point of B cell differentiation. PCs produce large amounts of antibodies capable of neutralizing antigens. As terminally differentiated cells, PCs remain quiescent, unable to proliferate.
B-cell activation results in the formation of either short-lived or long-lived PCs. B cells activated in the presence of T cells providing help through ligation of CD40 generate prolonged responses characterized by the presence of germinal centers (GCs) where activated B cells endure somatic hypermutation and isotype switching.Citation1 Those that compete successfully and receive survival signals exit the GC and continue to mature as either plasmablasts (PBs) then early PCs, or memory B cells (MBCs). These PBs migrate to medullary cords, exit into the lymph through a sphingosine phosphate gradient and reach the peripheral blood.Citation2 Circulating PBs/early PCs must find a specific niche in the bone marrow (BM) or mucosa that will provide them with the factors to survive and fully differentiate. Migration to specific microenvironment niches is controlled by chemokines and their receptors. Coordinated expression of chemokines and their receptors orchestrates the movement of PBs to the BM where growth factors produced by the PC niche control PC survival.Citation2 Factors that promote the differentiation of PBs into bone marrow PCs (BMPCs) are poorly identified in vivo, due to the rarity of BMPCs and the difficulties to harvest them.
Using in vitro models of B to PCs differentiation, we and others have shown that in vitro generated PCs are early PCs with a phenotype close to that of circulating PBs and PCs in healthy individuals.Citation3,4 Recently, we and others also demonstrated that IL-6 in combination with APRIL, BAFF, or soluble stromal cell factors supports the generation and survival of long-lived PCs (LLPCs).Citation5,6
In mammals, oxygen distribution is robustly regulated by the vascular system, which continually distributes oxygen-saturated red blood cells throughout the body. Despite the sturdiness of this system, oxygen distribution in the body is not uniform. Following air inspiration [partial pressure of oxygen (PO2) of 159 mmHg], red blood cells absorb oxygen resulting in a maximal PO2 in the pulmonary vasculature of 98 mmHg. As oxygen is delivered to tissue, PO2 decrease to 50 mmHg in the BM, and 40 mmHg in venous blood.Citation7 Previous studies have shown that a large drop of pO2 occurred across blood/tissue interface, reaching hypoxic levels from 30–40 µm from blood vessels.Citation8 In a recent paper, Abbott, et al.Citation9 showed that most of GC were located > 40 µm from the nearest blood vessel and that GC B cells from spleens, Peyer's patches and mesenteric lymph nodes were hypoxic. These unique microenvironments affect cell phenotype, gene expression profiles, and functional behavior.Citation10 In mice, hypoxic parts of germinal centers are associated with accelerated class switch recombination and antibody secretion.Citation9
Thus, the generation of PCs from B cells occurs in hypoxic conditions in vivo and the relevance of O2 pressure in PC generation remains unknown. We therefore sought to evaluate the impact of decreased oxygen partial pressures in plasma cell generation.Citation3,6,11
Results
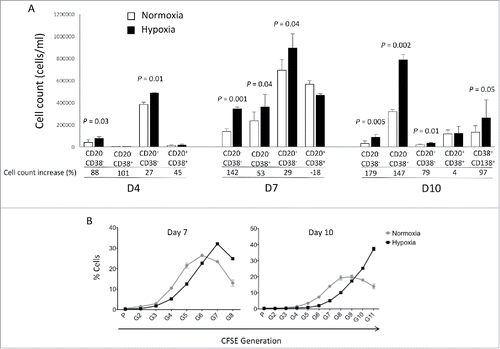
Bone marrow microenvironment is physiologically hypoxic. This hypoxic microenvironment is important for normal bone marrow hematopoiesis. We investigated the impact of decreased oxygen partial pressures in plasma cell generation using our in vitro modelCitation3,6,11,12 at each step (day 4, day 7 and day 10) of B cell differentiation (). Five percent oxygen (hypoxia) culture compared with 20% oxygen (normoxia) led to an increased generation of CD20−CD38− prePBs and CD20−CD38+ PBs at day 7 starting from MBCs by triggering CD40 and Toll-like receptor 9 and adding IL2, IL10 and IL15 (P = 0.04). A 2-fold increase in CD20−CD38+ PB generation was maintained at day 10 (P = 0.002) (). Of note, the increase in PBs count under hypoxic conditions resulted in a significant increase in the generation of CD138+ PCs at day 10 (P = 0.05) (). Reduced PO2 pressure (1% or 3%) did not increase further PB generation, but on the contrary led to decreased cell survival (Fig. S1). Using CFSE incorporation at day 0 and monitoring its level throughout the differentiation process, cells growing in hypoxia were roughly one division ahead of the normoxic cells at day 7 (). Moreover, these cells continued cycling at day 10, unlike the normoxic ones, explaining the increase in PB cell count until day 10.
