ABSTRACT
p53R2 is a p53-inducible ribonucleotide reductase subunit involved in deoxyribonucleotide biosynthesis and DNA repair. Although p53R2 has been linked to human cancer, its role in cervical cancer remains unknown. In this study, we investigated the expression and clinical significance of p53R2 in early-stage cervical cancer. p53R2 expression is significantly upregulated at both mRNA and protein levels in cervical cancer cells and tissues, compared with that in matched normal cervical cells and tissues, respectively. p53R2 overexpression is associated with increased risk of pelvic lymph node metastasis (PLNM, p = 0.001) and cancer relapse (p = 0.009). Patients with high p53R2 expression have a shorter overall survival (OS) and disease-free survival (DFS). p53R2 is an independent factor for predicting OS and DFS of cervical cancer patients. We further show that p53R2 is important for oncogenic growth, migration and invasion in cervical cancer cells. Mechanistically, p53R2 promotes Akt signaling and epithelial–mesenchymal transition (EMT). In conclusion, our study demonstrates for the first time that p53R2 protein is overexpressed in early-stage cervical cancer and unravels some unconventional oncogenic functions of p53R2. p53R2 may be a useful prognostic biomarker and therapeutic target for cervical cancer.
Introduction
Cervical cancer is the fourth most frequent cancer among women worldwide. In 2012, there was an estimated 527,600 new cases and 265,700 deaths from cervical cancer in the world.Citation1 Treatment options for cervical cancer include surgery, chemotherapy, radiation therapy or a combination of these strategies. Although the clinical outcome for early stages of cervical cancer is excellent after treatments, the survival rate is low for late stage diseases even with treatment.Citation2 Therefore, it is necessary to identify reliable biomarkers for prognosis and new therapeutic targets of cervical cancer.
Ribonucleotide reductase (RNR) catalyzes the conversion of ribonucleotides into 2′-deoxyribonucleotides (dNDPs), which is important for DNA synthesis and repair.Citation3 Human RNR consists of 3 subunits, the large subunit M1 (RRM1) and 2 small subunits M2 (RRM2) and M2B (p53R2). RRM1 is the catalytic subunit consisting of the catalytic site, 2 allosteric effector-binding sites, and redox-active disulfides that participate in the reduction of substrates. RRM2 and p53R2 are the regulatory subunits containing the diferric tyrosyl radical cofactor required for the enzyme activity. RRM1 interacts with either RRM2 or p53R2 to become the catalytically active form of eukaryotic RNR.Citation3-8
Consistent with a vital role of RNR in DNA synthesis and cell proliferation, dysregulation of RNR has been reported to be involved in tumorigenesis of several types of cancers. However, the 3 subunits of RNR seem to play different roles in different cancers. RRM1 appears to act as a tumor suppressor that inhibits the progression and metastasis of many cancers.Citation9-15 For example, in human colorectal cancer (CRC), ectopic overexpression of RRM1 prevents the migration and invasion of CRC cells by promoting PTEN expression.Citation9 On the other hand, RRM2 is oncogenic by promoting cancer cell proliferation.Citation16-20 A high level of RRM2 expression is reported to correlate with tumor growth,Citation17 invasiveness and metastasis,Citation18 tumor angiogenesisCitation19 and poor outcome.Citation21
Although p53R2 and RRM2 share 80% amino acid sequence similarity, they have distinct functions in human cancers.Citation21 p53R2 is a direct target of the tumor suppressor p53 and is involved in DNA replication and repair, cell cycle and mitochondrial homeostasis. p53R2 overexpression promotes the accumulation of p21, G1 or G2 arrest and DNA repair.Citation22-24 It appears to have a complex role in human cancer. In CRC cancer cells, high expression of p53R2 is negatively correlated with cancer cell proliferation, invasion and metastasis.Citation25,26 Disruption of the p53R2-mediated DNA repair in ulcerative colitis is implicated in the initiation of colon tumorigenesis.Citation27 In liver cancer, overexpression of p53R2 suppresses cell migration and spreading by modulating the Egr-1/PTEN/Akt1 pathway.Citation28 On the other hand, p53R2 appears to act as an oncogene with elevated expression in lung cancer and esophageal squamous cell carcinoma, and is associated with the progression of these cancers.Citation22,29,30 Furthermore, downregulation of p53R2 by siRNA or mutation inhibits cancer cell growth and sensitizes cancer cells to anticancer agents or ionizing radiation.Citation31-33
To date, the role of p53R2 in cervical cancer has not been studied. Because of its important role in tumorigenesis and therapy, we investigated the expression and clinical significance of p53R2 in a large cohort of human cervical cancer samples, and explored its biologic functions and potential regulatory mechanism in cervical cancer models.
Results
p53R2 is overexpressed in cervical cancer
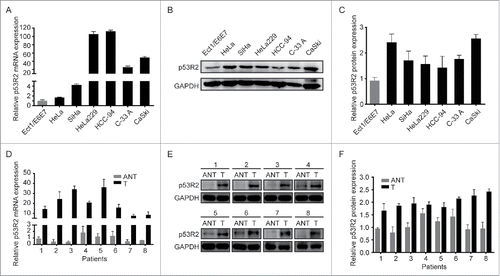
To investigate the potential role of p53R2 in cervical cancer, we analyzed p53R2 expression in immortalized normal cervical cell line Ect1/E6E7 and cervical cancer cell lines including HeLa, SiHa, HeLa229, HCC-94, C-33 A and CaSki. Both p53R2 mRNA and protein levels were higher in cancer than the immortalized cells (). Consistently, both p53R2 mRNA and protein expression were also much higher in human cervical tumors compared with the paired adjacent noncancerous tissues, as detected by qRT-PCR and Western Blot, respectively (). Interestingly, the increase in p53R2 mRNA was much greater than that in p53R2 protein, suggesting that p53R2 expression is differentially regulated at the transcriptional and translational levels.
