Abstract
Normally, hepatic progenitor cells (HPCs) are activated and differentiate into hepatocytes or bile ductular cells to repair liver damage during liver injury. However, it remains controversial whether the abnormal differentiation of HPCs occurs under abnormal conditions. Lipopolysaccharide (LPS), a component of the microenvironment, promotes liver fibrosis. In the present study, HPCs promoted liver fibrosis in rats following carbon tetrachloride (CCl4) treatment. Meanwhile, the LPS level in the portal vein was elevated and played a primary role in the fate of HPCs. In vitro, LPS inhibited the hepatobiliary differentiation of HPCs. Concurrently, HPCs co-cultured with LPS for 2 weeks showed a tendency to differentiate into myofibroblasts (MFs). Thus, we conclude that LPS promotes the aberrant differentiation of HPCs into MFs as a third type of descendant. This study provides insight into a novel differentiation fate of HPCs in their microenvironment, and could thus lead to the development of HPCs for treatment methods in liver fibrosis.
Introduction
Liver fibrosis is a complex fibrogenic and inflammatory process that results from various forms of chronic hepatic disease and represents an early step in the progression of liver cirrhosis.Citation1 Liver fibrosis occurs during the repair of liver damage. If the damaged factors cannot be removed for an extended period of time, the process of fibrosis continues to develop into long-term cirrhosis of the liver and leads to an increased risk of hepatocellular carcinoma.Citation2 Liver cirrhosis is a major health problem worldwide, which can ultimately cause organ failure and death, owing to the lack of effective treatment methods. Moreover, owing to the lack of an effective liver source, liver transplantation is also limited. Therefore, it is necessary to recognise the progression of liver fibrosis to prevent further deterioration.
Hepatic progenitor cells (HPCs) are liver-derived stem cells with the ability to self-renew and possess a bipotential capacity to differentiate into both hepatocytes and bile ductular cells.Citation3-5 Several studies have shown that activated HPCs release cytokines to activate quiescent hepatic stellate cells to promote liver fibrosis.Citation6 In addition, the activation and differentiation of HPCs are closely related to liver fibrosis.Citation7,8 HPCs, as stem cells, are considered the source of tumour-initiating cells or cancer stem cells under abnormal conditionsCitation9,10, and HPCs have been shown to repair liver damage in a liver resection experiment.Citation11,12 HPCs may play a similar role in fibrosis. Several studies have reported that HPCs are embedded in the extracellular matrix and are surrounded by myofibroblasts (MFs), which are presumably the source of the new matrix following liver injury via a choline-deficient, ethionine-supplemented diet. Moreover, alpha-smooth muscle actin (α-SMA)-positive MFs and the matrix likely form a “leading edge” ahead of the expanding progenitor cells.Citation13 α-sma is a major marker for fibrosis and the matrix is mainly produced by mesenchymal cells. Therefore, HPCs appears to be associated with fibrosis in a fibrotic environment. The role of HPCs in liver fibrosis remains controversial; therefore, the ultimate goal of our study was to determine the role that HPCs play and how HPCs change in fibrosis.
Stem cell differentiation is affected by the surrounding microenvironment, including the activation of various inflammatory cells and the accumulation of toxins due to metabolismCitation14. Several components of the liver microenvironment, including epidermal growth factor (EGF), hepatocyte growth factor (HGF) and Kupffer cells, amongst others, affect the activation and differentiation of HPCs.Citation15,16 Lipopolysaccharide (LPS), a cell wall component of Gram-negative bacteria, plays a crucial role in liver fibrosis.Citation17 The LPS level is elevated in a variety of liver diseases, probably because it is involved in the development and progression of chronic liver injury.Citation18 A close relationship between augmented circulating LPS levels and fibrosis severity has been reported both in human subjects and in animal models.Citation19 Currently, little is known regarding the effect of LPS on the differentiation of HPCs. Therefore, we postulated that LPS plays an important role in the function of HPCs in liver fibrosis.
Several reports have shown that LPS promotes liver fibrosis and HPCs are closely related to the progression of liver fibrosis. Therefore, we hypothesised that LPS may affect the functions of HPCs. In the present work, we investigated the effect of LPS on the fate of HPCs in vivo and in vitro. We found that LPS induced the differentiation of HPCs into MFs; moreover, the Hedgehog signalling pathway is involved in this process. These results indicate a novel role for LPS in the fate of HPCs regarding the progression of liver fibrosis and provide insight into a novel treatment method by blocking the process of liver fibrosis.