Figure 1. Hypoxia promotes PB generation in vitro and induces cell cycle progression. (A) Number of cells obtained at days 4, 7 and 10 in vitro either under hypoxic conditions (5% O2) or normoxic ones (20% O2). At D4, D7 and D10 of cell culture, the cells were counted and CD20, CD38 and CD138 expressions assessed by flow cytometry. The percentage of cell count increase in hypoxic conditions compared with normoxic conditions is indicated. Results are the means ± SD of 4 experiments. Statistical significance was tested using a Student's t-test for pairs. (B) Percentages of live cells according to their CFSE fluorescence obtained at days 7 and 10 in vitro under hypoxic conditions or normoxic ones following MBC CFSE incorporation at day 0. The statistical comparisons were done using a t-test.
Analyzing gene expression profiles of sorted CD20−CD38+ PBs at day 7, 175 genes were differentially expressed between normoxic and hypoxic PBs (fold change ≥ 1.5, P ≤ 0.0001, Supplementary Tables S1 and S2) including 74 genes with increased expression and 101 with a significant downregulation in hypoxic condition. Reactome analysis demonstrated a significant enrichment of genes involved in HIF1α and HIF2α transcription factor network, metabolism and cancer related pathways in hypoxic PB compared with normoxic ones ().
Table 1. REACTOME analysis of genes significantly overexpressed in hypoxic PBs compared with normoxic PBs.
HIF1α and HIF2α are the main regulators of oxygen homeostasis. When complexed with ARNT, they activate target gene transcription involved in glycolysis, iron transport, cell proliferation and survival and angiogenesis such as vascular endothelial growth factor (VEGF).Citation13,14 Among the genes significantly upregulated in hypoxic PBs, HIF2A (EPAS1) (fold change = 5.3) and VEGFA (fold change = 1.5) were identified (Supplementary Table S1 and Fig. S2A) and validated by quantitative RT-PCR (Supplementary Fig. S2B). No significant changes in HIF1A and ARNT gene expression was identified between normoxic and hypoxic PBs (Supplementary Fig. S2A). Cancer related pathways were also enriched in hypoxic PB (). Interestingly, HIF2α was shown to promote cell-cycle progression of hypoxic VHL-deficient renal cell carcinoma cells through c-Myc activation.Citation15 Therefore, a significant induction of c-MYC gene expression was identified in hypoxic PBs (fold change = 1.9) compared with normoxic PBs (Fig. S3). c-MYC upregulation in hypoxic conditions was validated by quantitative RT-PCR (Supplementary Fig. S3).
Hypoxic PBs are characterized by CD19 (2.1-fold), CD69 (1.9-fold), CD52 (1.6-fold) and HLA-DQB1 B cell markers overexpression (11.5-fold) suggesting hypoxic PBs are less differentiated than normoxic ones.
Normoxic PB overexpressed genes are significantly enriched in mitotic genes and nucleosome assembly (Supplementary ).
A score equal to the sum of standardized expressions of these 175 genes differentially expressed between hypoxic and normoxic PBs was high in germinal center or bone marrow sorted primary samples (centroblasts, centrocytes and BMPCs) compared with blood circulating cells and cells that have undergone normoxic differentiation in vitro (naive B cells, MBCs and in vitro PCs, Supplementary Fig. S4).
