ABSTRACT
It was reported that γ-irradiation had a controversial therapeutic effect on glioma cells. We aimed to investigate the cytotoxic effect on the glioma cells induced by γ-irradiation and explore the treatment to rescue the phenotype alteration of remaining cells. We used transwell assay to detect the glioma cell invasion and migration capacity. Cell proliferation and apoptosis were tested by the CCK-8 assay and flow cytometry respectively. Western Blot was used to detect the activity of Hedgehog signaling pathway and Epithelial-to-Mesenchymal Transition (EMT) status. γ-irradiation showed cytotoxic effect on LN229 cells in vitro, whereas this contribution was limited in U251 cells. However, it could significantly stimulated EMT process in both LN229 and U251. Curcumin (CCM) could rescue EMT process induced by γ-irradiation via the suppression of Gli1 and the upregulation of Sufu. The location and expression of EMT markers were also verified by Immunofluorescence. Immunohistochemistry assay was used on intracranial glioma tissues of nude mice. The capacities of cell migration and invasion were suppressed with combined therapy. This research showed Curcumin could rescue the EMT process induced by γ-irradiation via inhibiting the Hedgehog signaling pathway and potentiate the cell cytotoxic effect in vivo and in vitro.
Introduction
Glioma is the most common primary malignant tumor in central nervous system. Now the γ-irradiation therapy for glioma is considered to be controversial.Citation1 Increasing the dose of γ-irradiation to heighten the effect of therapy would cause unexpected complications such as cerebral edema or necrosis.Citation2 Besides, some glioma cells even have a resistance to the cytotoxic effect caused by γ-irradiation. However, the cytotoxic effect on the glioma cells induced by γ-irradiation needs further study. The resistance to γ-irradiation in some cells needs to be confirmed. In the present study, we found that γ-irradiation had differential effect on the proliferative and apoptotic capacity and induced EMT of glioma cell lines. Thus, a novel therapeutic method to rescue the EMT caused by γ-irradiation was important. Hedgehog signaling pathway has been reported as an important role in EMT. Down-regulation of Hedgehog signaling pathway proteins could cause an suppression to EMT.Citation3 Gli1, considered as a key marker of the Hedgehog signaling pathway activation, is implicated in EMT through a series of complex interactions.Citation4-Citation6 In our previous researches, we illustrated that Curcumin, the ingredient of turmeric, suppressed the activity of Hedgehog signaling pathway by mediating the protein expression including Gli1, SMO and Sufu.Citation7,Citation8 And Curcumin could also prevent the EMT in different tumor cells.Citation9,Citation10 With the interruption of EMT, Curcumin might cover the shortage of γ-irradiation (induction of EMT). In this research, we had experiments on LN229 and U251 cells, which represented to be sensitive and insensitive to cytotoxic effect of γ-irradiation, respectively. However, both LN229 and U251 showed the induction of EMT caused by γ-irradiation. We added Curcumin into the remaining cells survived from γ-irradiation, and explored their the phenotype alteration. After the introduction of Curcumin treatment, this induction of EMT was prevented, and the activity of Hedgehog signaling pathway was also attenuated. The cell migration and invasion abilities were suppressed. In both LN229 and U251, cell proliferation was inhibited in combined therapy group. Taken together, we illustrated that Curcumin had an interaction with the induction of EMT caused by γ-irradiation, via restricting the Hedgehog signaling pathway. Combined treatment with Curcumin and γ-irradiation would restrain EMT process in both irradiation sensitive and insensitive glioma cells, and would make γ-irradiation more efficiency in irradiation sensitive glioma cells.
Results
γ-irradiation had differential effects on the proliferation and apoptosis of glioma cell
LN229 showed both dose-dependent and time-dependent manner after γ-irradiation treatment (). When the LN229 cells were treated with γ-irradiation for less than 72 hours, the cell proliferation might not be significantly impacted. However, γ-irradiation could inhibit the cell growth after 96 hours (P < 0.05) and 120 hours (P < 0.05), regardless of the dose (). On the opposite, γ-irradiation didn't significantly inhibit the cell growth of another glioma cell line U251 neither in 72 hours nor after 96 hours ().
Figure 1. γ-irradiation had a cytotoxic effect on LN229 and U251 cells. (A) γ-irradiation had a manner of time-depend and dose-depend effect on LN229 cell proliferation. LN229 cells treated with 6, 9, 12, 15, 18, 21 Gy γ-irradiation were detected to have a reduction on cell proliferation ability at the time point of 24, 48, 72, 96, 120 hours. (B) Compared with LN229, U251 had a resistance to γ-irradiation. Cell proliferation assay showed that U251 treated with γ-irradiation did not have obvious differentiation compared with control group. There existed a clear difference in the sensitivity of LN229 and U251. (C) In apoptosis assay, LN229 had the manner of time-depend and dose-depend to γ-irradiation. LN229 cells with a larger dose of irradiation showed a tendency to early apoptosis at 96 and 120 hours, while this effect could not be detected in U251 cells. Data are mean ± SD of 3 independent experiments. * meant P value was less than 0.05 compared with NC group.
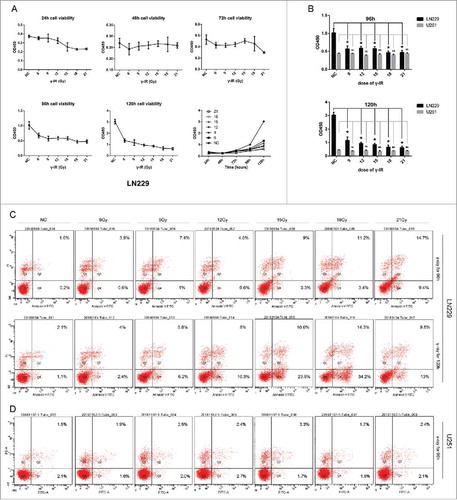
Flow cytometry showed that γ-irradiation could induce the cell apoptosis with dose of 18 Gy and 21 Gy at the time points of 96 hours and 120 hours in LN229 cell line (). But γ-irradiation couldn't significantly induce the U251 cells apoptosis (). These data suggested that γ-irradiation might inhibit the cell proliferation and induce the cell apoptosis, depending on different glioma intrinsic genomic alteration.
γ-irradiation induced Epithelial-to-Mesenchymal Transition in glioma cell lines
It had been reported that γ-irradiation could induce the Epithelial-to-Mesenchymal Transition in tumors.Citation11,Citation12 To investigate whether γ-irradiation influenced the EMT in glioma cells, cells were treated with 18 Gy and 21 Gy γ-irradiation, and the EMT markers were tested after 48 (when the γ-irradiation might not affect the glioma cell growth) and 96 hours ().
Figure 2. γ-irradiation could induce the Epithelial-to-Mesenchymal Transition. Both LN229 and U251 cells manifested the EMT proteins expression changed. β-catenin and Vimentin were upregulated, while E-cadherin and Claudin were suppressed. GAPDH was used as reference. This phenomenon was more significant at 96 hours than 48 hours. According to these changes, EMT was launched after γ-irradiation. * meant P value was less than 0.05 compared with NC group.