Results
HPC transplantation facilitated liver fibrosis in vivo
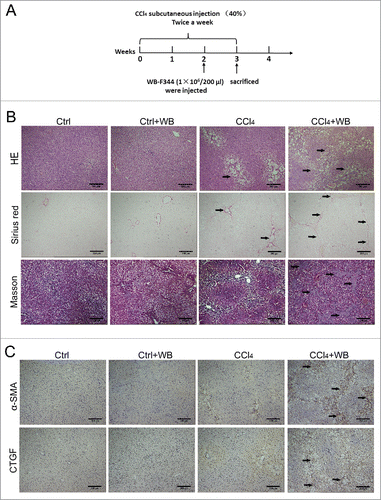
Liver fibrosis is the major complication of many types of chronic liver injury. We established a CCl4-induced liver fibrosis model in rats. To assess the effect of HPCs in CCl4-induced liver fibrosis in rats, we selected HPCs of rats, WB-F344 cells, as the study subjects. WB-F344 cells were injected into the tail vein after 2 weeks of treatment with CCl4 (). During the third week, we examined the degree of liver fibrosis after injection with WB-F344 cells. Liver paraffin sections stained with haematoxylin and eosin (HE) and Sirius Red revealed that the transplantation of WB-F344 cells significantly aggravated liver fibrosis in the CCl4-treated group (). In the CCl4 group, the hepatocytes were steatotic, whereas those in the WB-F344 cell transplantation group exhibited a fibrous cord-like structure,suggesting that the degree of fibrosis was more serious. We also examined the extent of collagen deposition by Masson's trichrome staining. Compared to the control group, WB-F344 cells clearly facilitated collagen deposition in the livers of the CCl4-treated group. We also examined the expression of α-SMA and connective tissue growth factor (CTGF) by immunohistochemistry (). In the CCl4-induced liver fibrosis model, the transplantation of WB-F344 cells increased the expression of liver fibrosis markers. These results suggest that WB-F344 cells promote liver fibrosis in CCl4-exposed rats.
Figure 1. HPC transplantation aggravated rat liver fibrosis in the CCl4-induced rat liver fibrosis model. (A) Schematic of the animal experiment (see Methods for details). (B) HE and Sirius Red staining indicated the extent of liver fibrosis, and collagen deposition was examined by Masson's trichrome staining.(C) The expression of α-SMA and CTGF was determined by immunohistochemical staining, brown showed positive expression (n = 5).
LPS is involved in the promotion of liver fibrosis in HPCs
WB-F344 cell transplantation did not aggravate rat liver fibrosis in non-CCl4-treated rats. To identify the factors that promoted liver fibrosis of WB-F344 cells, the LPS concentration was measured in portal venous blood. Enzyme-linked immunosorbent assay (ELISA) revealed increased LPS concentrations in the CCl4-treated group relative to the non-CCl4-treated group (). To investigate whether the effect of LPS on HPCs promoted liver fibrosis in rats, rats were given antibiotic-water for 4 weeks to eliminate gut-derived LPS (). The level of LPS decreased following treatment (), indicating that the effect of WB-F344 cells on liver fibrosis may be associated with the level of LPS. Liver tissues from the antibiotic-treated and untreated groups were stained with HE and analysed by immunohistochemistry. The fibrosis observed in the treated group was significantly less than that of the untreated group (), and the expression of α-SMA and CTGF was reduced in the untreated group (). These results indicate that LPS enhances the effect of WB-F344 cells to promote liver fibrosis in rats.
Figure 2. LPS is involved in liver fibrosis in rats and may influence the final fate of HPCs in the CCl4-induced model.(A) Concentration of LPS in portal vein serum was detected using a rat endotoxin ELISA test kit.(B) Schematic of the animal experiment with antibiotic pretreatment (see Methods for details).(C) The level of LPS changed after antibiotic treatment. (D) HE staining indicated the change in liver fibrosis after antibiotic pretreatment.(E) The expression of α-SMA and CTGF was determined by immunohistochemical staining after antibiotic pretreatment.(F) Frozen sections of WB-F344 cells exhibiting green fluorescence in the liver. Data are presented as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, n = 5.
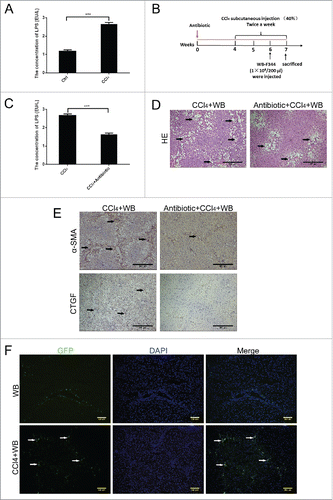
WB-F344 cells promoted liver fibrosis in rats with a high level of LPS. We next focused on determining the factor(s) that caused WB-F344 cells to promote liver fibrosis. The fate of WB-F344 cells was examined in rats following liver damage. Following transfection with green fluorescent protein (GFP) lentivirus, WB-F344 cells were injected into Fisher 344 rats and liver damage was induced by CCl4 treatment. Frozen liver sections were harvested 3 weeks later. WB-F344 cells spelled as green fluorescence in the liver plate, rather than in the liver parenchyma, indicating that WB-F344 cells had a tendency to differentiate into mesenchymal cells ().