Discussion
Altogether, our data demonstrated that hypoxia increases the generation of PBs starting from MBCs by activating cell cycle and division number. Hypoxic PBs are less differentiated than normoxic PBs and continue cycling at day 10, favoring the generation of PCs in vitro. Our results are in agreement with recent data revealing a critical role of hypoxia in germinal center B cell differentiation.Citation9 We also confirmed that the genes upregulated in hypoxic PBs are also significantly overexpressed in germinal center B cells compared with circulating naive B cells (Supplementary Fig. S5). Furthermore, hyperoxia induced a significant inhibition of germinal center response and antibody production after immunization in mice.Citation9
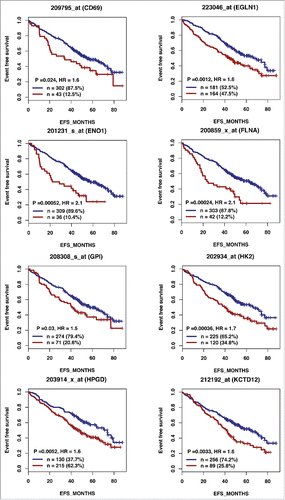
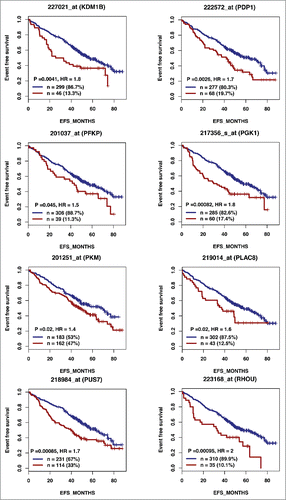
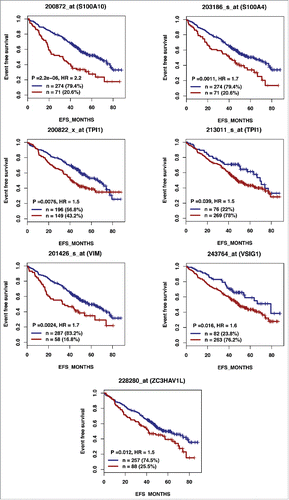
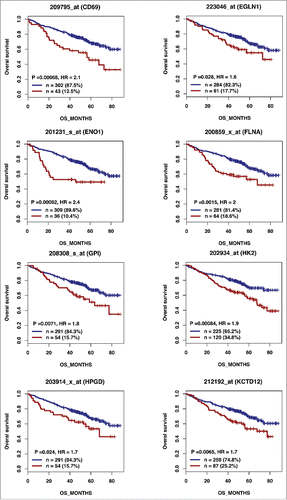
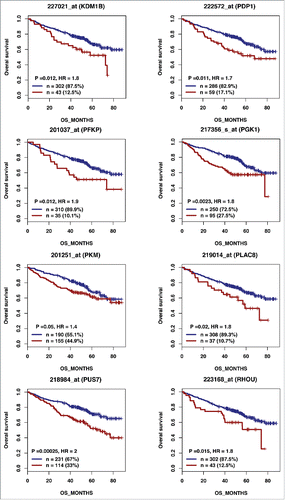
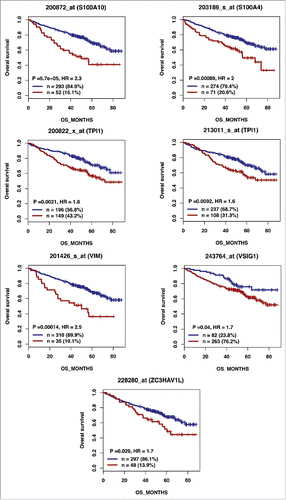
According to the role of hypoxia in supporting PB generation through cell cycle activation, it could be involved in plasma cell tumorigenesis or autoimmune diseases. HIF1α and HIF2α immunostaining in bone biopsies from multiple myeloma (MM) patients demonstrated a high level of HIF1α in 33% and of HIF2α in 13% of the cases.Citation16 Furthermore, overexpression of HIF1α and HIF2α in MM cells, resulted in significant induction of MM-induced angiogenesis in xenograft model.Citation17 Hypoxic PBs are characterized by c-MYC and HIF2A gene activation in association with an induction of genes enriched in metabolism and cancer pathways. Abnormal activity of several transcription factors including c-MYC have been implicated in MM development.Citation18,19 HIF2α can promote c-MYC-induced transcriptional modifications through binding and stabilization of MYC-MAX heterodimer.Citation15 These support the role of HIF2α in driving hypoxia-dependent proliferation.Citation15 Interestingly, a significant enrichment (P < 0.0001) of genes associated with a poor prognosis (event free survival and overall survival), in newly diagnosed MM patients (LR-TT2 cohort, n = 345), was identified among genes overexpressed in hypoxic PBs ( and and and ). These genes are significantly enriched in genes involved in HIF signaling pathway and glycolysis (ALDOA, HK2, PKM, TPI1, ENO1, PFKP, GPI and PGK1) (). Hypoxia-induced metabolic re-writing is known to be involved in tumorigenesis and cancer progression.Citation20 Furthermore, altered tumor cell metabolism is essential