ABSTRACT
Chemotherapy is a crucial adjuvant therapy of advanced nasopharyngeal carcinoma (NPC). However, enhancing sensitivity and tolerance of chemotherapeutics in NPC treatment have been challenging. Both Bcl-2 and Mcl-1, 2 pro-survival proteins of Bcl-2 family, play essential roles on the chemotherapy tolerance of numerous cancers. In the present study, we explored the influences of TW-37, a small molecule inhibitor of Bcl-2 and Mcl-1, on the efficiency of chemotherapy for NPC. Oncomine cancer database shows that NPC tissues have higher expression of Bcl-2 and Mcl-1 than those of normal nasopharyngeal epithelial (NPE) tissues. And our results reveal that chemotherapeutics, Cisplatin (CDDP) and 5-Fluoracil (5-FU), result in the greater decrease of protein level of Bcl-2 and Mcl-1 in NPC cells than those in NPE cells. TW-37 does not have significant impact on the chemotherapeutics-treated NPE cell viability at a dosage that efficiently reduces chemotherapeutics-treated NPC cell viability. Moreover, impacts of TW-37 on the cell viability of chemotherapeutics-treated NPC cells are dependent on the expression of Bcl-2 and Mcl-1 in NPC cells. Further explorations suggest that TW-37 prominently promotes apoptosis in NPC cells under chemotherapeutics treatments but not in NPE cells. Meanwhile, TW-37 also remarkably reduces colony formation ability of chemotherapeutics-treated NPC cells. Importantly, in vivo models, TW-37 observably increases chemosensitivity of NPC tumors but has not markedly influence on the normal tissues in mice. In conclusion, our results point to TW-37 as a promising ancillary drug for the chemotherapy of NPC.
Introduction
Nasopharyngeal carcinoma (NPC) is a type of head and neck cancer originating from nasopharyngeal epithelium. Epstein-Barr virus (EBV) infection is the primary risk factor of NPC tumorigenesis.Citation1 Globally, about 86,000 new case of NPC are reported annually and approximately 50,000 patients die from this cancer.Citation2 Almost 70% of newly diagnosed cases were reported in the east and southeast of Asia, especially in China. In 2015, China had 60.6 thousand estimated new NPC cases and 34.1 thousand estimated mortality of NPC.Citation3 Since the location of NPC is difficult to perform surgery and NPC is a relatively radiosensitive cancer, radiotherapy is the principal treatment of non-metastatic NPC. However, in the newly diagnosed NPC patients, more than 70% of cases belong to locoregionally advanced NPC.
Combination of chemotherapy and radiotherapy, mainly concurrent chemoradiotherapy, without or with adjuvant chemotherapy, plays an indispensable role in the treatment of advanced types of NPC.Citation4 However, therapy tolerance and treatment toxicities still limit the effect of chemotherapy in NPC. Therefore, novel and safe therapeutic approaches for enhancing the chemotherapy sensitivity of the NPC have yet to be explored.
Bcl-2 family members play crucial roles in tumor resistance to chemotherapy. The Bcl-2 family is a protein group of up to 20 members that have sequence motif termed Bcl-2 homology (BH) domain. Bcl-2 family members can be mainly divided into 3 groups, anti-apoptotic proteins (e.g. Bcl-2, Mcl-1 and Bcl-xl), pro-apoptotic BH3-only proteins (e.g., Bim, Bid, Noxa and Bad) and pro-apoptotic effectors (Bax and Bak). Since the crucial role on suppressing apoptosis, the anti-apoptotic Bcl-2 family members have been considered promising targets for anti-cancer drugs.Citation5 TW-37, a non-peptide pleiotropic BH3 mimetic, mainly binds to Bcl-2 and Mcl-1 with a Ki of 290 nmol/L and 260 nmol/L, respectively.Citation6 Previous studies show that TW37 promotes tumor cell apoptosis in melanoma,Citation7 lymphoma,Citation6 pancreatic carcinoma,Citation8 malignant rhabdoid tumorCitation9 and oophoroma.Citation10 However, effects of TW-37 in NPC treatment remain unclear.
Therefore, in the present study, we sought to explore the impacts of TW-37 in NPC cells and whether TW-37 could enhance the sensitivity of NPC cells to chemotherapeutics, Cisplatin (CDDP) and 5-Fluoracil (5-FU). Meanwhile, we also paid attention to whether the dose of TW-37 that enhances the chemosensitivity of NPC is tolerable for normal cells under chemotherapeutics treatment.