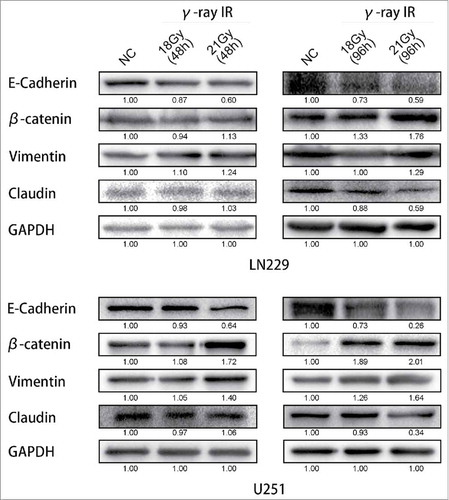
At the time point of 48 hours after treated with γ-irradiation, the expression of EMT markers in both LN229 and U251 were not changed remarkable. In LN229, comparing between NC and 18 Gy (48 h) groups, E-cadherin, β-catenin, Vimentin and Claudin had the same expression level. But comparing between NC and 21 Gy (48 h) groups, E-cadherin was downregulated. β-catenin and Vimentin were upregulated. In U251, it had the similar results. Only the β-catenin was upregulated compared between NC and 21 Gy (48 h) groups. However, with more time after treated with γ-irradiation, the proteins' expression changed more notably. In LN229 NC, 18 Gy (96 h) and 21 Gy (96 h) groups, E-cadherin and Claudin were downregulated, at the same time β-catenin and Vimentin were upregulated. These changes had a dose-dependent manner. We also validated the results in U251 cells. In U251 cells treated with 18 Gy (96 h) and 21 Gy (96 h), we could also find that E-cadherin and Claudin expressions were suppressed significantly but β-catenin and Vimentin were increased comparing with the control group. These results suggested that the induction of EMT process in glioma cell lines was caused by γ-irradiation, and this change of EMT markers' expression would not be observed significantly until 48 hours.
Curcumin rescued the induction of EMT caused by γ-irradiation via suppressing the Hedgehog signaling pathway
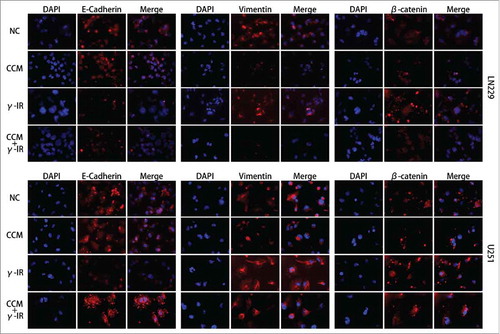
To rescue the glioma cells EMT induced by γ-irradiation, we used 20 μM Curcumin to treat the glioma cells for 24 hours after γ-irradiation, as other scientists reported.Citation13 Then we used Western Blot to test the EMT markers and the Hedgehog signaling pathway, which was reported to be the target signaling pathway of Curcumin. We chose 96 hours as a time period after γ-irradiation. When cells got γ-irradiation after 72 hours, Curcumin was added to CCM group and CCM+γ-IR group for extra 24 hours. Then at the time point of 96 hours, cells were harvested for Western Blot testing. We observed that γ-irradiation did not affect the Hedgehog signaling pathway protein expression significantly, however, increased β-catenin, Vimentin protein expression and decreased E-cadherin, Claudin protein expression (). In LN229 γ-IR group, the proteins of Hedgehog signaling pathway were not affected significantly. The alteration of EMT induced by γ-irradiation was confirmed again. In CCM group, the Hedgehog signaling pathway were suppressed. Gli1 and SMO, the effector proteins of Hedgehog signaling pathway were attenuated. Sufu, the suppressor protein of Gli1, was upregulated. In LN229 CCM+γ-IR group, we observed that Gli1, SMO, β-catenin and Vimentin were attenuated compared with NC group or γ-IR group. On the opposite, Sufu, E-cadherin and Claudin were upregulated compared with NC group. In U251, this kind of change was similar. Immunofluorescence assay was performed to verify the location and the expression of E-cadherin, Vimentin and β-catenin. We observed that the expression of E-cadherin was induced significantly in combined therapy group in both LN229 and U251. On the contrary, Vimentin showed the opposite tendency. It could be found that besides the downregulation of β-catenin in combine therapy group, the transfer of β-catenin from cytoplasm to the nucleus was limited either, which meant the EMT process was suppressedCitation14,Citation15 (). In conclude, the EMT process was rescued by Curcumin via the suppressing of Hedgehog signaling pathway. With 20 μM Curcumin and 18 Gy γ-irradiation treated for 96 hours, the combined therapy group showed less tendency on EMT process.
Figure 3. Curcumin could rescue the induction of EMT via suppressing of Hedgehog signaling pathway. With the treatment of 18 Gy γ-irradiation for 96 hours, EMT process of cells was reversed. The expression of β-catenin and Vimentin were restrained. The expression of E-cadherin and Claudin were recovered. The expression of Gli1 and SMO were inhibited, and that of Sufu which acted as an inhibitor of Gli1 was increased. * meant P value was less than 0.05 compared with NC group.
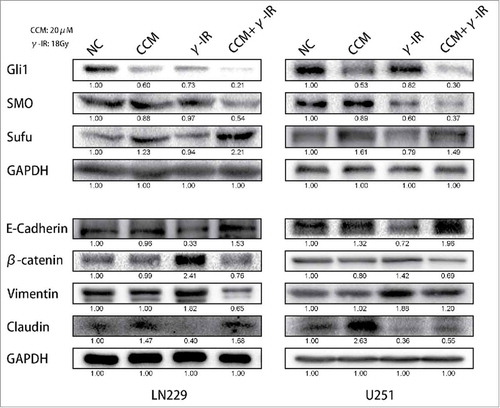
Figure 4. The Immunofluorescence assay showed that the induction of EMT was rescued by Curcumin. Nucleus were stained with DAPI (blue), while E-cadherin, Vimentin and β-catenin were shown in red. The merged pictures were combining with DAPI and the target proteins. γ-IR group showed the induction of EMT in both LN229 and U251, however the CCM+γ-IR group had restrained the process of EMT. All images were taken microscopically (40 ×).