for MM cell drug resistance.Citation21 Annexin A2 (ANXA2) and KDM1B were also identified among the genes induced by hypoxia in PBs and associated with an adverse prognosis in MM (). ANXA2 is known to promote MM cell growth and survival, stimulate angiogenesis and increase osteoclast formation.Citation22-25 In MM, high ANXA2 expression is associated with an adverse prognosis, high-risk proliferation and high-risk gene expression-based signatures.Citation26 KDM1B is a lysine-specific protein demethylaseCitation27 that functions as an enhancer of gene transcription by modulating H3K4 methylation.Citation28 Interestingly, KDM1B was shown to control NFκB target gene activation,Citation29 a pathway playing a critical role in MM survival and proliferation.Citation30,31 Our data underline a potential role of hypoxia in plasma cell tumorigenesis through c-MYC activation, metabolic modifications and cancer related pathways induction.
Table 2A. High expression of genes induced by hypoxia in PBs could predict for shorter event free survival in myeloma patients. Patients of the LR-TT2 cohort (n = 345) were ranked according to increasing gene expression and a maximum difference in event free survival (EFS) was obtained using the Maxstat R function.
Table 2B. High expression of genes induced by hypoxia in PBs could predict for shorter overall survival in myeloma patients. Patients of the LR-TT2 cohort (n = 345) were ranked according to increasing gene expression and a maximum difference in overall survival (OS) was obtained using the Maxstat R function.
Figure 2A. The expression of 28 out of the 74 genes overexpressed in hypoxic PBs compared with normoxic ones is associated with a poor prognosis in MM. High expression of 28 out of the 74 genes induced by hypoxia in PBs could predict for shorter overall and event free survival in myeloma patients. Patients of the LR-TT2 cohort (n = 345) were ranked according to increasing gene expression and a maximum difference in event free survival (EFS) was obtained using the Maxstat R function.
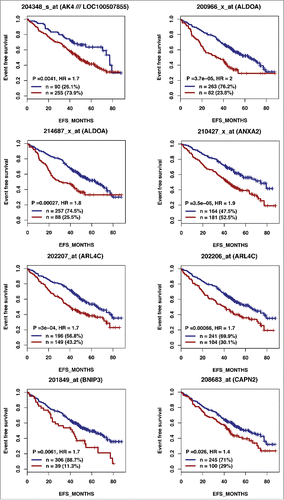
Figure 2B. The expression of 28 out of the 74 genes overexpressed in hypoxic PBs compared with normoxic ones is associated with a poor prognosis in MM. High expression of 28 out of the 74 genes induced by hypoxia in PBs could predict for shorter overall survival in myeloma patients. Patients of the LR-TT2 cohort (n = 345) were ranked according to increasing gene expression and a maximum difference in overall survival (OS) was obtained using the Maxstat R function.