Results
Chemotherapeutics significantly decrease Bcl-2 and Mcl-1 expressions in NPC cells
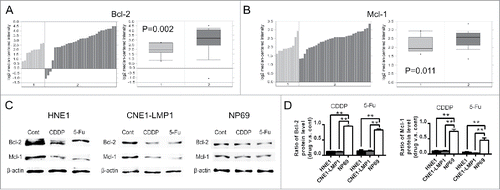
Since Bcl-2 and Mcl-1 are the major targets of TW-37, we first studied the expression of Bcl-2 and Mcl-1 in normal nasopharynx and NPC tissues. The analyses of the publicly available microarray data sets from Oncomine cancer database demonstrate that mRNA expression of Bcl-2 and Mcl-1 in NPC samples was 2.009- and 1.305- fold compared with those in nasopharynx samples, respectively ( and ). Then, we further chose EBV-positive NPC cell line CNE-LMP1,Citation11 EBV-negative NPC cell line HNE112 and immortalized nasopharyngeal epithelial (NPE) cell line NP69 to explore how chemotherapeutics influence on the expression of Bcl-2 and Mcl-1 in NPC and normal NPE cells. Immunoblot results revealed that both CDDP and 5-FU led to the decrease of protein level of Bcl-2 and Mcl-1 in NPC cells (). Moreover, although chemotherapeutics also reduced expression of Bcl-2 and Mcl-1 in NP69 cells (), NPC cells had a greater reduction in protein level of Bcl-2 and Mcl-1 than those of NP69 cells after chemotherapeutics treatments ().
Figure 1. Bcl-2 and Mcl-1 expressions in the normal nasopharynx and NPC under normal and chemotherapeutics-treated conditions. (A and B) The mRNA expression of Bcl-2 (A) and Mcl-1 (B) in normal nasopharynx samples (group 1, n = 10) and NPC samples (group 2, n = 31) from a public microarray data set was analyzed by Oncomine platform. (C and D) HNE1, CNE1-LMP1 and NP69 cells were processed by CDDP (2 μM) and 5-Fu (10 μM). After 24 hours, protein level of Bcl-2 and Mcl-1 in the indicated groups was detected with western blot (C). Fold reduction of Bcl-2 (Left) and Mcl-1 (Right) protein level (drug v.s. untreated) in the indicated cells after CDDP and 5-Fu treatments was presented in graphs. The data are shown as mean ± SEM (n = 3; **, P < 0.01).
TW-37 reduces cell viabilities of chemotherapeutics-treated NPC cells through the Bcl-2- and Mcl-1-dependent manner
Next, we examined the influence of TW-37 on the cell viabilities of chemotherapeutics -treated NPC and NPE cells. After pretreatment with TW-37 for 24 hours, NPC and NP69 cells were treated with various dosages of CDDP (0, 1, 2 and 3 μM). Cell viabilities of the indicated cells were evaluated by Cell Counting Kit-8 (CCK8) assay 48 hours after CDDP administration. The data showed that both 1 and 2 μM of TW-37 significantly enhanced the CDDP (1, 2 or 3 μM)-induced decrease of NPC cell viabilities. Additionally, 2 μM TW-37 alone led to the reduction of NPC cell viabilities (, left and middle). Meanwhile, although 4 μM TW-37 also could decrease cell viabilities of CDDP (2 or 3 μM)-treated NP69 cells, both 2 and 3 μM of TW-37 had no prominent impact on the cell viabilities of CDDP-treated NP69 cells (, right). We further studied the impact of TW-37 on the cell viabilities of 5Fu-treated NPC and NP69 cells. The results revealed that both 1 and 2 μM of TW-37 brought about a remarkable reduction of the half maximal inhibitory concentration (IC50) value of 5-Fu in the NPC cells but not in NP69 cells (). These data indicate that TW-37 exacerbates the decrease of NPC cell viabilities resulting from chemotherapeutics treatment, and NPE cells have lower sensitivity of TW-37 compared with that of NPC cells.