Figure 1. p53R2 expression is elevated in cervical cancer cell lines and tissues. (A) Relative p53R2 mRNA expression in normal cervical cell and cervical cancer cell lines. (B) Western blotting analysis of p53R2 protein expression in immortalized cervical cell and cervical cancer cell lines. (C) Quantification of results from . (D) Relative p53R2 mRNA expression level in 8 pairs of matched cervical cancer tissues (T) and adjacent noncancerous cervical tissues (ANT). (E) Western blotting analysis of p53R2 protein expression in 8 pairs of matched cervical cancer tissues (T) and adjacent noncancerous cervical tissues (ANT). (F) Quantification of results from . The expression of p53R2 was normalized against GAPDH.
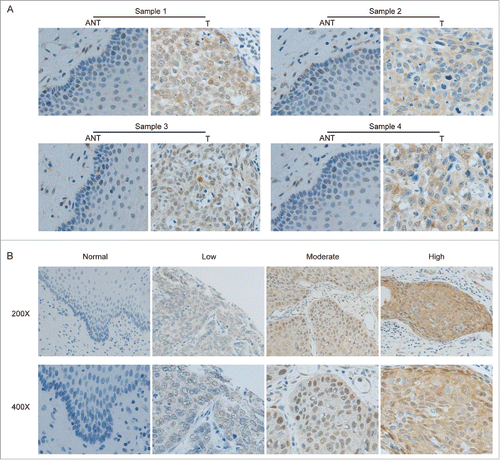
To further explore the clinical significance of p53R2 overexpression in cervical cancer, we performed IHC staining for p53R2 expression in 275 paraffin-embedded cervical tumor samples. p53R2 was highly expressed in over half of the patients (58.5%, 161/275; see Material and Methods for the IHC score criteria). In contrast, p53R2 was not detectable or marginally detectable in the normal cervical tissues or the areas surrounding the cancerous tissues (). There was considerable variability in the level of p53R2 among different tissue samples, ranging from negative, low, moderate, to high IHC staining (). Interestingly, p53R2 was distributed in both the cytoplasm and nucleus (), and in some instances, prominently concentrated in the nuclei of tumor tissues (e.g. , moderate panel). The nuclear p53R2 nuclear localization was previously observed, but the significance remains largely unknown.Citation25
Figure 2. Immunohistochemistry (IHC) staining of p53R2 in matched cervical cancer tissues (T) and adjacent noncancerous cervical tissues (ANT). (A) Representative IHC staining of p53R2 in matched cervical cancer tissues (T) and adjacent noncancerous cervical tissues (ANT). (B) IHC staining of p53R2 expression in adjacent normal cervical tissues and cervical cancer tissues with different staining intensity.
p53R2 overexpression is associated with tumor progression, metastasis and poor survival
To evaluate the clinical significance of p53R2, we investigated the relationship between p53R2 expression and clinicopathological features in cervical cancer. p53R2 overexpression was significantly associated with pelvic lymph node metastasis (PLNM, p = 0.001) and cancer relapse (p = 0.009) (). A log-rank test and Kaplan-Meier analysis were used to calculate the effect of p53R2 on cervical cancer patients' survival. Patients with high p53R2 expression had a significantly shorter overall survival (OS) (HR 2.727, 95% CI 1.190–6.246, p = 0.018) and disease free survival (DFS) (HR 2.458, 95% CI 1.358–4.451, p = 0.003) than those with low p53R2 expression (). In our study cohort, 207 patients received adjuvant chemoradiotherapy after radical hysterectomy and 68 patients did not. Higher p53R2 expression was also significantly correlated with a shorter OS (HR 2.709, 95% CI 1.081–6.794, p = 0.034) and a shorter DFS (HR 2.517, 95% CI 1.294–4.894, p = 0.007) in this subgroup of patients with adjuvant therapy ( and ). Additionally, multivariate analysis indicated that p53R2 expression is an independent prognostic factor for OS () and DFS () in the patients.
Table 1. Correlation between p53R2 expression and clinicopathological characteristics in cervical cancer patients.
Figure 3. Kaplan–Meier analysis of cervical cancer patients' survival rates in association with p53R2 expression. (A) and (B). Overall survival rate (OS) and Disease free survival rate (DFS) in 275 patients with high or low p53R2 expression. (C) and (D). Overall survival rate (OS) and Disease free survival rate (DFS) in patient subgroups with or without adjuvant therapy.