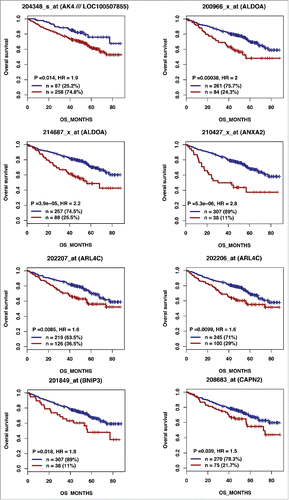
Table 3. REACTOME analysis of genes significantly overexpressed in hypoxic PBs compared with normoxic PBs and associated with an adverse prognosis in MM.
Materials and methods
Cell cultures
Peripheral blood cells from healthy volunteers were purchased from the French Blood Center (Toulouse, France) and CD19+ CD27+ MBCs purified (> 95% purity) as described.Citation11 Standard normoxic culture conditions comprised 21% O2, 5% CO2 and 37°C. Hypoxic culture conditions (5% O2, 5% CO2; 37°C) were obtained by oxygen displacement by nitrogen using the muti-Gas MCO-5M-PE Panasonic Biomedical incubator (Avons, France). PCs were generated as reported.Citation3,6 All cultures were performed in Iscove's modified Dulbecco medium (IMDM, Invitrogen) and 10% FCS.Citation3,6,11 Purified peripheral blood MBCs (1.5 × 105/ml) were activated for 4 d by CpG oligodeoxynucleotide and CD40 ligand (sCD40L) −10 µg/ml of phosphorothioate CpG oligodeoxynucleotide 2006 (Sigma), 50 ng/ml histidine tagged sCD40L, and anti-poly-histidine mAb (5 µg/ml), (R&D Systems) - with IL-2 (20 U/ml), IL-10 (50 ng/ml) and IL-15 (10 ng/ml) in 6-well culture plates. PBs were generated by removing CpG oligonucleotides and sCD40L and changing the cytokine cocktail (IL-2, 20 U/ml, IL-6, 50 ng/ml, IL-10, 50 ng/ml and IL-15, 10 ng/ml). PBs were differentiated into PCs adding IL-6 (50 ng/ml), IL-15 (10 ng/ml) and IFN-α (500 U/ml) for 3 d.
Reagents
Human recombinant IL-2 was purchased from R&D Systems (Minneapolis, MN), IFN-α (IntronA) from Merck Canada Inc. (Kirckland, Canada), IL-6 and IL-15 from AbCys SA (Paris, France), IL-10, from Peprotech (Rocky Hill, NJ, USA). Mouse (or rat when indicated) mAbs conjugated to allophycocyanin (APC), fluorescein isothiocyanate (FITC), peridinin chlorophyll protein-cyanin 5.5 (PerCP-Cy5.5), phycoerythrin (PE), specific for human CD19 (clone HIB19), CD27 (clone M-T271), CD38 (clone HIT2) and CD138 (clone RF8B2) were purchased from BD Biosciences (Le Pont De Claix, France); CD20 (clone B9E9) from Beckman Coulter (Fullerton, CA).
Real-time RT-PCR
RNA was converted to cDNA using the Qiagen QuantiTect Rev. Transcription Kit (Qiagen, Hilden, Germany). The assays-on-demand primers and probes and the TaqMan Universal Master Mix were used according to the manufacturer's instructions (Applied Biosystems, Courtaboeuf, France). The measurement of gene expression was performed using the Roche LC480 Sequence Detection System. For each primer, serial dilutions of a standard cDNA were amplified to create a standard curve, and values of unknown samples were estimated relative to this standard curve to assess PCR efficiency. Ct values were obtained for GAPDH and the respective genes of interest during log phase of the cycle. Gene expression was normalized to that of GAPDH (dCt = Ct gene of interest – Ct GAPDH) and compared with the values obtained for a known positive control using the following formula: 100/2ddCt where ddCt = dCt unknown – dCt positive control.