Figure 2. Effect of TW-37 on the cell viabilities of NPC and NPE cells under CDDP and 5-Fu treatments. (A) After pretreatment with the indicated concentration of TW-37 for 24 hours, HNE1 (left), CNE1-LMP1 (middle) and NP69 (right) cells were treated with the indicated concentration of CDDP. Cell viabilities of the indicated cells were detected with CCK8 assay 48 hours after CDDP treatment. The data are shown as mean ± SEM (n = 6; *, P < 0.05; **, P < 0.01). (B) After pretreatment with the indicated concentration of TW-37 for 24 hours, the indicated cells were treated with 5-Fu of different dosages for 48 hours, and IC50 of the indicated cells to 5-Fu was assessed by CCK8 assay. The data are presented as the mean ± SEM (n = 6; *, P < 0.05; **, P < 0.01). (C) After pretreatment with TW-37 (1 μM) for 24 hours, HNE1 (upper) and CNE-LMP1 (lower) cells were treated with the indicated chemotherapeutics (CDDP, 2 μM; 5-Fu, 10 μM) for the next 24 hours. Then, protein level of Bcl-2 and Mcl-1 in the indicated cells was examined by western blotting. (D and F) CNE-LMP1 cells were transfected with the indicated recombinant adenoviral vectors (D) or siRNA (F). After 48 (D) or 72 (F) hours post transfection, protein level of Bcl-2 and Mcl-1 in the indicated cells was examined by western blotting. (E and G) The transfected CNE-LMP1 cells were pretreated with TW-37 (1 μM) 48 hours after transfection. After the following 24 hours, cells were treated with CDDP (2 μM, left) or 5-Fu (10 μM, right). Cell viabilities of the indicated cells were assessed by CCK8 assay 48 hours after the indicated treatment. The data are shown as mean ± SEM (n = 6; *, P < 0.05; **, P < 0.01; n.s, no significant).
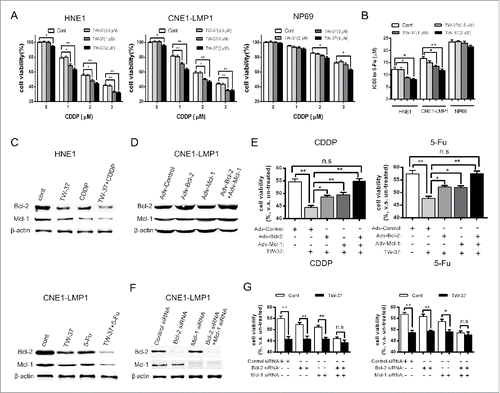
Previous study showed that the effects of TW-37 mainly depend on inhibiting Mcl-1 but not Bcl-2.Citation13 Therefore, we further detect the dependency of TW-37 on Bcl-2 and Mcl-1 in NPC cells. Although TW-37 mainly inhibits activity of Bcl-2 and Mcl-1, some studies report that TW-37 suppresses expression of Bcl-2 and Mcl-1.Citation10,14 Our results also revealed that TW-37 decreased protein level of Bcl-2 and Mcl-1 in both untreated and chemotherapeutics -treated NPC cells (). Then, we increased expression of Bcl-2 and Mcl-1 by infection of specific recombinant adenovirus (). CCK8 assay showed that both Bcl-2 and Mcl-1 overexpressions antagonized the influence of TW-37 in the chemotherapeutics-treated NPC cells. Moreover, Bcl-2 and Mcl-1 co-overexpression fully reversed TW-37-induced decrease of cell viability in the chemotherapeutics-treated NPC cells (). These data hint that both Bcl-2 and Mcl-1 have influence on the effect of TW-37 treatment in the chemotherapeutics-treated NPC cells. We further used specific siRNA to efficiently decrease protein level of Bcl-2 and Mcl-1 (). After CDDP treatment, TW-37 significantly reduced cell viability of Bcl-2- or Mcl-1-inhibited NPC cells, but had not prominently impact on the cell viability of Bcl-2 and Mcl-1 co-inhibited NPC cells. Meanwhile, we obtain approximate results after 5-Fu treatment (). These data suggest that both Bcl-2 and Mcl-1 expressions are necessary for the influence of TW-37 on the cell viability of NPC cells under chemotherapeutics treatment.