Combination of Curcumin and γ-irradiation therapy changed the biologic phenotype of glioma cells
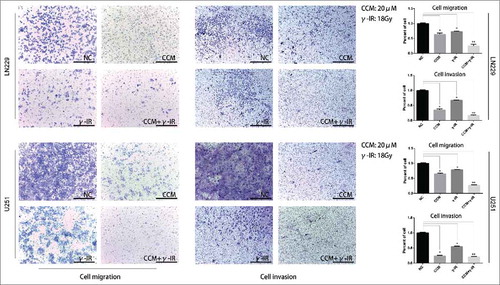
It is well known that LN229 and U251 presented highly invasive growth characteristics. Our previous works had shown that Curcumin might rescue the induction of EMT process caused by γ-irradiation. We performed cell migration and cell invasion assays to confirm that whether cell migration and cell invasion were attenuated. In , LN229 cell migration assay showed that with separated Curcumin and γ-irradiation treatment, cells showed low migration ability compared with NC group. But compared with CCM group, γ-irradiation group showed enhanced migration ability. In CCM+γ-IR group, cells showed minimum migration ability. We also observed the similar results in the cell invasion assay. After 96 hours treated with combined therapy, the abilities of migration and invasion were attenuated significantly. We repeated the experiments in U251 cell line subsequently. The results were similar to LN229.
Figure 5. The cell migration and invasion assays revealed that the metastasis ability was restricted in combined therapy group in both LN229 and U251 cells. The γ-irradiation (18Gy) group showed more tendency to metastasize than NC group and CCM (20 μM) group. Adding with Curcumin (20 μM), combined therapy showed the least metastasis ability. * meant P value was less than 0.05 compared with NC group. ** meant P value was less than 0.01 compared with NC group.
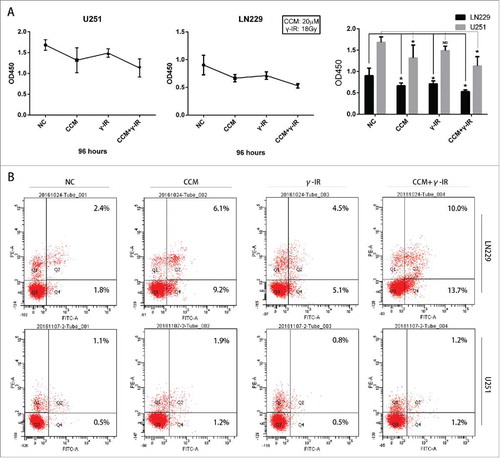
Then we focused on the cytotoxic effect of combined therapy treatment (). The cell proliferation assay showed that in LN229 and U251 cells, CCM+γ-IR group had a lower level of cell proliferation compared with NC group. Histogram was used for a more visible comparison (). The cell apoptosis assay showed that in LN229 we had more percentage of cells in Q4 compared with Q4 of NC, CCM, γ-IR groups. That meant combined therapy had an induction to early apoptosis in LN229. Compared with U251 NC group, there were more cells of CCM+γ-IR group arrested in early apoptosis phage (). However, compared with CCM group, the percentage of early apoptosis cell did not change significantly. Further work was needed to explain this phenomenon.
Figure 6. In addition with Curcumin (20 μM) and γ-irradiation (18Gy) as combined therapy, Curcumin did not reverse the formation of early apoptosis promoted by γ-irradiation. In LN229, Curcumin could advance the cytotoxic effect of γ-irradiation remarkably, however this phenomenon was slightly in U251. (A) The cell proliferation assay showed the promotion of Curcumin was remarkable in LN229, while that of U251 was slightly. The bar graph merged 2 cell line together for a more intuitive observation. (B) Cell apoptosis assay showed the combined therapy had limited influence on early apoptosis of U251, however it was more effective in LN229. * meant P value was less than 0.05 compared with NC group. (C) A model for Curcumin increasing the efficiency of γ-irradiation by regulating Hedgehog signaling pathway.
γ-irradiation combined with Curcumin therapy inhibited the EMT process in vivo
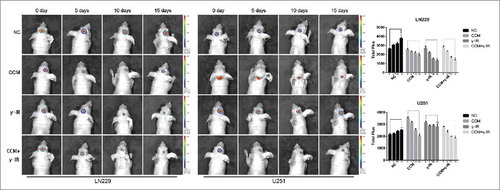
To investigate whether combined therapy with γ-IR and Curcumin could affect the tumor growth and EMT process, the intracranial glioma models of nude mice were built. The size of tumors was compared between each group every 5 d by xenograft study. In tumors formed by LN229, comparing with NC group, the tumors of CCM group started to reduce after treating for 10 d. However, the tumors of γ-IR group started decreasing after 5 days, which was same as the results in vitro. The combine therapeutic treatment had a significant cytotoxic effect on implanted tumors (), especially after 15 d of treatments. On the contrary, tumors formed by U251 were not sensitive with γ-IR. Even in the combined therapy group, the reduction of U251 tumors was limited. The reduction was supposed to be mainly contributed by Curcumin.
Figure 7. The bioluminescence images showed that tumors formed by LN229 were sensitive to γ-irradiation. The size of tumors were detected every 5 d and had decreased gradually in CCM group, γ-IR group, CCM+γ-IR group in 15 d. The tumors formed by U251 were resist to γ-irradiation. The columns on the right represented the fluorescence intensity of tumors. * meant P value was less than 0.05 compared with NC group.
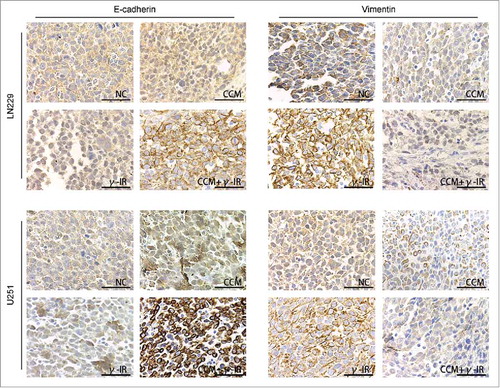
After 15 days, all mice were executed and the brain tissues were stored in paraformaldehyde for Immunohistochemistry. E-cadherin and Vimentin were chose as markers of epithelium type and mesenchyme type respectively (). In tumor tissues formed by either LN229 or U251, E-cadherin was upregulated in CCM group and was downregulated in γ-IR group. In combined therapy group, E-cadherin was significantly induced, which demonstrated that tumor tissues showed more epithelium type. This consequence was converse in the results of Vimentin. In tumor tissues formed by either LN229 or U251, Vimentin was restrained in CCM group and was stimulated in γ-IR group. In combined therapy group, Vimentin was restrained significantly, which demonstrated that mesenchymal phenotypic characteristics of tumor tissues was restricted. All results above suggested that combined therapy with Curcumin and γ-irradiation had a restriction on the process of EMT in vivo. Even γ-irradiation had an induction on EMT, Curcumin could rescue this induction and slightly improve the cytotoxic effect. In general, Curcumin could improve the efficiency of γ-irradiation therapy by rescuing the induction of EMT.
Figure 8. The Immunohistochemistry showed the effect of Curcumin on induction of EMT in vivo. All images was taken microscopically (40 ×). The expression of E-cadherin was upregulated in CCM+γ-IR group and Vimentin was downregulated compared with NC group. Both LN229 and U251 showed the similar results.