Elevated levels of LPS promoted the differentiation of HPCs into MFs and inhibited their differentiation into hepatobiliary cells
Since HPCs can differentiate into mesenchymal cells, we first needed to determine the type of cell that HPCs had differentiated into and the change(s) that resulted in their abnormal differentiation. WB-F344 cells were cultured with different concentrations of LPS. During the second week, WB-F344 cell morphology appeared similar to that of MFs when the LPS concentration was 10 μg/mL (), and WB-F344 cells showed rapid death when cultured at LPS concentrations as high as 500 μg/mL. Therefore, we selected a concentration of 10 μg/mL to culture WB-F344 cells.
Figure 3. LPS induced the differentiation of WB-F344 cells into myofibroblasts in vitro.(A) Morphological changes of WB-F344 cells in the presence of different concentrations of LPS. (B) qRT-PCR revealed the expression of fibrosis markers (α-SMA, BFGF, CTGF and TGF-β1) in WB-F344 cells treated with LPS for 14 days.(C, D) Western blot analysis and immunofluorescence staining of fibrosis markers (α-SMA and CTGF) in WB-F344 cells treated with LPS for 14 days. Data are presented as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, NS: no significance.
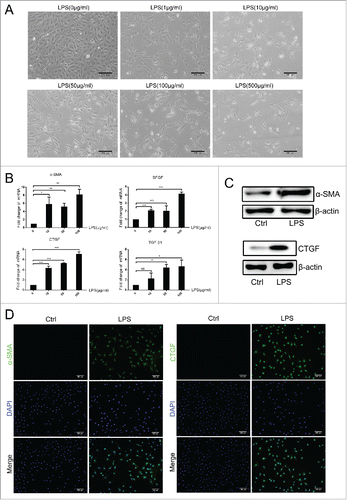
We next wanted to determine the type of cell that HPCs differentiated into under the action of elevated LPS. WB-F344 cells were cultured in the presence of LPS, and the expression of liver fibrosis markers was examined by reverse transcription polymerase chain reaction (RT-PCR) and Western blot analysis after 2 weeks (, ), as well as immunofluorescence (). WB-F344 cells showed a trend to differentiate into MFs under the action of LPS.
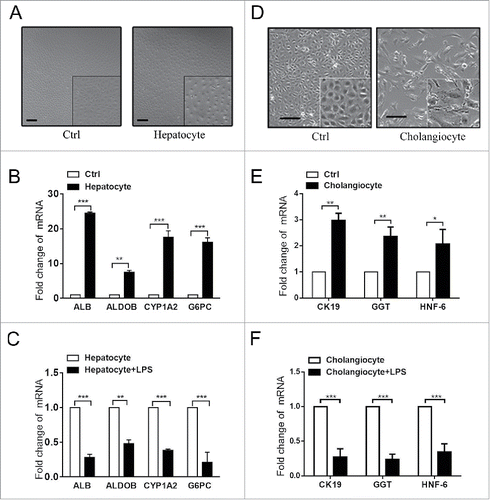
To understand the normal differentiation of HPCs, WB-F344 cells were induced to differentiate into hepatocytes by culturing with hepatocyte differentiation induction medium. On the seventh day, the morphology of the WB-F344 cells had changed significantly, similar to that of hepatocytes (), and the mRNA levels of hepatocyte markers Alb, Aldob, Cyp1a2, and G6pc had increased significantly according to RT-PCR (). When WB-F344 cells were co-cultured with LPS, LPS inhibited the process of differentiation (). Similarly, culturing WB-F344 cells in bile ductular cell differentiation induction medium successfully induced the differentiation of HPCs into bile ductular cells. LPS also inhibited their biliary differentiation (-F), indicating that HPCs differentiated into other cell types, and further confirming that HPCs differentiate into MFs.
Figure 4. LPS inhibited the differentiation of WB-F344 cells into hepatobiliary cells in vitro.(A) Morphological changes of WB-F344 cells cultured in hepatocyte differentiation induction medium for 7 days. (B) RT-PCR revealed the expression of hepatocyte markers (Alb, Aldob, cyp1a2 and G6pc) in WB-F344 cells cultured in hepatocyte differentiation induction medium for 7 days.(C) RT-PCR revealed changes in hepatocyte markers in WB-F344 cells cultured with LPS.(D) Morphological changes of WB-F344 cells cultured in bile ductular cell differentiation induction medium for 4 days.(E) RT-PCR revealed the expression of bile ductular cells markers (CK19, GGT and HNF-6) in WB-F344 cells.(F) RT-PCR revealed changes in bile ductular cell markers upon culture with LPS.