Microarray hybridization and bioinformatics analysis
RNA was extracted and hybridized to human genome U133 Plus 2.0 GeneChip microarrays, according to the manufacturer's instructions (Affymetrix, Santa Clara, CA). Gene expression data are available on GenomicScape (http://www.genomicscape.com).Citation32 Gene expression data were normalized using MAS5 algorithm and analyzed with GenomicScape (http://www.genomicscape.com).Citation32 Gene expression profiles (GEP) of hypoxic and normoxic plasmablasts were analyzed with Affymetrix GCOS (GeneChip Operating Software) software using Wilcoxon test and Benjamini-Hochberg multiple testing correction and a fold change of 1.5.Citation33 The molecular pathways, encoded by the genes differentially expressed between hypoxic and normoxic PBs, were pointed out using reactome FI (Functional Interaction) cytoscape plugin (http://wiki.reactome.org/index.php/ Reactome_FI_Cytoscape_Plugin). Pathways significantly enriched in a given cell subpopulation with a FDR < 0.001 are selected.
GEP of MMCs were obtained from large patients' cohort from the University of Arkansas for Medical Sciences (UAMS, Little Rock, USA, cohort treated with total therapy 2, N = 345) (GEO, http://www.ncbi.nlm.nih.gov/geo/, accession number GSE2658). Centroblast and centrocytes GEP are publicly available from GEO database (http://www.ncbi.nlm.nih.gov/geo/; accession number GSE15271) and naive B cell GEP from ArrayExpress database (http://www.ebi.ac.uk/arrayexpress/; accession number E-MTAB-1771).
Statistical analysis
Statistical comparisons were made using a student's t-test on Prism software. P values ≤ .05 were considered as significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
MS performed the experiments and wrote the paper. MJ developed the in vitro model for plasma cell generation. AB participated in research. AK provided help for microarray analysis and wrote the paper.
BK and JM are the senior investigators who designed research and wrote the paper.
1317408_Supplemental_Material.zip
Download Zip (281.1 KB)Funding
This work was supported by grants from French INCA (Institut National du Cancer) Institute (2012–109/087437 and PLBIO15–256), Languedoc Roussillon CRLR (R14026FF), Fondation de France (201400047510), FFRMG (132554) and ITMO Cancer (MM&TT) and SIRIC Montpellier INCa-DGOS-Inserm 6045.
References
- O'Connor BP, Gleeson MW, Noelle RJ, Erickson LD. The rise and fall of long-lived humoral immunity: terminal differentiation of plasma cells in health and disease. Immunol Rev 2003; 194:61-76; PMID:12846808; https://doi.org/10.1034/j.1600-065X.2003.00055.x
- Tangye SG. Staying alive: regulation of plasma cell survival. Trends Immunol 2011; 32:595-602; PMID:22001488; https://doi.org/10.1016/j.it.2011.09.001
- Jourdan M, Caraux A, De Vos J, Fiol G, Larroque M, Cognot C, Bret C, Duperray C, Hose D, Klein B. An in vitro model of differentiation of memory B cells into plasmablasts and plasma cells including detailed phenotypic and molecular characterization. Blood 2009; 114:5173-81; PMID:19846886; https://doi.org/10.1182/blood-2009-07-235960
- Mei HE, Yoshida T, Sime W, Hiepe F, Thiele K, Manz RA, Radbruch A, Dorner T. Blood-borne human plasma cells in steady state are derived from mucosal immune responses. Blood 2009; 113:2461-9; PMID:18987362; https://doi.org/10.1182/blood-2008-04-153544