Materials and methods
Cells and reagents
The glioma cell lines (U251, LN229) were purchased from Chinese Academy of Sciences Cell Bank. U251 cells were cultured in DMEM supplement, while LN229 cells were in DMEM/F12 50/50 supplement. All supplement contained 10% fetal bovine serum. Both U251 and LN229 cells were incubated with 5% CO2 and 95% air and routinely passaged every other day or 2 d interval. Cells centrifuged at 1,300 rpm for 5 minutes that concentrated at the bottom of 1.5 ml EP pipes were prepared for γ-irradiation therapy. All these treatments were performed in room temperature.
γ-irradiation therapy
Different doses of γ-irradiation were delivered to the 75% isodose line by using a 8-mm collimator from the 201-source 60Co gamma unit. This radiation treatment was performed using the Leksell Gamma Knife. After γ-irradiation therapy, cells were seeded back to Petri dishes immediately with the conditions described above.
Cell proliferation assay
The Cell Counting Kit-8 (CCK-8) reagent was used for cell proliferation assay. Cells with various doses of γ-irradiation treatments were planted into 96-well, as well as Curcumin treatments. Each well contains 1,000 cells and 100 μL medium. At right time, 10 μL CCK-8 was added into each well, then cells were incubated for another 2 hours. The proliferation rate was assessed using Tecan infinite F50 microplate reader with 450 nm absorbance.
Migration and invasion assays
Cell migration and invasion assays were performed by 24-well transwell chambers and reconstituted extracellular matrix membrane (Matrigel). The cell invasion chambers were prepared by placing 40 μL Matrigel of 1:7 dilution onto the filters at 37°C for at least 30 minutes to make Matrigel polymerization. The cell migration chambers contained no Matrigel. 5 × 105 cells with or without treatments were seeded into the upper side of chambers as 200 μL cell suspension in culture with 2% fetal bovine serum for invasion assay, while 1 × 105 cells for migration assay, and 500 μL culture with 20% fetal bovine serum was added into the wells outside the chambers for both experiments. Chambers with cells were incubated for 24 hours for migration and 48 hours for invasion, at 37°C in a humid atmosphere of 5% CO2 and 95% air. All chambers were fixed in methanol for 10 minutes and stained in Giemsa (Biotopped) for 15 minutes. The upper surfaces of the filters were scraped slightly with wet cotton swaps to remove the non-migrated cells. Stick the chambers upside-down on microscope slide for observation. Triplicate wells were repeated for each group, and migrated cells were counted with microscopically (40 ×) in 5 different fields per filter.
Cell apoptosis assay
U251 and LN229 cells with or without treatments were planted in 6-well plates. By the specific time for different purposes, 1 × 106 cells for each group were harvested and transferred into 5 ml culture tubes. BD Pharmingen™ FITC Annexin V Apoptosis Detection Kit II was used for cell apoptosis assay. Following the instruction of manufacturer, after washing cells with PBS twice and resuspending in 300 μL 1x Annexin V binding buffer, 5 μL Annexin V for each group was added into cell suspension for 15 minutes in the dark, then 5 μL PI for each group was added for another 15 minutes, following by gently vortex. Cells were analyzed by flow cytometer (FACS Canto II, BD Biosciences).
Western blot assay
Cells with or without treatments were scraped in Thermo scientific RIPA buffer (Solarbio) with 1% protease inhibitor. After centrifugation of 140,000 rpm for 30 minutes, with temperature of 4°C, total cellular protein concentration was measured by NanoDrop 2000C spectrophotometer (NanoDrop Products) according to the manufacturer's instructions. All samples were subjected in 7.5%/10%/12.5% sodium dodecyl sulfate polyacrylamide gel (EpiZyme Scientific) electrophoresis, and the gel was blotted onto PVDF membrane (Millipore, USA). The membrane was blocked in 5% milk-TBST solution and incubated separately with rabbit antihuman Gli1 (1:1000, Cell Signaling Technology), rabbit antihuman SMO (1:1000, Cell Signaling Technology), rabbit antihuman Sufu (1:1000, Cell Signaling Technology), rabbit antihuman N-cadherin (1:1000, Cell Signaling Technology), rabbit antihuman β-catenin (1:1000, Cell Signaling Technology), rabbit antihuman Vimentin (1:1000, Cell Signaling Technology), rabbit antihuman Claudin (1:500, Cell Signaling Technology), rabbit antihuman E-cadherin (1:500, Proteintech), mouse antihuman GAPDH (1:1000, Cell Signaling Technology). Following incubation with HRP-labbled secondary antibody (Introvigen), protein bands were detected with SuperEnhanced chemiluminescence detection reagents (Applygen Technologies Inc.) in ChemiDoc™ MP Imaging System (BioRad).
Immunofluorescence assay
Cells (1 × 105) with or without treatments were transported to glass coverslips to grow overnight. On the second day, cells were fixed in 2% paraformaldehyde, permeabilized, blocked, and incubated with antibodies, such as rabbit antihuman E-cadherin (1:100, Proteintech), rabbit antihuman Vimentin (1:100, Cell Signaling Technology) and rabbit antihuman β-catenin (1:100, Cell Signaling Technology). After washing, the cells were incubated with Alexa488 labeled secondary antibody. The slides were analyzed by confocal laser scanning microscopy (Olympus FV1000 Laser Scanning Microscope).
Tumor xenograft study
LN229 and U251 cells transfected with luciferase lentivirus (LN229-luc and U251-luc) were used to be injected into the intracranial site of 4-week old female nude mice as described.Citation16 All mice were separated into 8 groups randomly, named as LN229-luc-NC, LN229-luc-CCM, LN229-luc-γ-IR, LN229-luc-CCM+γ-IR, U251-luc-NC, U251-luc-CCM, U251-luc-γ-IR and U251-luc-CCM+γ-IR. After 14 days, intracranial tumors were confirmed to be formed using IVIS Lumina Imaging System (Xenogen). The LN229-luc-γ-IR group, LN229-luc-CCM+γ-IR group, U251-luc-γ-IR group and U251-luc-CCM+γ-IR group were anaesthetized and fixed on Nuclear Magnetic Resonance to locate the tumors. With data transmission back to Leksell Gamma Knife machine, 18 Gy dose of γ-irradiation was delivered to these mice by using a 8-mm collimator from the 201-source 60Co gamma unit. At the same time, Curcumin was injected intraperitoneally with the dose of 60mg/kg to LN229-luc-CCM group, LN229-luc-CCM+γ-IR group, U251-luc-CCM group and U251-luc-CCM+γ-IR group every day. Other mice were injected with solvent DMSO as control. The tumor size was detected by IVIS Lumina Imaging System every 5 d. All mice were executed at the 15th day after treatments.
These procedures were performed following approval by the Harbin Medical University Institutional Animal Care and Use Committee.
Immunohistochemistry (IHC) assay
IHC analysis was performed followed the procedure described previously.Citation13,Citation17 Briefly, the tumor tissues were deparaffinaged, rehydrated, treated with 0.3% hydrogen peroxide, and processed with antigen retrieval using heat induction for about 10 minutes. Samples were stained with anti-E-cadherin antibody (1:200, Proteintech) and anti-Vimentin antibody (1:200, Cell Signaling Technology). The expression were detected using peroxidase conjugated AffiniPure goat anti-rabbit IgG (ZSGB-BIO) and 3,3′-diaminoben-zidine (DAB substrate kit, ZSGB-BIO). The slides were dehydrated, mounted, and observed with an Olympus CX31 Microscope (Olympus Corporation).