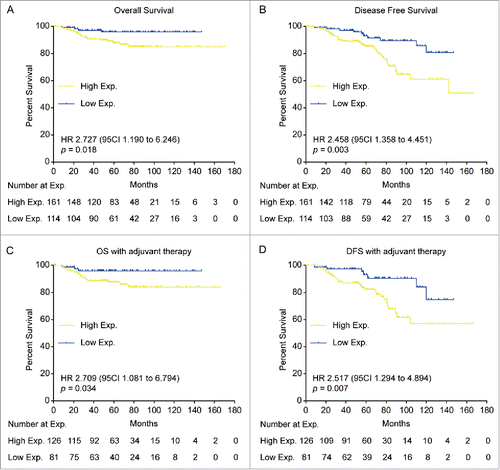
Table 2. Univariate and multivariate analysis of p53R2 expression and clinicopathological features on the overall survival in cervical cancer patients.
Table 3. Univariate and multivariate analysis of the effects of P53R2 expression and clinicopathological features on disease free survival in patients with cervical cancer.
p53R2 is crucial for the proliferation of cervical cancer cells
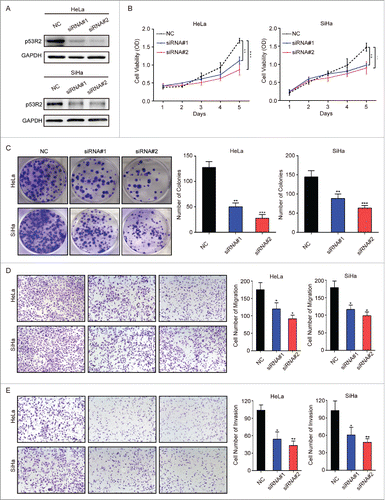
p53R2 is overexpressed in cervical cancer, which is correlated with tumor progression and poor survival, suggesting that p53R2 is important for this cancer. To understand the role of p53R2, we used previously validated siRNAsCitation34 to knock down p53R2 in HeLa and SiHa, 2 cervical cancer cell lines with high p53R2 protein expression. p53R2-specific siRNAs resulted in significant reduction in p53R2 expression as determined by Western blot (). Down-regulation of p53R2 markedly inhibited the growth of these cells (). Consistently, p53R2 knockdown also attenuated colony formation of these cervical cancer cells, as indicated by the decrease in colony size and number (). These results demonstrate that p53R2 plays a vital role in promoting cell growth and proliferation of cervical cancer cells.
Figure 4. p53R2 is important for the growth, proliferation, migration and invasion of cervical cancer cells. (A) HeLa and SiHa cells were transfected with siRNAs and analyze for p53R2 expression by Western blot. (B) The growth of HeLa and SiHa cells transfected with siRNAs was measured by the MTS assay. (C) HeLa and SiHa cells transfected with siRNAs was measured for their ability to form colonies. (D) HeLa and SiHa cells transfected with siRNAs was measured for their mobility by the transwell migration assay. (E) HeLa and SiHa cells transfected with siRNAs was measured for their invasive ability by the transwell invasion assay. NC: cells transfected with scramble siRNAs; siRNA#1 and #2: cells transfected with 2 human siRNA sequences to repress p53R2 expression, respectively. *p < 0.05, **p < 0.01, ***p < 0.001.
p53R2 is required for the migration and invasion of cervical cancer cells
Our study of clinical samples indicates that p53R2 overexpression is associated with metastasis. To verify this, we used the transwell assay to assess the role of p53R2 in cervical cancer cell migration and invasion. p53R2 knockdown by siRNAs in HeLa and Siha cells attenuated the migration and invasion of cervical cancer cells ( and ). These results show that p53R2 is important for the motility and invasiveness of cervical cancer cells.
Down-regulation of p53R2 leads to inhibition of Akt signaling and reversal of epithelial-mesenchymal transition (EMT)
PI3K-Akt signaling pathway is frequently deregulated in human cancer, including cervical cancer.Citation35-39 Aberrant activation of the PI3K-Akt signaling contributes to cervical cancer cell proliferation, survival and angiogenesis.Citation40 To investigate the mechanism by which p53R2 promote cervical cancer cell growth and proliferation, we examined the effect of p53R2 knockdown on Akt signaling. Down-regulation of p53R2 expression inhibited Akt phosphorylation, but not Akt protein expression (), suggesting that p53R2 promotes Akt signaling in cervical cancer cells. PTEN is an inhibitor of the PI3K/Akt pathway by antagonizing the PI3K action upstream of Akt.Citation28,41 Interestingly, p53R2 knockdown leads to upregulation of PTEN expression, suggesting that p53R2 blocks Akt signaling through PTEN ().
Figure 5. p53R2 promotes Akt signaling and reversed EMT in cervical cancer cells. (A) Western blotting analysis of the expression of p53R2, P-Akt, Akt and PTEN. (B) The expression of EMT markers as determined by Western blot. NC: cells transfected with scramble siRNAs; siRNA#1 and #2: cells transfected with 2 human siRNA sequences to repress p53R2 expression, respectively.
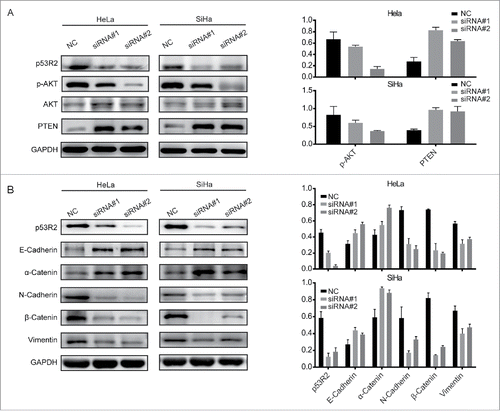
The PI3K/Akt pathway plays an important role in EMT,Citation42 which stimulates cancer cell invasion and metastasis through reduced cell adhesion and enhanced cancer cell dissemination. The key characteristic of EMT is the loss of epithelial markers such as E-cadherin and α-catenin, and the increase of mesenchymal markers such as N-cadherin, vimentin and β-catenin. To investigate whether p53R2 regulates the EMT process, we examined the expression of EMT markers in HeLa and SiHa cells after p53R2 knockdown. The loss of p53R2 decreased the expression of mesenchymal markers N-cadherin, β-catenin and vimentin, while increased the expression of epithelial markers E-cadherin and a-catenin (). Together, these results show that p53R2 activates Akt signaling and EMT.