- Cocco M, Stephenson S, Care MA, Newton D, Barnes NA, Davison A, Rawstron A, Westhead DR, Doody GM, Tooze RM. In vitro generation of long-lived human plasma cells. J Immunol 2012; 189:5773-85; PMID:23162129; https://doi.org/10.4049/jimmunol.1103720
- Jourdan M, Cren M, Robert N, Bollore K, Fest T, Duperray C, Guilloton F, Hose D, Tarte K, Klein B. IL-6 supports the generation of human long-lived plasma cells in combination with either APRIL or stromal cell-soluble factors. Leukemia 2014; 28(8):1647-56; PMID:24504026; https://doi.org/10.1038/leu.2014.61
- Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO(2) distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophys J 2001; 81:685-96; PMID:11463617; https://doi.org/10.1016/S0006-3495(01)75733-5
- Tsai AG, Friesenecker B, Mazzoni MC, Kerger H, Buerk DG, Johnson PC, Intaglietta M. Microvascular and tissue oxygen gradients in the rat mesentery. Proc Natl Acad Sci U S A 1998; 95:6590-5; PMID:9618456; https://doi.org/10.1073/pnas.95.12.6590
- Abbott RK, Thayer M, Labuda J, Silva M, Philbrook P, Cain DW, Kojima H, Hatfield S, Sethumadhavan S, Ohta A, et al. Germinal Center Hypoxia Potentiates Immunoglobulin Class Switch Recombination. J Immunol 2016; 197:4014-20; PMID:27798169; https://doi.org/10.4049/jimmunol.1601401
- Ricciardi A, Elia AR, Cappello P, Puppo M, Vanni C, Fardin P, Eva A, Munroe D, Wu X, Giovarelli M, et al. Transcriptome of hypoxic immature dendritic cells: modulation of chemokine/receptor expression. Mol Cancer Res 2008; 6:175-85; PMID:18314479; https://doi.org/10.1158/1541-7786.MCR-07-0391
- Jourdan M, Caraux A, Caron G, Robert N, Fiol G, Reme T, Bollore K, Vendrell JP, Le Gallou S, Mourcin F, et al. Characterization of a transitional preplasmablast population in the process of human B cell to plasma cell differentiation. J Immunol 2011; 187:3931-41; PMID:21918187; https://doi.org/10.4049/jimmunol.1101230
- Schoenhals M, Jourdan M, Seckinger A, Pantesco V, Hose D, Kassambara A, Moreaux J, Klein B. Forced KLF4 expression increases the generation of mature plasma cells and uncovers a network linked with plasma cell stage. Cell Cycle 2016; 15(14):1919-28: 0; PMID:27230497; https://doi.org/10.1080/15384101.2016.1191709
- Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol 2003; 23:9361-74; PMID:14645546; https://doi.org/10.1128/MCB.23.24.9361-9374.2003
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003; 3:721-32; PMID:13130303; https://doi.org/10.1038/nrc1187
- Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell 2007; 11:335-47; PMID:17418410; https://doi.org/10.1016/j.ccr.2007.02.006
- Giatromanolaki A, Bai M, Margaritis D, Bourantas KL, Koukourakis MI, Sivridis E, Gatter KC. Hypoxia and activated VEGF/receptor pathway in multiple myeloma. Anticancer Res 2010; 30:2831-6; PMID:20683019
- Martin SK, Diamond P, Williams SA, To LB, Peet DJ, Fujii N, Gronthos S, Harris AL, Zannettino AC. Hypoxia-inducible factor-2 is a novel regulator of aberrant CXCL12 expression in multiple myeloma plasma cells. Haematologica 2010; 95:776-84; PMID:20015878; https://doi.org/10.3324/haematol.2009.015628
- Dean M, Kent RB, Sonenshein GE. Transcriptional activation of immunoglobulin alpha heavy-chain genes by translocation of the c-myc oncogene. Nature 1983; 305:443-6; PMID:6413865; https://doi.org/10.1038/305443a0
- Shaffer AL, Emre NC, Lamy L, Ngo VN, Wright G, Xiao W, Powell J, Dave S, Yu X, Zhao H, et al. IRF4 addiction in multiple myeloma. Nature 2008; 454:226-31; PMID:18568025; https://doi.org/10.1038/nature07064