Statistical assay
All results with mean and SEM in this research were calculated using GraphPad Prism software (version 6.0). T-test was used for comparing between each treatments data with control. P values less than 0.05 were considered to be significant.
Discussion
We observe that patients with glioblastoma always have poor prognosis in clinical work.Citation18 Most patients would be suggested to have chemotherapy, radiation therapy or concomitant radiochemotherapy.Citation19,Citation20 According to clinical observation, treatment outcome with single therapy method could not always be satisfied.Citation21 In fact, some of patients are sensitive to γ-irradiation while others are not, even though γ-irradiation is one of the effective methods of radiation therapy.Citation22,Citation23 As known, γ-irradiation and other ionizing radiation work by damaging the DNA double strand of cancer cells leading to cellular death.Citation24,Citation25 After that, DNA damage repair has been launched and genes associated with DNA repair will be activated, such as p53, ATM and ATR. Cells with mutant type of these genes would be resistant to irradiation. In clinical work, there has minority patients with glioblastoma reoccurred in situ or in adjacent areas after γ-irradiation therapy. This phenomenon has few reports, neither the validations. Therefore, there is a pressing need to search for novel strategies to increase the efficiency of γ-irradiation. Researchers have performed so many essays noticing that proteins or other cellular functional and biologic macromolecules have cross effect on resistance to radiation.Citation26 Qiang Jia et al. have attempted to transfected shRNA to downregulated the expression of Ku70, for a purpose to enhance the radiosensitivity of glioma cells.Citation27 Xuefeng Gu et al. have experimented to silencing of R-Spondin1 (Rspo1) to increase radiosensitivity.Citation28 Valproic acid (VPA) treatment is reported to protect hippocampal neurons from damage induced by radiation in vivo and in vitro.Citation29 Different ideas are studied.
According to other researches, EMT process could be induced after radiation.Citation11,Citation30,Citation31 It is considered that therapeutic effect of γ-irradiation can be more stable with recall of EMT process. In this research, we verified that γ-irradiation produced a delayed cytotoxic effect on LN229 cell proliferation suppression and apoptosis induction until 48 hours after γ-irradiation treatment. With a larger dose of γ-irradiation, the influences were more remarkable. The most satisfied dose for therapy was 21Gy, while the most convenient dose for research observation was 18Gy. Considering that glioma cells with irradiation would be out of vitality after 120 hours, we chose 96 hours as a period of time for γ-irradiation effect. For a better confirmation, we chose U251 cell to repeat the experiments. Interestingly, the influence of γ-irradiation was limited in U251 cells. There was a theory on molecular biology that U251 had mutant type p53 which caused the differential expression of P53, ATM and ATR after γ-irradiation.Citation32 In this research, we also set U251 to γ-irradiation in dose of 9, 12, 15, 18, 21Gy. We performed cell proliferation assay in 96, 120 hours and cell apoptosis assay in 96 hours. The results showed that the influence of γ-irradiation was not significant compared with U251 NC group. Subsequently we had a test on EMT process markers by Western Blot. As a result, E-cadherin and Claudin were downregulated, and Vimentin and β-catenin were upregulated. These results indicated that whether cells had resistance to γ-irradiation or not, EMT process would be induced after γ-irradiation treatment. High level of expression of β-catenin might because of the enrichment of β-catenin in nucleus.Citation33,Citation34 This was the initial stage of EMT process. E-cadherin maintained a tight connection between cells and cells. Vimentin played a significant role in supporting and anchoring the position of the organelles in the cytosol. The increasing expression level of Vimentin represented that cells were more likely to be mesenchymal. Claudin was recognized as a transcription factor of E-cadherin.Citation35,Citation36 In summary, we had a conclusion that EMT process was induced after γ-irradiation, and especially in U251. This result was regardless with the resistance to γ-irradiation.
Hedgehog signaling pathway has been a research focus recently.Citation37 Some other scientists have reported that in gliomas Hedgehog signaling pathway is activated abnormally. SMO and Gli1 are recognized as positive markers of Hedgehog signaling pathway, and Sufu as a negative marker. In some recent studies, SMO is considered as a poor prognosis factor and a potential therapeutic target in glioma.Citation38 Especially for high-grade gliomas, the activation or inhibtion of the Hedgehog signaling pathway has a remarkable impact on cell proliferation.Citation39 There are also numbers of research revealed that Curcumin has specific regulation to Hedgehog signaling pathway and EMT processCitation7,Citation40,Citation41 in several cancer cells including pancreas, colon, leukemia, prostate carcinoma and glioblastomas.Citation8,Citation42-Citation44 Islam SS et al. and Xu X et al.Citation9,Citation10 point out that the Hedgehog signaling pathway activates EMT process to promote tumorigenicity and stemness. It is potent that Tsai, C. L.Citation5 et al. demonstrate that Hedgehog signaling pathway inhibition could be as a strategy to augment radiosensitivity,Citation45 especially in non-small cell lung cancers.Citation46 Gli1 proteins which are effectors of Hedgehog (Hh) signaling pathway, primarily regulate S100A family members via cis-acting elements. S100A4 and VIM gene are upregulated significantly by Shh/Gli1 increasing expression, and E-cadherin coded by CDH1 is reduced at the same time. In a dose-dependent manner of Gli1 expression, the migration ability of cells is increased. Inhibitor of Hedgehog signaling pathway can reversed this response to EMT. Overall, it is believed that Curcumin and its target Hedgehog signaling pathway would be key roles to redeem EMT process caused by γ-irradiation, and in this manner they would enhance the radiosensitivity without impaction on the cytotoxic results. Considering these theories, we chose the Hedgehog signaling pathway for testing the government of EMT induction after γ-irradiation. Curcumin was used as an inhibitor to Hedgehog signaling pathway.Citation47,Citation48 In some other research, 20–40 μM dose of Curcumin is used as a approperate dose for inhibiting EMT.Citation49,Citation50 According to other research and our prior work, 20 μM Curcumin acted for 24 hours was used as a treatment in vitro, and 60 mg/kg/d was the dose used for intraperitoneal injection in vivo. With dose of 20 μM, the therapeutic effect of Curcumin would not cover the effect of γ-irradiation, but highlight the efficiency of combined therapy group. At 72 hours, 20 μM CCM was added to the supplement of CCM group and CCM+γ-IR group. With incubating for extra 24 hours, cells were harvested for assays. Gli1 and SMO were suppressed in combined therapy group (CCM+γ-IR), while Sufu was inspired.Citation51 This represented the inactivation of Hedgehog signaling pathway. The expression level of E-cadherin and Claudin in CCM+γ-IR group were increased compared with γ-IR group, while either of them was still lower than NC group. Vimentin and β-catenin showed the opposite appearance with other 2 proteins. For a further study on the location and expression of EMT markers, Immunofluorescence was performed. Comparing with NC group of LN229 cells, there was less E-cadherin located on the membrane of cells in γ-IR group, and the expression was downregulated either. The CCM group and CCM+γ-IR group had the opposite consequence. The results of Vimentin were on the contrary of E-cadherin. More staining could be found in γ-IR group, but in the CCM group and CCM+γ-IR group there were fewer. β-catenin, which was reported to act as a trigger of EMT,Citation52,Citation53 and translocate into nucleus in the initial stage of EMT,Citation14,Citation54 was detected to have more expression in both nucleus and cytoplasm in γ-IR group, but less expression in CCM group and CCM+γ-IR group. This provided an convincing result that Curcumin could cover the induction of EMT which caused by γ-irradiation in the protein level.