Discussion
The role of p53R2 in human cancer is complex, which is dependent on the tumor type and stages.Citation43,44 While it has been reported to be a tumor suppressor in certain cancers such as human colon adenocarcinoma and lung cancer, it appears to act as an oncogene in other cancers such as esophageal squamous cell carcinoma and melanoma. Previous studies primarily focused on the DNA repair functions using in vitro cell models. The clinical significance of p53R2 is largely unknown. Only a small cohort of human non-small cell lung cancer (NSCLC) has been previously investigated.Citation22 In this study, we investigated the potential role of p53R2 in cervical cancer and found that p53R2 expression is upregulated in cervical cancer and that overexpression of p53R2 was significantly correlated with pelvic lymph node metastasis (PLNM, p = 0.001) and relapse (p = 0.009). Multivariate and univariate analysis demonstrate that high p53R2 expression is an independent prognostic factor for poor OS and DFS in patients with early-stage cervical cancer, indicating that p53R2 is a potentially useful biomarker for cervical cancer.
p53R2 is essential for cervical cancer cell proliferation, suggesting that it is a potential therapeutic target. Consistently, it was reported that pharmacological inhibition of RNR with 3-aminopyridine-2-carboxaldehyde thiosemicarbazone significantly enhances radiation-related cytotoxicity in cervical and colon cancer cells in a p53-independent mechanism,Citation45 and that down-regulating p53R2 by siRNA also increases radiosensitivity in esophageal squamous cell carcinoma.Citation30 It was shown that silencing p53R2 expression sensitizes CRC cancer cells to undergo apoptotic cell death in the presence of DNA damage agent.Citation46 These findings indicate that targeting p53R2 could enhance the effectiveness of ionizing radiation or DNA-damaging chemotherapy. Interestingly, in some tumors, p53R2 nuclear localization is much stronger than the cytoplasmic localization (). A previous study shows that p53R2 is normally localized in the cytoplasm of several cancer cell lines, but becomes enriched in the nucleus upon radiation-induced DNA damage.Citation24,47 These studies suggest that nuclear localization of p53R2 directs localized reduction of ribonucleotides to generate deoxyribonucleotides that are important for supporting DNA repair. It will be interesting to determine in the future studies whether nuclear enrichment of p53R2 is associated with radiation and/or chemotherapy resistance.
Although p53R2 is clearly important for DNA repair, there are some evidence that p53R2 is involved in growth regulation. For example, p53R2 knockout mice show growth retardation.Citation48 However, such non-DNA repair functions remain obscure. We have investigated the biologic function and mechanism of p53R2 in cervical cancer. Here we show that p53R2 promotes cervical cancer through at least 2 distinct mechanisms. First, p53R2 stimulates PI3K-Akt signaling as evidenced by the dependency of Akt phosphorylation and repression of PTEN expression on p53R2. Interestingly, Akt phosphorylation is also regulated by p53R2 in human chondrocytes in response to mechanical stress,Citation49 suggesting that p53R2-dependent regulation of Akt is a common mechanism to control cell growth and survival. Second, p53R2 enhances EMT process. EMT is known to initiate the invasiveness of cancer cells, causing the primary tumor to lose cell-cell adhesion through E-cadherin repression. This provides a mechanistic explanation for increased metastasis in high p53R2 expressing cervical cancer patients. In conclusion, we demonstrate that p53R2 is overexpressed in cervical cancer, and meanwhile it may be a potential prognostic biomarker of cervical cancer. Inhibition of p53R2 may improve the treatment effect of cervical cancer. p53R2 is a potential novel molecular target for cervical cancer therapy.
Materials and methods
Clinical samples
Current study used tumor samples surgically removed from 275 early-stage cervical cancer patients who received radical hysterectomy and lymphadenectomy in the Department of Gynecologic Oncology in Sun Yat-sen University Cancer Center (SYSUCC, Guangzhou, China) enrolled between January 2000 to December 2010. They were pathologically diagnosed as cervical cancer without radiotherapy, chemotherapy, or hormonal therapy before surgery. The clinical staging and pathological grading were determined according to the 2009 FIGO criteria. Clinical/pathological features of the cervical cancer cohort are summarized in . 60 samples of paired adjacent noncancerous cervical tissues or chronic cervicitis tissues from hysteromyoma patients were obtained as controls by simple hysterectomy. 15 pairs of fresh cervical cancer tissues and paired adjacent noncancerous cervical tissues were obtained from the Department of Tumor Resource Library of SYSUCC for qPCR and Western blotting analysis. All the paraffin embedded tissues and fresh tissues were taken obtained the written informed consent of each patient.