LPS action supported the differentiation of HPCs into MFs via activation of the Hedgehog signalling pathway
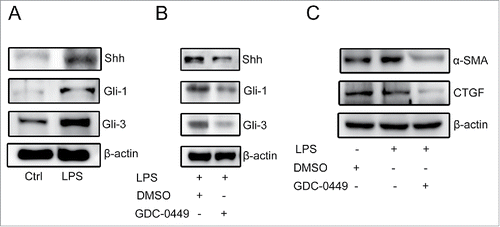
LPS promoted the differentiation of WB-F344 cells into MFs whilst inhibiting their hepatobiliary differentiation. Recent studies have shown that LPS-induced liver fibrosis can be facilitated by the Hedgehog signalling pathway.Citation20,21 Treatment with LPS up-regulated the expression of key molecules involved in the Hedgehog signalling pathway (). To determine the relationship between the Hedgehog signalling pathway and the expression of fibrosis markers, WB-F344 cells were cultured with different concentrations of GDC-0449 (an inhibitor of the Hedgehog pathway). GDC-0449 is a novel and specific Hedgehog inhibitor, blocking the activities of the Hedgehog-ligand cell surface receptors Patch and/or Smo, and suppressing the expression of downstream genes (e.g., Gli-1 and Gli-3). When the concentration of GDC-0449 was 20 μM, the expression of downstream genes was significantly inhibited (). Treatment with GDC-0449 also significantly reduced the expression of liver fibrosis markers (). These results indicate that LPS induces the differentiation of WB-F344 cells into MFs via activation of the Hedgehog signalling pathway.
Figure 5. LPS induced the differentiation of WB-F344 cells into myofibroblasts via the Hedgehog signalling pathway.(A) Western blot analysis of key molecules involved in the Hedgehog signalling pathway (Gli-1, Gli-2 and Shh) in WB-F344 cells with or without LPS treatment.(B) Western blot analysis revealed the inhibitory effect of GDC-0449 in WB-F344 cells.(C) Western blot analysis revealed changes in the expression of fibrosis markers (α-SMA and CTGF) in WB-F344 cells after treatment with GDC-0449.
Discussion
Liver fibrosis is the common pathology in all types of chronic liver disease. There are many animal models of liver fibrosis. The rat liver fibrosis model induced by CCl4 treatment is a quite mature model.Citation22-24 In this study, we demonstrated that WB-F344 cells aggravated liver fibrosis in rats following CCl4 treatment. Meanwhile, the level of LPS in the portal vein was also elevated. Further research revealed that LPS induced the differentiation of HPCs into MFs via activation of the Hedgehog signalling pathway.
Many studies have demonstrated that HPCs play an important role in promoting fibrosis in complex microenvironments.Citation25 Other studies have also indicated that many chronic liver diseases are often accompanied by elevated levels of LPSCitation26,27, which is known to promote liver fibrosis. For example, clinical studies have shown increased LPS plasma levels in the systemic and portal circulation of patients with cirrhosis.Citation28 Therefore, we hypothesised that LPS is involved in the promotion of liver fibrosis induced by HPCs. Yu and colleagues reported that LPS can be eliminated by using antibiotic regimens in rats.Citation29 We found that HPC-induced liver fibrosis was attenuated by a decrease in LPS concentration. These results confirmed our previous assumptions that HPCs promote liver fibrosis under the action of LPS.
It is well known that MFs play a key role in fibrotic diseases and that MFs can evolve from a variety of cell types.Citation30 The fate of HPCs is related to the microenvironment in which they reside.Citation31 In our work, fluorescent HPCs localised around the liver, indicating that HPCs became stromal cells. Further in vitro experiments indicated that HPCs have a tendency to evolve into MFs. Several studies have suggested that HPCs transit through a mesenchymal phase before differentiating into hepatocytes during liver regeneration, and HPCs gain increased migratory ability and express mesenchymal markers during their activation.Citation32 Previous reports have also shown that HPCs can serve as a source of MFs that may create a microenvironment for tumour development arising through the progression of chronic liver injury.Citation33 Our in vivo and in vitro experiments showed that HPCs differentiate into MFs via the action of LPS. Meanwhile, hepatobiliary differentiation was inhibited, indicating that HPCs aggravate liver fibrosis due to their abnormal differentiation into MFs under the influence of LPS.
The complexity of signalling pathways that regulate HPCs and liver fibrosis has been reported.Citation34,35 Omenetti and colleagues established that Hedgehog signals are activated in various types of liver injury in adults in both experimental models and patient livers.Citation36,37 Additionally, published studies have demonstrated that LPS activates components of the Hedgehog signalling pathway in human peripheral blood monocytes.Citation38 In our study, the Hedgehog signalling pathway was activated, and was determined to be involved in the differentiation of HPCs and affect the progression of liver fibrosis. Therefore, further development of fibrosis could be prevented by inhibiting the Hedgehog signalling pathway.
In conclusion, HPCs can differentiate into MFs with elevated levels of LPS and the Hedgehog signalling pathway is involved in the progression of liver fibrosis. These findings reveal a novel fate of HPCs and could aid in the development of a treatment method that makes better use of the ‘stemness’ characteristics of HPCs. However, it will be necessary to perform lineage-tracing studies to provide additional proof of the existence of progenitor cell plasticity. The relationship between HPCs and MFs provides insight into a reliable anti-fibrotic strategy that would not negatively affect liver regeneration.