Cell migration and invasion abilities were suppressed. Observing with 40 × microscope, we believed that Curcumin rescued the induction of EMT process caused by γ-irradiation via suppressing Hedgehog signaling pathway. In addition, we indicated that Curcumin heightened the effect of γ-irradiation in cell proliferation and apoptosis assays. With combined therapy treatment, cell proliferation of LN229 and U251 was inhibited. Cell apoptosis assay showed the combined therapy had limited influence on early apoptosis of U251, however it was more effective in LN229.
As more convincing results were needed, nude mice with xenograft gliomas were used as animal models for research in vivo. In tumors formed by LN229, there was a significant reduction in tumor size compared with γ-IR group and combined therapy group. However, in tumors formed by U251, the tumor size changed little. This consequence demonstrated that different biologic phenotypes of glioma cells showed different sensitivity to γ-irradiation. Subsequently, IHC analysis of these xenograft tumor tissues showed that Curcumin could also rescue the induction of EMT cause by γ-irradiation in vivo.
As a conclusion, we demonstrated that Curcumin increased the effect of γ-irradiation in glioma by inhibiting the Hedgehog signaling pathway in vitro and in vivo. These findings provided evidences of combined therapy in clinical work, and could serve as a potential therapeutic method for patients with glioma.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by these grants:
The Research Special Fund For Public Welfare Industry of Heath (No. 201402008);
The National Key Research and Development Plan (No. 2016YFC0902500);
National Natural Science Foundation of China (No. 81572701, No. 81372700);
Special Fund Project of Translational Medicine in the Chinese-Russian Medical Research Center (No. CR201417)
Research Project of Chinese Society of Neuro-oncology, CACA (CSNO-2014-MSD08);
Heilongjiang Postdoctoral Fund (No.LBH-Z12154);
Science and Technology Research Project of Education Department of Heilongjiang Province (No.12531302);
Research Fund for the Doctoral Program of The Second Affiliated Hospital of Harbin Medical University (BS2012–12).
References
- Ryu S, Buatti JM, Morris A, Kalkanis SN, Ryken TC, Olson JJ, A.C.J.G. Committee. The role of radiotherapy in the management of progressive glioblastoma : a systematic review and evidence-based clinical practice guideline. J Neuro-Oncol 2014; 118(3):489-99; PMID:24728785; https://doi.org/10.1007/s11060-013-1337-6
- Matsunaga S, Shuto T, Takase H, Ohtake M, Tomura N, Tanaka T, Sonoda M. Semiquantitative analysis using thallium-201 SPECT for differential diagnosis between tumor recurrence and radiation necrosis after gamma knife surgery for malignant brain tumors. Int J Radiat Oncol Biol Phys 2013; 85(1):47-52; PMID:22541963; https://doi.org/10.1016/j.ijrobp.2012.03.008
- Chong Y, Tang D, Gao J, Jiang X, Xu C, Xiong Q, Huang Y, Wang J, Zhou H, Shi Y, et al. Galectin-1 induces invasion and the epithelial-mesenchymal transition in human gastric cancer cells via non-canonical activation of the hedgehog signaling pathway. Oncotarget 2016; 7(50):83611-26.
- Chun HW, Hong R. Significance of the hedgehog pathway-associated proteins Gli-1 and Gli-2 and the epithelial-mesenchymal transition-associated proteins Twist and E-cadherin in hepatocellular carcinoma. Oncology Letters 2016; 12(3):1753-62; PMID:27602109
- Tsai CL, Hsu FM, Tzen KY, Liu WL, Cheng AL, Cheng JC. Sonic Hedgehog inhibition as a strategy to augment radiosensitivity of hepatocellular carcinoma. J Gastroenterol Hepatol 2015; 30(8):1317-24; PMID:25682950; https://doi.org/10.1111/jgh.12931
- Sims-Mourtada J, Izzo JG, Apisarnthanarax S, Wu TT, Malhotra U, Luthra R, Liao Z, Komaki R, van der Kogel A, Ajani J, et al. Hedgehog: an attribute to tumor regrowth after chemoradiotherapy and a target to improve radiation response. Clin Cancer Res 2006; 12(21):6565-72; PMID:17085672; https://doi.org/10.1158/1078-0432.CCR-06-0176
- Cao L, Xiao X, Lei J, Duan W, Ma Q, Li W. Curcumin inhibits hypoxia-induced epithelialmesenchymal transition in pancreatic cancer cells via suppression of the hedgehog signaling pathway. Oncol Reports 2016; 35(6):3728-34; PMID:27035865
- Milacic V, Banerjee S, Landis-Piwowar KR, Sarkar FH, Majumdar AP, Dou QP. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res 2008; 68(18):7283-92; PMID:18794115; https://doi.org/10.1158/0008-5472.CAN-07-6246
- Xu X, Su B, Xie C, Wei S, Zhou Y, Liu H, Dai W, Cheng P, Wang F, Xu X, et al. Sonic hedgehog-Gli1 signaling pathway regulates the epithelial mesenchymal transition (EMT) by mediating a new target gene, S100A4, in pancreatic cancer cells. PloS One 2014; 9(7):e96441; PMID:25072505; https://doi.org/10.1371/journal.pone.0096441
- Islam SS, Mokhtari RB, Noman AS, Uddin M, Rahman MZ, Azadi MA, Zlotta A, van der Kwast T, Yeger H, Farhat WA. Sonic hedgehog (Shh) signaling promotes tumorigenicity and stemness via activation of epithelial-to-mesenchymal transition (EMT) in bladder cancer. Mol Carcinogenesis 2016; 55(5):537-51; PMID:25728352; https://doi.org/10.1002/mc.22300
- Cho JH, Hong WG, Jung YJ, Lee J, Lee E, Hwang SG, Um HD, Park JK. Gamma-Ionizing radiation-induced activation of the EGFR-p38/ERK-STAT3/CREB-1-EMT pathway promotes the migration/invasion of non-small cell lung cancer cells and is inhibited by podophyllotoxin acetate. Tumour Biol 2016; 37(6):7315-25; PMID:26671552; https://doi.org/10.1007/s13277-015-4548-y
- Pan Y, Zhou C, Yuan D, Zhang J, Shao C. Radiation exposure promotes hepatocarcinoma cell invasion through Epithelial Mesenchymal transition mediated by H2S/CSE pathway. Radiat Res 2016; 185(1):96-105; PMID:26727544; https://doi.org/10.1667/RR14177.1
- Du WZ, Feng Y, Wang XF, Piao XY, Cui YQ, Chen LC, Lei XH, Sun X, Liu X, Wang HB, et al. Curcumin suppresses malignant glioma cells growth and induces apoptosis by inhibition of SHH/GLI1 signaling pathway in vitro and vivo. CNS Neurosci Therapeutics 2013; 19(12):926-36; https://doi.org/10.1111/cns.12163
- Wang YP, Guo PT, Zhu Z, Zhang H, Xu Y, Chen YZ, Liu F, Ma SP. Pleomorphic adenoma gene like-2 induces epithelial-mesenchymal transition via Wnt/beta-catenin signaling pathway in human colorectal adenocarcinoma. Oncol Reports 2017; 37(4):1961-70.