This study was approved by the Institutional Review Boards of SYSUCC. All the patients were followed up regularly with the last follow-up in November 2016. Overall survival (OS) is defined as the time from surgery to the date of cervical cancer-associated death or last follow-up. Disease-free survival (DFS) is calculated from the date of surgery to the date of first cervical cancer-associated relapse, metastasis, death or the last follow-up.
Cell lines and antibodies
Immortalized human normal cervical cell line Ect1/E6E7 and cervical cancer cell lines HeLa, SiHa, HeLa229 and C-33A were cultured in Dulbecco's Modified Eagle Medium (GIBCO BRL). CaSki and HCC-94 were cultured in RPMI 1640 medium (GIBCO BRL). All cultures were supplemented with 10% fetal bovine serum (GIBCO BRL). Cell cultures were maintained in a humidified 95% air/5% CO2 atmosphere at 37°C. Rabbit anti-p53R2 (#ab154194) was purchased from Abcam; antibody for Akt (#4691), p-Akt(#4060), PTEN (#9188) and GAPDH (#5174) were obtained from Cell Signaling Technology. Mouse Anti-E-cadherin (#610181), N-cadherin (#610921), α-catenin (#610194), β-catenin (#610154) and vimentin (#550513) were purchased from BD Biosciences.
Immunohistochemistry (IHC) staining
IHC staining was performed on 4 μm thick paraffin-embedded tissue sections using rabbit anti-p53R2 antibody (1:750 dilution, #ab8105, Abcam) as previously reported.Citation34 Tissue sections omitting the primary antibody were used as the negative control. Two independent pathologists, blinded to the histopathological features and patients' data of the samples, scored the IHC staining intensity based on the percentage of positive tumor cells multiplied by the intensity of staining. The percentage of positive tumor cells in the sections was scored as 1 (< 10%), 2 (11–50%), 3 (51–75%) and 4 (> 75%). The staining intensity was graded as 0 (no staining), 1 (weak), 2 (moderate) or 3 (strong). The final immunostaining score for each section ranged from 0 to 12. Tissue sections scored 0–3 were considered as low expression, and 4–12 was considered as high expression.
RNA preparation and quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
Total RNA from cultured cells and fresh tissues were extracted using the Trizol reagent (15596018, Invitrogen) according to the manufacturer's instruction. Two-step RT-qPCR was performed to assess p53R2 mRNA level as described previously.Citation34 GAPDH was used as the internal control and all qRT-PCR reactions were done in triplicate. The primer sequences used as follows: p53R2, 5′-AGGAGGTGCAGGTTCCAGAG-3′ (forward) and 5′- CTGCTATCCATCGCAAGGC-3′ (reverse); GAPDH, 5′-TGCACCACCAACTGCTTAGC-3′ (forward) and 5′-GGCATGGACTGTGGTCATGAG-3′ (reverse). Relative mRNA expression level was presented by the 2−ΔΔCT method.
Transfection of p53R2 siRNA
siRNA transfection was performed as described previously.Citation34 Briefly, cervical cancer cells were grown to 40–60% confluence, and then were incubated with siRNAs at a final concentration of 0.1μM using LipofectamineTM 3000 (L3000015, Invitrogen) according to the manufacturer's protocol. Silencer select siRNAs against p53R2 were purchased from Gene Pharma. The siRNA sequences are as follows: siRNA-1, 5′-GUACCCUGAUAUUUGGAAATT-3′; siRNA-2, 5′-GAGAUGUACAGUUUGCUGATT-3′; The negative control siRNA, 5′-UUCUCCGAACGUGUCACGUTT-3′.
Proliferation assay
Cell proliferation was assessed by the MTS method using the CellTiter 96® AQueous One Solution Cell Proliferation Assay kit (G3580, Promega) according to the manufacturer's instructions. Briefly, cells were plated in 96-well plates at a concentration of 2000 cells per well in 200μL of culture medium. Each plate was taken every 24 h for the MTS assay. The absorbance was measured at 490 nm using a multifunctional microplate reader.
Colony formation assay
Cells transfected with p53R2 siRNAs or a control siRNA were plated in a fresh 6-well plate (1000 cells/well) and maintained in culture medium containing 10% FBS for 2 wk until visible clones were formed. Colonies were fixed with 4% paraformaldehyde for 10 min and stained with 0.1% crystal violet solution for 30 min. After washing with PBS, the number of colonies (> 50 cells/colony) was counted with a microscope.
Cell migration and invasion assays
Transwell migration and invasion assays were performed in Boyden chambers without (for migration) or with (for invasion) Matrigel according to the manufacturer's protocol (Invitrogen). Briefly, 5 × 104 cells in 250μL serum-free medium were seeded in the upper chamber. 750μL medium with 10% FBS was added to the lower chamber in a 24 well plate. The plates were then incubated at 37°C in a 5% CO2 incubator for 24h. The migrated or invaded cells through the pores were fixed and stained using 0.1% crystal violet, and counted in 5 random microscopic fields. Each experiment was done in triplicate.
Protein extracts and western blotting
Tumor cells or tissues were lysed using RIPA buffer. Protein concentrations were determined using the BCA Protein Assay Kit (KGP902, KeyGEN BioTECH) according to the manufacturer's instructions. Equal amount of protein lysates were mixed with the loading buffer, resolved on SDS-PAGE gels and transferred to PVDF membranes (Millipore). After incubation with a specific primary antibody at 4°C overnight and then a secondary antibody, the signals were detected using an ECL Kit (35055, Thermo Scientific). The Western blot signal intensity was quantified using image analysis software (Image-J, ver. 1.44; NIH) and normalized by the GAPDH intensity.