Experimental Procedures
Reagents
LPS was purchased from Sigma-Aldrich (L2880, Sigma). The Rat Endotoxin (ET) Elisa Assay Kit (H178) was purchased from Jiancheng, Nanjing, China. The α-SMA (ab5694, Abcam), CTGF (NB100–724, Novus), Gli-1 (sc-20687, Santa Cruz), Gli-3 (sc-20688, Santa Cruz), Shh (sc-1194, Santa Cruz), goat anti-rabbit IgG (BS13271, Bioworld), anti-goat IgG h+l (RGHL-50P, ICL), Alexa Fluor 488 goat anti-rabbit and β-actin (AP0060, Bioworld) antibodies were used for Western blotting or cellular immunofluorescence staining. GDC-0449 (s1082) was obtained from Selleck. Sodium butyrate (s9637), Vitc (A4403) and nicotinamide (N0636) were purchased from Sigma-Aldrich. HGF (315–23) and EGF (400–25) were obtained from Peprotech. OSM (495-MO) was purchased from R&D Systems. ITS-X (51500–056) was obtained from Lifetech. 2-Mercaptoethanol (21985023) was purchased from Thermo Fisher Scientific. Dexamethasone, gentamicin, amoxicillin, gentamicin, metronidazole and vancomycin were purchased from Eastern Hepatobiliary Surgery Hospital, Shanghai, China.
Animals and experimental design
All Fisher 344 rats (male, 180 ± 15 g body weight) were purchased from the Shanghai Experimental Animal Center at the Chinese Academy of Sciences in Shanghai, China. Animals were kept in ordinary cages at room temperature (25 ± 3°C) with a 12 h dark/light cycle, with free access to standard laboratory feed and water. Twenty Fisher 344 rats were randomly divided into four groups: control, control + WB-F344 cells, CCl4 and CCl4 + WB-F344 cells. Fisher 344 rats received a subcutaneous injection of CCl4 (1 mL/kg, Sigma-Aldrich) diluted 2:5 in olive oil twice weekly for 2 weeks (2 injections each week on Tuesdays and Fridays). Control rats received injections of olive oil alone. During the third week, rats were injected with WB-F344 cells (1 × 106) through the tail vein. The same cell culture medium without WB-F344 cells was injected into the control group (CCl4 was injected subcutaneously as usual). Rats were killed 3 weeks after injection of WB-F344 cells. To detect the endotoxin level in portal vein blood, 20 rats were randomly divided into three groups and treated as follows: normal group (n = 5, subcutaneous olive oil), CCl4 group (n = 5, subcutaneous CCl4) and antibiotics/CCl4 group (n = 5, antibiotic-water (amoxicillin 1 g/L, gentamicin 1 g/L, metronidazole 1 g/L, and vancomycin 0.5 g/L) and subcutaneous CCl4). All animals were sacrificed after 4 weeks. The same groups were injected with WB-F344 cells to observe changes in liver fibrosis.
Endotoxin assay
At the time of sacrifice, blood was collected from the inferior vena cava and serum was collected by centrifugation at 3,000 × g and 4°C for 10 min. The serum levels of endotoxin were measured using the Rat Endotoxin (ET) Elisa Assay Kit.
Histological examination
Liver tissues for histopathological examination were fixed with 10% neutral-buffered formalin, processed and trimmed, embedded in paraffin, sectioned to a thickness of approximately 5 μm, and stained with HE following a standard protocol. Liver sections were stained with Masson's trichrome and Sirius Red following a standard protocol for collagen determination and examined under a light microscope.
Immunohistochemistry
Immunohistochemical examinations were carried out to detect the expression of α-SMA and CTGF. Liver samples were fixed in 4% paraformaldehyde and embedded in paraffin. Sections of tissues (5 μm thick) were dewaxed and rehydrated with freshly distilled water. Sections underwent inactivation of endogenous peroxidase for 20 min and were washed with distilled water three times, followed by exposure to primary antibodies after a specific step of antigen retrieval (0.1 mol/L citrate buffer solution, pH = 6.0). Negative controls were incubated with isotype-matched immunoglobulin instead of the specific antibody and sections were incubated overnight at 4°C. The following day, sections were washed in phosphate-buffered saline (PBS) and incubated at 37°C for 30 min with biotinylated goat anti-rabbit IgG. After washing with PBS, sections were incubated with streptavidin-peroxidase for 10 min, stained with diaminobenzidine for 10 min and washed for 5 min. Sections were stained with haematoxylin, and were dehydrated and mounted for microscopic examination. Images were obtained using Image-Pro Plus 4.5 software (Media Cybernetics, USA) with brown staining under light microscopy, which indicated a positive reaction to α-SMA or CTGF.