- Cho YH, Cha PH, Kaduwal S, Park JC, Lee SK, Yoon JS, Shin W, Kim H, Ro EJ, Koo KH, et al. KY1022, a small molecule destabilizing Ras via targeting the Wnt/beta-catenin pathway, inhibits development of metastatic colorectal cancer. Oncotarget 2016; 7(49):81727-40; PMID:27835580
- Perry MC, Demeule M, Regina A, Moumdjian R, Beliveau R. Curcumin inhibits tumor growth and angiogenesis in glioblastoma xenografts. Mol Nutr Food Res 2010; 54(8):1192-201.
- Zhang J, Han L, Ge Y, Zhou X, Zhang A, Zhang C, Zhong Y, You Y, Pu P, Kang C. miR-221/222 promote malignant progression of glioma through activation of the Akt pathway. Int J Oncol 2010; 36(4):913-20; PMID:20198336
- Wen PY, Kesari S. Malignant gliomas in adults. N Eng J Med 2008; 359(5):492-507; PMID:18669428; https://doi.org/10.1056/NEJMra0708126
- Dong Y, Pan L, Wang B, Wang E, Zhang N, Cai P, Dai J. Stereotactic radiosurgery in the treatment of primary central nervous system lymphoma. Chinese Medical J 2003; 116(8):1166-70; PMID:12935403
- Li Y, Jia Q, Zhang J, Han L, Xu D, Zhang A, Zhang Y, Zhang Z, Pu P, Kang C. Combination therapy with Gamma Knife radiosurgery and antisense EGFR for malignant glioma in vitro and orthotopic xenografts. Oncol Reports 2010; 23(6):1585-91; PMID:20428813
- Lim YJ, Leem W. Two cases of Gamma Knife radiosurgery for low-grade optic chiasm glioma. Stereotactic Functional Neurosurg 1996; 66 Suppl 1:174-83; PMID:9032859
- Elaimy AL, Mackay AR, Lamoreaux WT, Demakas JJ, Fairbanks RK, Cooke BS, Lamm AF, Lee CM. Clinical outcomes of gamma knife radiosurgery in the salvage treatment of patients with recurrent high-grade glioma. World Neurosurg 2013; 80(6):872-8; PMID:23403349; https://doi.org/10.1016/j.wneu.2013.02.030
- Liang CL, Lu K, Liliang PC, Chen HJ. Gamma Knife surgery for optic glioma. Report of 2 cases. J Neurosurg 2010; 113 Suppl:44-7.
- Alfano L, Costa C, Caporaso A, Antonini D, Giordano A, Pentimalli F. HUR protects NONO from degradation by mir320, which is induced by p53 upon UV irradiation. Oncotarget 2016; 7(47):78127-39.
- Ryabova A, Mukae K, Cherkasov A, Cornette R, Shagimardanova E, Sakashita T, Okuda T, Kikawada T, Gusev O. Genetic background of enhanced radioresistance in an anhydrobiotic insect: transcriptional response to ionizing radiations and desiccation. Extremophiles 2017; 21(1):109-120; PMID:27105509; https://doi.org/10.1007/s00792-016-0888-9
- Li X, Chen F, Zhu Q, Ding B, Zhong Q, Huang K, Jiang X, Wang Z, Yin C, Zhu Y, et al. Gli-1/PI3K/AKT/NF-kB pathway mediates resistance to radiation and is a target for reversion of responses in refractory acute myeloid leukemia cells. Oncotarget 2016; 7(22):33004-15; PMID:27105509
- Jia Q, Li Y, Xu D, Li Z, Zhang Z, Zhang Y, Liu D, Liu X, Pu P, Kang C. Radiosensitivity of glioma to Gamma Knife treatment enhanced in vitro and in vivo by RNA interfering Ku70 that is mediated by a recombinant adenovirus. J Neurosurg 2010; 113 Suppl:228-35; PMID:21121806
- Gu X, Wang X, Xiao H, Ma G, Cui L, Li Y, Zhou H, Liang W, Zhao B, Li K. Silencing of R-Spondin1 increases radiosensitivity of glioma cells. Oncotarget 2015; 6(12):9756-65; PMID:25865226; https://doi.org/10.18632/oncotarget.3395
- Thotala D, Karvas RM, Engelbach JA, Garbow JR, Hallahan AN, DeWees TA, Laszlo A, Hallahan DE. Valproic acid enhances the efficacy of radiation therapy by protecting normal hippocampal neurons and sensitizing malignant glioblastoma cells. Oncotarget 2015; 6(33):35004-22; PMID:26413814
- Nambiar DK, Rajamani P, Singh RP. Silibinin attenuates ionizing radiation-induced pro-angiogenic response and EMT in prostate cancer cells. Biochem Biophys Res Commun 2015; 456(1):262-8; PMID:25446081; https://doi.org/10.1016/j.bbrc.2014.11.069
- de Marcondes PG, Morgado-Diaz JA. The role of EphA4 signaling in radiation-induced EMT-Like phenotype in colorectal cancer cells. J Cell Biochem 2016; PMID:27632701
- Miao W, Liu X, Wang H, Fan Y, Lian S, Yang X, Wang X, Guo G, Li Q, Wang S. p53 upregulated modulator of apoptosis sensitizes drug-resistant U251 glioblastoma stem cells to temozolomide through enhanced apoptosis. Mol Med Reports 2015; 11(6):4165-73; PMID:25625235
- Cheung CT, Bendris N, Paul C, Hamieh A, Anouar Y, Hahne M, Blanchard JM, Lemmers B. Cyclin A2 modulates EMT via beta-catenin and phospholipase C pathways. Carcinogenesis 2015; 36(8):914-24; PMID:25993989; https://doi.org/10.1093/carcin/bgv069
- Chen HN, Yuan K, Xie N, Wang K, Huang Z, Chen Y, Dou Q, Wu M, Nice EC, Zhou ZG, et al. PDLIM1 stabilizes the E-Cadherin/beta-Catenin complex to prevent Epithelial-Mesenchymal transition and metastatic potential of colorectal cancer cells. Cancer Res 2016; 76(5):1122-34; PMID:26701804; https://doi.org/10.1158/0008-5472.CAN-15-1962
- Bhat AA, Ahmad R, Uppada SB, Singh AB, Dhawan P. Claudin-1 promotes TNF-alpha-induced epithelial-mesenchymal transition and migration in colorectal adenocarcinoma cells. Exp Cell Res 2016; 349(1):119-27; PMID:27742576; https://doi.org/10.1016/j.yexcr.2016.10.005