TW-37 decreases survival and clonogenic capacity of chemotherapeutics-treated NPC cells
To deeply explore the impacts of TW-37 on the chemotherapeutics-treated NPC cells, we evaluated these cells by apoptosis detection and colony formation assay. Annexin V/PI double staining assay revealed that pretreatment of TW-37 brought about the increase of percentage of apoptotic NPC cells under CDDP treatment ( and ). Meanwhile, 5-Fu administration showed similar data (). Notably, TW-37 had not affected the apoptosis of NP69 cells under chemotherapeutics administration (). Besides, Lactate dehydrogenase (LDH) release assay also suggested that TW-37 pretreatment aggravated chemotherapeutics-induced apoptosis of NPC cells but not that of NPE cells ().
Figure 3. Impacts of TW-37 on the apoptosis of NPC and NPE cells and on the colony formations of NPC cells under CDDP and 5-Fu treatments. (A and B) After pretreatment with TW-37 (1 μM) for 24 hours, HNE1, CNE1-LMP1 and NP69 cells were received with CDDP treatment (2 μM) for the following 48 hours. Then, percentage of cell apoptosis of the indicated cells were detected with Annexin V/ PtdIns double staining assay (A). The data are shown as mean ± SEM (n = 3; **, P < 0.01) (B). (C) After pretreatment with TW-37 (1 μM) for 24 hours, HNE1, CNE1-LMP1 and NP69 cells were received 5-Fu (10 μM) treatment. After 48 hours, percentage of cell apoptosis in the indicated cells was detected and the data are shown as mean ± SEM (n = 3;**, P < 0.01). (D) HNE1, CNE1-LMP1 and NP69 cells were treated with TW-37 (1 μM) for 24 hours and CDDP (2 μM, left) or 5-Fu (10 μM, right) for the next 48 hours. Then, cells in the indicated groups were examined with LDH cytotoxicity detection assay. The data are shown as mean ± SEM (n = 6; n.s, no significant; **, P < 0.01). (E and F) After pretreatment with TW-37 (1 μM) for 24 hours, HNE1 and CNE1-LMP1 cells were treated with CDDP (2 μM). After 14 days, number of colonies per sample was examined (E). The data are shown as mean ± SEM (n = 3; *, P < 0.05; **, P < 0.01) (F). (G) After pretreatment with TW-37 (1 μM) for 24 hours, HNE1 and CNE1-LMP1 cells were received with 5-Fu (10 μM) treatment. After 14 days, number of colonies per sample was recorded and the data are shown as mean ± SEM (n = 3; **, P < 0.01). (H) HNE1 and CNE1-LMP1 cells were treated with TW-37 (1 μM) for 24 hours and CDDP (2 μM, left) or 5-Fu (10 μM, right) for the next 72 hours. Then, NPC cells in the indicated groups were harvested and reseeded in 6-well plates. After 10 days, number of colonies per sample was examined. The data are shown as mean ± SEM (n = 3; *, P < 0.05; **, P < 0.01).
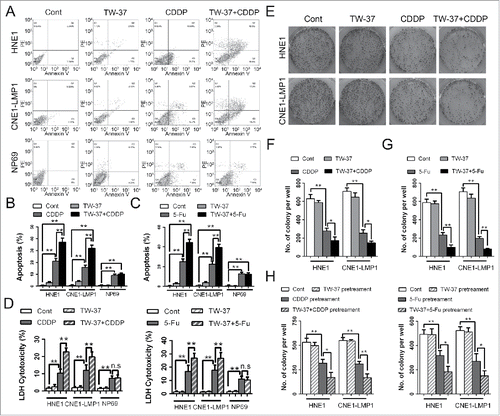
Assessment of clonogenic capacity showed that CDDP treatment remarkably reduced colony formation of NPCs. Moreover, pretreatment of TW-37 decreased clonogenic capacity of CDDP-treated NPC cells ( and ). Meanwhile, TW-37 pretreatment also promoted the decrease of colony formation caused by 5-Fu treatments in the NPC cells (). To further confirm the influence of TW-37 on the clonogenic capacity of chemotherapeutics –treated NPC cells, the NPC cells treated with TW-37 and/or chemotherapeutics were re-seeded and detected their clonogenic capacity. The data revealed that clonogenic capacity of TW-37-chemotherapeutics-co-pretreated NPC cells was significantly lower than that of chemotherapeutics -pretreated NPC cells (). These results demonstrate that TW-37 reduces cell survival and colony formation of NPC cells under chemotherapeutics treatment.