Cell culture and treatment
WB-F344 cells were purchased from the Chinese Academy of Sciences, Shanghai. WB-F344 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 4.5 g/L glucose and 10% foetal bovine serum (FBS) at 37°C with 5% CO2. The treatment group was incubated with LPS (10 μg/mL) for 2 weeks. WB-F344 cells were cultured with DMEM/F12 supplemented with 4.5 g/L glucose, 10% FBS, 0.1 mmol/L 2-mercaptoethanol, 1 × ITS-X, 10 ng/mL HGF, 20 ng/mL EGF, 20 ng/mL OSM, 0.5 mmol/L Vitc, 50 μg/mL gentamicin, 10 mmol/L nicotinamide and 10−6 mol/L dexamethasone for 7 days for hepatocyte differentiation. WB-F344 cells were also cultured in DMEM supplemented with 4.5 g/L glucose, 10% FBS and 3.75 mmol/L sodium butyrate for 4 days for bile ductular cell differentiation.
Real-time quantitative PCR
To determine the levels of mRNA expression, total RNA from different groups of cells was extracted using TRIzol reagent (Invitrogen, USA) according to the manufacturer's protocol. RNA was quantified on an ND-2000 spectrophotometer (Nanodrop Technologies), and complementary DNA synthesis was performed using the PrimeScript RT Reagent Kit (Takara, Japan). The original amount of the specific transcript was detected via real-time PCR using an SYBR Green PCR Kit (Applied BI), and the results were normalised to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Primer sequences for these transcripts are listed in .
Table 1. Primers used for quantitative real-time RT-PCR.
Cellular immunofluorescence staining
Cells were fixed in 4% paraformaldehyde solution for 20 min when the density reached approximately 60%. Cells were then treated with 0.5% Triton-X 100 solution in PBS (v/v) after washing with PBS. α-SMA and CTGF antibodies were added to cells and incubated overnight at 4°C. Alexa Fluor 488 goat anti-rabbit IgG secondary antibody was used at a dilution of 1:200. Nuclei were stained with DAPI (1 μg/mL, Sigma-Aldrich). Fluorescence intensity was evaluated using a confocal microscope (Leica TCS SP2).
Western blot analysis
Treated cells were washed with PBS and lysed with RIPA buffer containing PMSF at a ratio of 100:1 to obtain total protein for Western blot analysis. An equal amount of protein was separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride membrane. The membrane was then blocked in 5% fat-free milk/1 × TBS/0.1% Tween-20 for 2 h at room temperature, and incubated with the appropriate primary antibody overnight at 4°C. The following day, the membrane was washed with 1 × TBS/0.1% Tween-20 and incubated with the appropriate secondary antibody (1:10,000) for 1 h at room temperature. Immunoblots were developed using the BeyoECL (Beyotime) and Tanon 5200 system. The expression of α-SMA (1:600), CTGF (1:1000), Gli-1 (1:200), Gli-3 (1:200), Shh (1:200) and β-actin (1:5000) was examined.
Statistical analysis
Analysis of variance was performed using GraphPad Prism 6.0 (GraphPad Software). Quantitative data are expressed as the mean ± standard deviation (SD) for each experiment. Significance between groups was determined using Student's t-test. For all analyses, p < 0.05 was considered statistically significant.
Ethics approval
Experiments and all procedures involving animals were performed in accordance with the institutional animal welfare guidelines of Second Military Medical University and approved by the Animal Ethics Committee of Eastern Hepatobiliary Surgery Hospital, Shanghai, China.
Disclosure of potential conflicts of interest
The authors declare that they have no competing interests
Author's Contribution
Xiaorong Pan, Yingying Jing and Wenting Liu performed the research, analyzed data, and participated in writing the paper. Zhipeng Han, Jingni Zhu, Xiaoyong Li and Peipei Li analyzed data and composed this paper. Yang Yang and Rong Li participated in performing this study. Lixin Wei conceived this study, provided funding, and gave final approval of this paper.
Funding
This project was supported by National Natural Science Foundation of China (Grant No. 81401308, 81402026, 81402454, 81402018, 81402020, 81630070, 81372312, 81472737, 81572444, 81502417); Special Funds for National Key Sci-Tech Sepcial Project of China (Grant No. 2016ZX10002019-005-002); Shanghai Science and Technology Committee (Grant No. 15PJ1410600, 14ZR1409200, 16ZR1400200, 16JC1405200, 14ZD1900403, 16YF1415000); Science Fund for Creative Research Groups, NSFC, China (Grant NO. 81521091).