- Figiel S, Vasseur C, Bruyere F, Rozet F, Maheo K, Fromont G. Clinical significance of epithelial-mesenchymal transition (EMT) markers in prostate cancer. Hum Pathol 2017; 61:26-32; PMID:27818287; https://doi.org/10.1016/j.humpath.2016.10.013
- Cherepanov SA, Baklaushev VP, Gabashvili AN, Shepeleva II, Chekhonin VP. Hedgehog signaling in the pathogenesis of neuro-oncology diseases. Biomeditsinskaia Khimiia 2015; 61(3):332-42; PMID:26215410; https://doi.org/10.18097/pbmc20156103332
- Tu Y, Niu M, Xie P, Yue C, Liu N, Qi Z, Gao S, Liu H, Shi Q, Yu R, et al. Smoothened is a poor prognosis factor and a potential therapeutic target in glioma. Sci Reports 2017; 7:42630; PMID:28195165; https://doi.org/10.1038/srep42630
- Cherepanov SA, Cherepanova KI, Grinenko NF, Antonova OM, Chekhonin VP. Effect of hedgehog signaling pathway activation on proliferation of high-grade gliomas. Bull Exp Biol Med 2016; 161(5):674-678; PMID:27709388; https://doi.org/10.1007/s10517-016-3483-2
- Sun XD, Liu XE, Huang DS. Curcumin reverses the epithelial-mesenchymal transition of pancreatic cancer cells by inhibiting the Hedgehog signaling pathway. Oncol Reports 2013; 29(6):2401-7; PMID:23563640
- Yoo YA, Kang MH, Lee HJ, Kim BH, Park JK, Kim HK, Kim JS, Oh SC. Sonic hedgehog pathway promotes metastasis and lymphangiogenesis via activation of Akt, EMT, and MMP-9 pathway in gastric cancer. Cancer Res 2011; 71(22):7061-70; PMID:21975935; https://doi.org/10.1158/0008-5472.CAN-11-1338
- Parasramka MA, Gupta SV. Synergistic effect of garcinol and curcumin on antiproliferative and apoptotic activity in pancreatic cancer cells. J Oncol 2012; 2012:709739; PMID:22685460; https://doi.org/10.1155/2012/709739
- Ng AP, Chng WJ, Khan M. Curcumin sensitizes acute promyelocytic leukemia cells to unfolded protein response-induced apoptosis by blocking the loss of misfolded N-CoR protein. Mol Cancer Res 2011; 9(7):878-88; PMID:21602299; https://doi.org/10.1158/1541-7786.MCR-10-0545
- Senft C, Polacin M, Priester M, Seifert V, Kogel D, Weissenberger J. The nontoxic natural compound Curcumin exerts anti-proliferative, anti-migratory, and anti-invasive properties against malignant gliomas. BMC Cancer 2010; 10:491; PMID:20840775; https://doi.org/10.1186/1471-2407-10-491
- Morgenroth A, Vogg AT, Ermert K, Zlatopolskiy B, Mottaghy FM. Hedgehog signaling sensitizes glioma stem cells to endogenous nano-irradiation. Oncotarget 2014; 5(14):5483-93; PMID:24978848; https://doi.org/10.18632/oncotarget.2123
- Zeng J, Aziz K, Chettiar ST, Aftab BT, Armour M, Gajula R, Gandhi N, Salih T, Herman JM, Wong J, et al. Hedgehog pathway inhibition radiosensitizes non-small cell lung cancers. Int J Radiat Oncol Biol Phys 2013; 86(1):143-9; PMID:23182391; https://doi.org/10.1016/j.ijrobp.2012.10.014
- Lian N, Jiang Y, Zhang F, Jin H, Lu C, Wu X, Lu Y, Zheng S. Curcumin regulates cell fate and metabolism by inhibiting hedgehog signaling in hepatic stellate cells. Lab Invest 2015; 95(7):790-803; PMID:25938627; https://doi.org/10.1038/labinvest.2015.59
- Elamin MH, Shinwari Z, Hendrayani SF, Al-Hindi H, Al-Shail E, Khafaga Y, Al-Kofide A, Aboussekhra A. Curcumin inhibits the Sonic Hedgehog signaling pathway and triggers apoptosis in medulloblastoma cells. Molecular Carcinogenesis 2010; 49(3): 302-14; PMID:20025076
- Gallardo M, Calaf GM. Curcumin inhibits invasive capabilities through epithelial mesenchymal transition in breast cancer cell lines. Int J Oncol 2016; 49(3):1019-27; PMID:27573203
- Jiao D, Wang J, Lu W, Tang X, Chen J, Mou H, Chen QY. Curcumin inhibited HGF-induced EMT and angiogenesis through regulating c-Met dependent PI3K/Akt/mTOR signaling pathways in lung cancer. Mol Therapy Oncolytics 2016; 3:16018; PMID:27525306; https://doi.org/10.1038/mto.2016.18
- Liu X, Wang X, Du W, Chen L, Wang G, Cui Y, Liu Y, Dou Z, Wang H, Zhang P, et al. Suppressor of fused (Sufu) represses Gli1 transcription and nuclear accumulation, inhibits glioma cell proliferation, invasion and vasculogenic mimicry, improving glioma chemo-sensitivity and prognosis. Oncotarget 2014; 5(22):11681-94; PMID:25373737; https://doi.org/10.18632/oncotarget.2585
- Pearlman RL, Montes de Oca MK, Pal HC, Afaq F. Potential therapeutic targets of epithelial-mesenchymal transition in melanoma. Cancer Letters 2017; 391:125-40; PMID:28131904; https://doi.org/10.1016/j.canlet.2017.01.029
- Lee HM, Hwang KA, Choi KC. Diverse pathways of epithelial mesenchymal transition related with cancer progression and metastasis and potential effects of endocrine disrupting chemicals on epithelial mesenchymal transition process. Mol Cell Endocrinol 2016; pii: S0303-7207(16)30549-4; PMID:28042023; https://doi.org/10.1016/j.mce.2016.12.026
- Li R, Dong T, Hu C, Lu J, Dai J, Liu P. Salinomycin repressed the epithelial-mesenchymal transition of epithelial ovarian cancer cells via downregulating Wnt/beta-catenin pathway. OncoTargets Ther 2017; 10:1317-25; PMID:28280366; https://doi.org/10.2147/OTT.S126463