Statistical analysis
All statistical analysis was performed using the SPSS statistical software program (Version 17.0 SPSS Inc.) and GraphPad Prism 6.0 Software. Paired Student's t test was used to analyze the difference in mRNA and protein expression between cervical cancer and paired adjacent noncancerous cervical tissues. The relationship between p53R2 expression and clinicopathological features was assessed using Chi-square test and Fisher's exact tests. Survival curves were calculated using the Kaplan-Meier method, and differences were assessed using a log-rank test. Univariable and multivariable survival analysis was performed using the Cox regression analysis to analyze the prognostic factors. The criterion for statistical significance was p < 0.05.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National Institutes of Health R01 grants (No: CA123391), the National Natural Science Foundation of China (No: 81572440 and 81372600), the Recruitment Program of Global Experts, the Leading Talent of Guangdong Province, the Natural Science Foundation of Guangdong Province for Distinguished Young Scholar (No: 2015A030306047) and the Research Fund of State Key Laboratory of Oncology in South China.
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: A Cancer J Clin 2015; 65:87-108.
- Boussios S, Seraj E, Zarkavelis G, Petrakis D, Kollas A, Kafantari A, Assi A, Tatsi K, Pavlidis N, Pentheroudakis G. Management of patients with recurrent/advanced cervical cancer beyond first line platinum regimens: Where do we stand? A literature review. Crit Rev Oncol Hematol 2016; 108:164-74; PMID:27931835; https://doi.org/10.1016/j.critrevonc.2016.11.006
- Nordlund P, Reichard P. Ribonucleotide reductases. Annu Rev Biochem 2006; 75:681-706; PMID:16756507; https://doi.org/10.1146/annurev.biochem.75.103004.142443
- Thelander L, Reichard P. Reduction of ribonucleotides. Annu Rev Biochem 1979; 48:133-58; PMID:382982; https://doi.org/10.1146/annurev.bi.48.070179.001025
- Eklund H, Uhlin U, Farnegardh M, Logan DT, Nordlund P. Structure and function of the radical enzyme ribonucleotide reductase. Prog Bio Mol Biol 2001; 77:177-268; PMID:11796141; https://doi.org/10.1016/S0079-6107(01)00014-1
- Zhou B, Shao J, Su L, Yuan YC, Qi C, Shih J, Xi B, Chu B, Yen Y. A dityrosyl-diiron radical cofactor center is essential for human ribonucleotide reductases. Mol Cancer Ther 2005; 4:1830-6; PMID:16373698; https://doi.org/10.1158/1535-7163.MCT-05-0273
- Cooperman BS, Kashlan OB. A comprehensive model for the allosteric regulation of Class Ia ribonucleotide reductases. Advances in Enzyme Regulation 2003; 43:167-82; PMID:12791390; https://doi.org/10.1016/S0065-2571(02)00035-3
- Kolberg M, Strand KR, Graff P, Andersson KK. Structure, function, and mechanism of ribonucleotide reductases. Biochim Biophys Acta 2004; 1699:1-34; PMID:15158709; https://doi.org/10.1016/S1570-9639(04)00054-8
- Qi H, Lou M, Chen Y, Liu X, Chen N, Shan J, Ling Z, Shen J, Zhu L, Yen Y, et al. Non-enzymatic action of RRM1 protein upregulates PTEN leading to inhibition of colorectal cancer metastasis. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 2015; 36:4833-42; PMID:25638032; https://doi.org/10.1007/s13277-015-3137-4
- Fan H, Huang A, Villegas C, Wright JA. The R1 component of mammalian ribonucleotide reductase has malignancy-suppressing activity as demonstrated by gene transfer experiments. Proceedings of the National Academy of Sciences of the United States of America 1997; 94:13181-6; PMID:9371820; https://doi.org/10.1073/pnas.94.24.13181
- Fan H, Villegas C, Wright JA. Ribonucleotide reductase R2 component is a novel malignancy determinant that cooperates with activated oncogenes to determine transformation and malignant potential. Proc Nat Acad Sci U S A 1996; 93:14036-40; PMID:8943056; https://doi.org/10.1073/pnas.93.24.14036
- Gautam A, Li Z-R, Bepler G. RRM1-induced metastasis suppression through PTEN-regulated pathways. Oncogene 2003; 22:2135-42; PMID:12687015; https://doi.org/10.1038/sj.onc.1206232
- Wang L, Meng L, Wang XW, Ma GY, Chen JH. Expression of RRM1 and RRM2 as a novel prognostic marker in advanced non-small cell lung cancer receiving chemotherapy. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 2014; 35:1899-906; PMID:24155212; https://doi.org/10.1007/s13277-013-1255-4