References
- Zhang CY, Yuan WG, He P, Lei JH, Wang CX. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J Gastroenterol 2016; 22:10512-22; PMID:28082803; https://doi.org/10.3748/wjg.v22.i48.10512
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014; 383:1749-61; https://doi.org/10.1016/S0140-6736(14)60121-5
- Español–Suñer R, Carpentier R, Van Hul N, Legry V, Achouri Y, Cordi S, Jacquemin P, Lemaigre F, Leclercq IA. Liver Progenitor Cells Yield Functional Hepatocytes in Response to Chronic Liver Injury in Mice. Gastroenterology 2012; 143:1564-75.e7; PMID:22922013; https://doi.org/10.1053/j.gastro.2012.08.024
- Itoh T, Miyajima A. Liver regeneration by stem/progenitor cells. Hepatology 2014; 59:1617-26; PMID:24115180; https://doi.org/10.1002/hep.26753
- Itoh T. Stem/progenitor cells in liver regeneration. Hepatology 2016; 64:663-8; PMID:27227904; https://doi.org/10.1002/hep.28661
- Chobert MN, Couchie D, Fourcot A, Zafrani ES, Laperche Y, Mavier P, Brouillet A. Liver precursor cells increase hepatic fibrosis induced by chronic carbon tetrachloride intoxication in rats. Lab Invest ; A J Tech Methods Pathol 2012; 92:135-50; PMID:21946857; https://doi.org/10.1038/labinvest.2011.143
- Clouston AD, Powell EE, Walsh MJ, Richardson MM, Demetris AJ, Jonsson JR. Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology 2005; 41:809-18; PMID:15793848; https://doi.org/10.1002/hep.20650
- Lukacs-Kornek V, Lammert F. The progenitor cell dilemma: cellular and functional heterogeneity in assistance or escalation of liver injury. J Hepatol 2017; 66:619-30.
- Chiba T, Zheng YW, Kita K, Yokosuka O, Saisho H, Onodera M, Miyoshi H, Nakano M, Zen Y, Nakanuma Y, et al. Enhanced self-renewal capability in hepatic stem/progenitor cells drives cancer initiation. Gastroenterology 2007; 133:937-50; PMID:17673212; https://doi.org/10.1053/j.gastro.2007.06.016
- Shi JH, Scholz H, Huitfeldt HS, Line PD. The effect of hepatic progenitor cells on experimental hepatocellular carcinoma in the regenerating liver. Scandinavian J Gastroenterol 2014; 49:99-108; PMID:24188385; https://doi.org/10.3109/00365521.2013.854406
- Shin S, Kaestner KH. The origin, biology, and therapeutic potential of facultative adult hepatic progenitor cells. Curr Topics Dev Biol 2014; 107:269-92; PMID:24439810
- Lu J, Zhou Y, Hu T, Zhang H, Shen M, Cheng P, Dai W, Wang F, Chen K, Zhang Y, et al. Notch signaling coordinates progenitor cell-mediated biliary regeneration following partial hepatectomy. Scientific Reports 2016; 6:22754; PMID:26951801; https://doi.org/10.1038/srep22754
- Van Hul NKM, Abarca-Quinones J, Sempoux C, Horsmans Y, Leclercq IA. Relation between liver progenitor cell expansion and extracellular matrix deposition in a CDE-Induced Murine model of chronic liver injury. Hepatology 2009; 49:1625-35; PMID:19296469; https://doi.org/10.1002/hep.22820
- Llorens-Bobadilla E, Martin-Villalba A. Adult NSC diversity and plasticity: the role of the niche. Curr Opin Neurobiol 2017; 42:68-74; PMID:27978480; https://doi.org/10.1016/j.conb.2016.11.008
- Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, Van Rooijen N, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med 2012; 18:572-9; PMID:22388089; https://doi.org/10.1038/nm.2667
- Kitade M, Factor VM, Andersen JB, Tomokuni A, Kaji K, Akita H, Holczbauer A, Seo D, Marquardt JU, Conner EA, et al. Specific fate decisions in adult hepatic progenitor cells driven by MET and EGFR signaling. Genes Dev 2013; 27:1706-17; https://doi.org/10.1101/gad.214601.113
- Roderburg C, Luedde T. The role of the gut microbiome in the development and progression of liver cirrhosis and hepatocellular carcinoma. Gut Microbes 2014; 5:441-5; PMID:25006881; https://doi.org/10.4161/gmic.29599
- Affo S, Morales-Ibanez O, Rodrigo-Torres D, Altamirano J, Blaya D, Dapito DH, Millan C, Coll M, Caviglia JM, Arroyo V, et al. CCL20 mediates lipopolysaccharide induced liver injury and is a potential driver of inflammation and fibrosis in alcoholic hepatitis. Gut 2014; 63:1782-92; PMID:24415562; https://doi.org/10.1136/gutjnl-2013-306098
- Ceccarelli S, Panera N, Mina M, Gnani D, De Stefanis C, Crudele A, Rychlicki C, Petrini S, Bruscalupi G, Agostinelli L, et al. LPS-induced TNF-a factor mediates pro-inflammatory and pro-fibrogenic pattern in non-alcoholic fatty liver disease. Oncotarget 2015; 6:41434-52; PMID:26573228