- Eales KL, Hollinshead KE, Tennant DA. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis 2016; 5:e190; PMID:26807645; https://doi.org/10.1038/oncsis.2015.50
- Maiso P, Huynh D, Moschetta M, Sacco A, Aljawai Y, Mishima Y, Asara JM, Roccaro AM, Kimmelman AC, Ghobrial IM. Metabolic signature identifies novel targets for drug resistance in multiple myeloma. Cancer Res 2015; 75:2071-82; PMID:25769724; https://doi.org/10.1158/0008-5472.CAN-14-3400
- D'Souza S, Kurihara N, Shiozawa Y, Joseph J, Taichman R, Galson DL, Roodman GD. Annexin II interactions with the annexin II receptor enhance multiple myeloma cell adhesion and growth in the bone marrow microenvironment. Blood 2012; 119:1888-96; PMID:22223826; https://doi.org/10.1182/blood-2011-11-393348
- Sharma MC, Sharma M. The role of annexin II in angiogenesis and tumor progression: a potential therapeutic target. Curr Pharmaceutical Design 2007; 13:3568-75; PMID:18220793; https://doi.org/10.2174/138161207782794167
- Menaa C, Devlin RD, Reddy SV, Gazitt Y, Choi SJ, Roodman GD. Annexin II increases osteoclast formation by stimulating the proliferation of osteoclast precursors in human marrow cultures. J Clin Invest 1999; 103:1605-13; PMID:10359570; https://doi.org/10.1172/JCI6374
- Li F, Chung H, Reddy SV, Lu G, Kurihara N, Zhao AZ, Roodman GD. Annexin II stimulates RANKL expression through MAPK. J Bone Miner Res 2005; 20:1161-7; PMID:15940368; https://doi.org/10.1359/JBMR.050207
- Seckinger A, Meissner T, Moreaux J, Depeweg D, Hillengass J, Hose K, Reme T, Rosen-Wolff A, Jauch A, Schnettler R, et al. Clinical and prognostic role of annexin A2 in multiple myeloma. Blood 2012; 120:1087-94; PMID:22705595; https://doi.org/10.1182/blood-2012-03-415588
- Burg JM, Link JE, Morgan BS, Heller FJ, Hargrove AE, McCafferty DG. KDM1 class flavin-dependent protein lysine demethylases. Biopolymers 2015; 104:213-46; PMID:25787087; https://doi.org/10.1002/bip.22643
- Fang R, Barbera AJ, Xu Y, Rutenberg M, Leonor T, Bi Q, Lan F, Mei P, Yuan GC, Lian C, et al. Human LSD2/KDM1b/AOF1 regulates gene transcription by modulating intragenic H3K4me2 methylation. Mol Cell 2010; 39:222-33; PMID:20670891; https://doi.org/10.1016/j.molcel.2010.07.008
- van Essen D, Zhu Y, Saccani S. A feed-forward circuit controlling inducible NF-kappaB target gene activation by promoter histone demethylation. Mol Cell 2010; 39:750-60; PMID:20832726; https://doi.org/10.1016/j.molcel.2010.08.010
- Demchenko YN, Kuehl WM. A critical role for the NFkB pathway in multiple myeloma. Oncotarget 2010; 1:59-68; PMID:20890394; https://doi.org/10.18632/oncotarget.109
- Demchenko YN, Glebov OK, Zingone A, Keats JJ, Bergsagel PL, Kuehl WM. Classical and/or alternative NF-kappaB pathway activation in multiple myeloma. Blood 2010; 115:3541-52; PMID:20053756; https://doi.org/10.1182/blood-2009-09-243535
- Kassambara A, Reme T, Jourdan M, Fest T, Hose D, Tarte K, Klein B. GenomicScape: an easy-to-use web tool for gene expression data analysis. Application to investigate the molecular events in the differentiation of B cells into plasma cells. PLoS Comput Biol 2015; 11:e1004077; PMID:25633866; https://doi.org/10.1371/journal.pcbi.1004077
- Feichtinger J, Thallinger G, McFarlane R, Larcombe L. Microarray meta-analysis: From data to expression to biological relationships. In: Trajanoski Z, editor, Computational Medicine. Vienna, Austria: Springer; 2012; 59-77.