- Zhang Q, Sun T, Kang P, Qian K, Deng B, Zhou J, Wang R, Jiang B, Li K, Liu F, et al. Combined analysis of rearrangement of ALK, ROS1, somatic mutation of EGFR, KRAS, BRAF, PIK3CA, and mRNA expression of ERCC1, TYMS, RRM1, TUBB3, EGFR in patients with non-small cell lung cancer and their clinical significance. Cancer Chemother Pharmacol 2016; 77:583-93; PMID:26842788; https://doi.org/10.1007/s00280-016-2969-y
- Zimling ZG, Santoni-Rugiu E, Bech C, Sorensen JB. High RRM1 Expression Is Associated with Adverse Outcome in Patients with Cisplatin/Vinorelbine-treated Malignant Pleural Mesothelioma. Anticancer Res 2015; 35:6731-8; PMID:26637889
- Lopez-Contreras AJ, Specks J, Barlow JH, Ambrogio C, Desler C, Vikingsson S, Rodrigo-Perez S, Green H, Rasmussen LJ, Murga M, et al. Increased Rrm2 gene dosage reduces fragile site breakage and prolongs survival of ATR mutant mice. Genes & development 2015; 29:690-5; https://doi.org/10.1101/gad.256958.114
- Yoshida Y, Tsunoda T, Doi K, Tanaka Y, Fujimoto T, Machida T, Ota T, Koyanagi M, Takashima Y, Sasazuki T, et al. KRAS-mediated up-regulation of RRM2 expression is essential for the proliferation of colorectal cancer cell lines. Anticancer Res 2011; 31:2535-9; PMID:21873171
- Liu X, Zhou B, Xue L, Yen F, Chu P, Un F, Yen Y. Ribonucleotide reductase subunits M2 and p53R2 are potential biomarkers for metastasis of colon cancer. Clin Colorectal Cancer 2007; 6:374-81; PMID:17311703; https://doi.org/10.3816/CCC.2007.n.007
- Zhang K, Hu S, Wu J, Chen L, Lu J, Wang X, Liu X, Zhou B, Yen Y. Overexpression of RRM2 decreases thrombspondin-1 and increases VEGF production in human cancer cells in vitro and in vivo: implication of RRM2 in angiogenesis. Molecular cancer 2009; 8:11; PMID:19250552; https://doi.org/10.1186/1476-4598-8-11
- Zhong Z, Cao Y, Yang S, Zhang S. Overexpression of RRM2 in gastric cancer cell promotes their invasiveness via AKT/NF-kappaB signaling pathway. Die Pharmazie 2016; 71:280-4; PMID:27348973
- Farquhar J, Savarino J, Jackson TL, Thiemens MH. Evidence of atmospheric sulphur in the martian regolith from sulphur isotopes in meteorites. Nature 2000; 404:50-2; PMID:10716436; https://doi.org/10.1038/35003517
- Hsu NY, Wu JY, Liu X, Yen Y, Chen CY, Chou MC, Lin CH, Lee H, Cheng YW. Expression status of ribonucleotide reductase small subunits hRRM2/p53R2 as prognostic biomarkers in stage I and II non-small cell lung cancer. Anticancer Res 2011; 31:3475-81; PMID:21965764
- Nakano K, Balint E, Ashcroft M, Vousden KH. A ribonucleotide reductase gene is a transcriptional target of p53 and p73. Oncogene 2000; 19:4283-9; PMID:10980602; https://doi.org/10.1038/sj.onc.1203774
- Yamaguchi T, Matsuda K, Sagiya Y, Iwadate M, Fujino MA, Nakamura Y, Arakawa H. p53R2-dependent pathway for DNA synthesis in a p53-regulated cell cycle checkpoint. Cancer Res 2001; 61:8256-62; PMID:11719458
- Liu X, Lai L, Wang X, Xue L, Leora S, Wu J, Hu S, Zhang K, Kuo ML, Zhou L, et al. Ribonucleotide reductase small subunit M2B prognoses better survival in colorectal cancer. Cancer Res 2011; 71:3202-13; PMID:21415168; https://doi.org/10.1158/0008-5472.CAN-11-0054
- Liu X, Zhou B, Xue L, Shih J, Tye K, Lin W, Qi C, Chu P, Un F, Wen W, et al. Metastasis-suppressing potential of ribonucleotide reductase small subunit p53R2 in human cancer cells. Clinical cancer research : an official journal of the American Association for Cancer Research 2006; 12:6337-44; PMID:17085643; https://doi.org/10.1158/1078-0432.CCR-06-0799
- Yoshida T, Haga S, Numata Y, Yamashita K, Mikami T, Ogawa T, Ohkusa T, Okayasu I. Disruption of the p53-p53r2 DNA repair system in ulcerative colitis contributes to colon tumorigenesis. International journal of cancer 2006; 118:1395-403; PMID:16206288; https://doi.org/10.1002/ijc.21538
- Tian H, Ge C, Li H, Zhao F, Hou H, Chen T, Jiang G, Xie H, Cui Y, Yao M, et al. Ribonucleotide reductase M2B inhibits cell migration and spreading by early growth response protein 1-mediated phosphatase and tensin homolog/Akt1 pathway in hepatocellular carcinoma. Hepatology 2014; 59:1459-70; PMID:24214128; https://doi.org/10.1002/hep.26929
- Liu X, Zhang H, Lai L, Wang X, Loera S, Xue L, He H, Zhang K, Hu S, Huang Y, et al. Ribonucleotide reductase small subunit M2 serves as a prognostic biomarker and predicts poor survival of colorectal cancers. Clini Sci 2013; 124:567-78; PMID:23113760; https://doi.org/10.1042/CS20120240
- Okumura H, Natsugoe S, Yokomakura N, Kita Y, Matsumoto M, Uchikado Y, Setoyama T, Owaki T, Ishigami S, Aikou T. Expression of p53R2 is related to prognosis in patients with esophageal squamous cell carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research 2006; 12:3740-5; PMID:16778101; https://doi.org/10.1158/1078-0432.CCR-05-2416