- Guy CD, Suzuki A, Zdanowicz M, Abdelmalek MF, Burchette J, Unalp A, Diehl AM, Nash CRN. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human nonalcoholic fatty liver disease. Hepatology (Baltimore, Md) 2012; 55:1711-21; PMID:22213086; https://doi.org/10.1002/hep.25559
- El-Agroudy NN, El-Naga RN, El-Razeq RA, El-Demerdash E. Forskolin, a hedgehog signalling inhibitor, attenuates carbon tetrachloride-induced liver fibrosis in rats. Br J Pharmacol 2016; 173:3248-60; PMID:27590029; https://doi.org/10.1111/bph.13611
- Sato R. Prevention of critical telomere shortening by oestradiol in human normal hepatic cultured cells and carbon tetrachloride induced rat liver fibrosis. Gut 2004; 53:1001-9; PMID:15194652; https://doi.org/10.1136/gut.2003.027516
- Kang JW, Hong JM, Lee SM. Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride-induced liver fibrosis. J Pineal Res 2016; 60:383-93; PMID:26882442; https://doi.org/10.1111/jpi.12319
- Rosado E, Rodriguez-Vilarrupla A, Gracia-Sancho J, Tripathi D, Garcia-Caldero H, Bosch J, Garcia-Pagan JC. Terutroban, a TP-receptor antagonist, reduces portal pressure in cirrhotic rats. Hepatology 2013; 58:1424-35; PMID:23703868; https://doi.org/10.1002/hep.26520
- Carpino G, Renzi A, Onori P, Gaudio E. Role of hepatic progenitor cells in nonalcoholic fatty liver disease development: Cellular cross-talks and molecular networks. Int J Mol Sci 2013; 14:20112-30; PMID:24113587; https://doi.org/10.3390/ijms141020112
- Anders LC, Lang AL, Anwar-Mohamed A, Douglas AN, Bushau AM, Falkner KC, Hill BG, Warner NL, Arteel GE, Cave M, et al. Vinyl chloride metabolites potentiate inflammatory liver injury caused by LPS in mice. Toxicol Sci 2016; 151:312-23; PMID:26962056; https://doi.org/10.1093/toxsci/kfw045
- Xie GX, Wang XN, Liu P, Wei RM, Chen WL, Rajani C, Hernandez BY, Alegado R, Dong B, Li DF, et al. Distinctly altered gut microbiota in the progression of liver disease. Oncotarget 2016; 7:19355-66. PMID: 27036035
- Chang LY, Li Y, Kaplan DE. Endotoxemia contributes to CD27+ memory B-cell apoptosis via enhanced sensitivity to Fas ligation in patients with Cirrhosis. Scientific Reports 2016; 6:36862; PMID:27857173; https://doi.org/10.1038/srep36862
- Yu LX, Yan HX, Liu Q, Yang W, Wu HP, Dong W, Tang L, Lin Y, He YQ, Zou SS, et al. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology (Baltimore, Md) 2010; 52:1322-33; PMID:20803560; https://doi.org/10.1002/hep.23845
- Kisseleva T. The origin of fibrogenic myofibroblasts in fibrotic liver. Hepatology 2017; 65:1039-43.
- Katoonizadeh A, Poustchi H. Adult Hepatic Progenitor Cell Niche: How it affects the progenitor cell fate. Middle East J Digest Dis 2014; 6:57-64. PMID:24872864
- Forbes SJ, Parola M. Liver fibrogenic cells. Best Practice Res Clin Gastroenterol 2011; 25:207-17; ; https://doi.org/10.1016/j.bpg.2011.02.006
- Sekiya S, Miura S, Matsuda-Ito K, Suzuki A. Myofibroblasts derived from hepatic progenitor cells create the tumor microenvironment. Stem Cell Reports 2016; 7:1130-9; PMID:27916538; https://doi.org/10.1016/j.stemcr.2016.11.002
- Spee B, Carpino G, Schotanus BA, Katoonizadeh A, Vander Borght S, Gaudio E, Roskams T. Characterisation of the liver progenitor cell niche in liver diseases: potential involvement of Wnt and Notch signalling. Gut 2010; 59:247-57; PMID:19880964; https://doi.org/10.1136/gut.2009.188367
- Lai L, Chen Y, Tian X, Li X, Zhang X, Lei J, Bi Y, Fang B, Song X. Artesunate alleviates hepatic fibrosis induced by multiple pathogenic factors and inflammation through the inhibition of LPS/TLR4/NF-kappaB signaling pathway in rats. European Journal Of Pharmacology 2015; 765:234-41; PMID:26318197; https://doi.org/10.1016/j.ejphar.2015.08.040
- Omenetti A, Diehl AM. The adventures of sonic hedgehog in development and repair. II. Sonic hedgehog and liver development, inflammation, and cancer. Am J Physiol Gastrointestinal Liver Physiol 2008; 294:G595-8; PMID:18218671; https://doi.org/10.1152/ajpgi.00543.2007
- Omenetti A, Porrello A, Jung Y, Yang L, Popov Y, Choi SS, Witek RP, Alpini G, Venter J, Vandongen HM, et al. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest 2008; 118:3331-42; PMID:18802480
- Wakelin SJ, Forsythe JL, Garden OJ, Howie SE. Commercially available recombinant sonic hedgehog up-regulates Ptc and modulates the cytokine and chemokine expression of human macrophages: an effect mediated by endotoxin contamination? Immunobiology 2008; 213:25-38; PMID:18207025; https://doi.org/10.1016/j.imbio.2007.06.006