- Yokomakura N, Natsugoe S, Okumura H, Ikeda R, Uchikado Y, Mataki Y, Takatori H, Matsumoto M, Owaki T, Ishigami S, et al. Improvement in radiosensitivity using small interfering RNA targeting p53R2 in esophageal squamous cell carcinoma. Oncology reports 2007; 18:561-7; PMID:17671702
- Yanamoto S, Iwamoto T, Kawasaki G, Yoshitomi I, Baba N, Mizuno A. Silencing of the p53R2 gene by RNA interference inhibits growth and enhances 5-fluorouracil sensitivity of oral cancer cells. Cancer Lett 2005; 223:67-76; PMID:15890238; https://doi.org/10.1016/j.canlet.2004.10.019
- Matsushita S, Ikeda R, Fukushige T, Tajitsu Y, Gunshin K, Okumura H, Ushiyama M, Akiyama S, Kawai K, Takeda Y, et al. p53R2 is a prognostic factor of melanoma and regulates proliferation and chemosensitivity of melanoma cells. J Dermatol Sci 2012; 68:19-24; PMID:22902076; https://doi.org/10.1016/j.jdermsci.2012.07.005
- Xu R, Hu JY, Zhang TS, Jiang C, Wang HY. TRIM29 overexpression is associated with poor prognosis and promotes tumor progression by activating wnt/beta-catenin pathway in cervical cancer. Oncotarget 2016; 7:28579-91; PMID:27081037
- Zhou Q, Lui VW, Yeo W. Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Future oncology 2011; 7:1149-67; PMID:21992728; https://doi.org/10.2217/fon.11.95
- Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan MB, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature 2008; 455:1069-75; PMID:18948947; https://doi.org/10.1038/nature07423
- Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 2004; 304:554; PMID:15016963; https://doi.org/10.1126/science.1096502
- Thomas RK, Baker AC, DeBiasi RM, Winckler W, LaFramboise T, Lin WM, Wang M, Feng W, Zander T, MacConnaill LE, et al. High-throughput oncogene mutation profiling in human cancer. Nature Genet 2007; 39:347-51; PMID:17293865; https://doi.org/10.1038/ng1975
- Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, et al. The genomic landscapes of human breast and colorectal cancers. Science 2007; 318:1108-13; PMID:17932254; https://doi.org/10.1126/science.1145720
- Mundi PS, Sachdev J, McCourt C, Kalinsky K. AKT in cancer: new molecular insights and advances in drug development. British J Clin Pharmacol 2016; 82:943-56; PMID:27232857; https://doi.org/10.1111/bcp.13021
- Horie Y, Suzuki A, Kataoka E, Sasaki T, Hamada K, Sasaki J, Mizuno K, Hasegawa G, Kishimoto H, Iizuka M, et al. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clini Invest 2004; 113:1774-83; PMID:15199412; https://doi.org/10.1172/JCI20513
- Larue L, Bellacosa A. Epithelial–mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene 2005; 24:7443-54; PMID:16288291; https://doi.org/10.1038/sj.onc.1209091
- Wang X, Zhenchuk A, Wiman KG, Albertioni F. Regulation of p53R2 and its role as potential target for cancer therapy. Cancer letters 2009; 276:1-7; PMID:18760875; https://doi.org/10.1016/j.canlet.2008.07.019
- Yousefi B, Rahmati M, Ahmadi Y. The roles of p53R2 in cancer progression based on the new function of mutant p53 and cytoplasmic p21. Life Sci 2014; 99:14-7; PMID:24486301; https://doi.org/10.1016/j.lfs.2014.01.063
- Kunos CA, Chiu SM, Pink J, Kinsella TJ. Modulating radiation resistance by inhibiting ribonucleotide reductase in cancers with virally or mutationally silenced p53 protein. Radiat Res 2009; 172:666-76; PMID:19929413; https://doi.org/10.1667/RR1858.1
- Qi JJ, Liu L, Cao JX, An GS, Li SY, Li G, Jia HT, Ni JH. E2F1 regulates p53R2 gene expression in p53-deficient cells. Mol Cell Biochem 2015; 399:179-88; PMID:25312903; https://doi.org/10.1007/s11010-014-2244-7
- Xue L, Zhou B, Liu X, Qiu W, Jin Z, Yen Y. Wild-Type p53 Regulates Human Ribonucleotide Reductase by Protein-Protein Interaction with p53R2 as well as hRRM2 Subunits. Cancer Res 2003; 63:980-6; PMID:12615712
- Kimura T, Takeda S, Sagiya Y, Gotoh M, Nakamura Y, Arakawa H. Impaired function of p53R2 in Rrm2b-null mice causes severe renal failure through attenuation of dNTP pools. Nat Genet 2003; 34:440-5; PMID:12858174; https://doi.org/10.1038/ng1212
- Kawakita K, Nishiyama T, Fujishiro T, Hayashi S, Kanzaki N, Hashimoto S, Takebe K, Iwasa K, Sakata S, Nishida K, et al. Akt phosphorylation in human chondrocytes is regulated by p53R2 in response to mechanical stress. Osteoarthritis Cartilage 2012; 20:1603-9; PMID:22954457; https://doi.org/10.1016/j.joca.2012.08.022