TW-37 enhances chemotherapeutics-induced inhibition of NPC tumor growth and is tolerated in mice at dosage that efficiently promotes chemosensitivity of NPC tumor
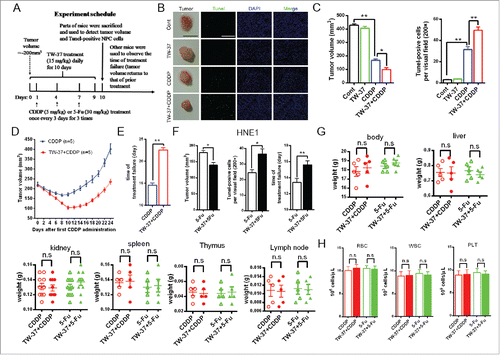
We further detected synergy effect of TW-37 and chemotherapeutics in vivo model of NPC. When volume of CNE1-LMP1 tumor reached approximately 200 cm3, the tumors were received TW-37 administration daily for 10 d and CDDP 3 times (once every 3 days) from the next day of the first TW-37 treatment. Parts of mice were killed to examine the influences of TW-37 on tumor volumes and tumor cell apoptosis under CDDP treatment 24 hours post last TW-37 administration (). The results showed that TW-37 significantly increased tumor cell apoptosis and reduced tumor volume under CDDP treatment ( and ). Time of treatment -failure of TW-37+CDDP group was also noteworthy longer compared with that of CDDP group ( and ). Meanwhile, the model of 5-Fu-treated HNE1 tumors showed similar results (). Finally, we used BALB/c mice to investigate the tolerance of chemotherapeutics-treated mice on TW-37 treatment. The results revealed that TW-37 had not prominent influence on the weight of whole body and key organs, including liver, kidney, spleen, thymus and lymph node, and amounts of main kinds of blood cells, including red cells, white cells and plantlet, in the CDDP- and 5-Fu-treated mice ( and ). These data demonstrate that TW-37 is tolerable and effective in chemotherapeutics-treated mice.
Figure 4. Influences of TW-37 on the chemotherapeutics-treated NPC tumors and normal tissues in vivo model. (A) The schedule of in vivo experiment. (B and C) Parts of CNE1-LMP1-tumor-bearing mice in the indicated groups were killed 24 hours after the last TW-37 administration. The representative tumor of indicated groups was exhibited (Black Bar, 1 cm) and apoptosis of tumors was evaluated by Tunel staining assay (magnification, 200; White Bar, 500 μm) (B). Tumor volumes (Left) and Tunel-positive cells per visual field (magnification, 200; Right) in the indicated groups represented in the graphs. The data are shown as mean ± SEM (n = 3; *, P < 0.05; **, P < 0.01) (C). (D and E) Tumor volumes of other CNE1-LMP1-tumor-bearing mice in the CDDP and TW-37+CDDP groups were measured and recorded daily from Day 0 to Day 24 (Day 0, first CDDP administration). The data of curves are shown as mean ± SEM (n = 5) (D). Times of treatment failure (from the day of the first CDDP administration to the day when tumor volume returns back to that of prior CDDP treatment) are shown as mean ± SEM (n = 5; **, P < 0.01) (E). (F) Parts of HNE1-LMP1-tumor-bearing mice in the 5-Fu and TW-37+5-Fu groups were killed 24 hours after the last TW-37 administration. Tumor volumes (Left) and Tunel-positive cells per visual field (magnification, 200; middle) in the indicated groups are shown as mean ± SEM (n = 3; *, P < 0.05). Tumor volumes of other HNE1-LMP1-tumor-bearing mice in the 5-Fu and TW-37+5-Fu groups were measured and recorded daily. Times of treatment failure in the indicated groups are shown as mean ± SEM (n = 5; **, P < 0.01; Right). (G and H) BALB/c mice received TW-37 treatment (15 mg/kg) daily for 10 d and drug treatment (CDDP 5 mg/kg or 5-Fu, 30 mg/kg) once every 3 d (3 times) from the second day of TW-37 administration. All mice were killed 24 hours after the last TW-37 administration. The weights of whole body and several organs, including liver, kidney, spleen, thymus and lymph node, in the mice of the indicated groups were measured and recorded. The data are shown as mean ± SEM (kidney, n = 10; others, n = 5; n.s, no significant) (G). Blood cells, including red cells (left), white cells (middle) and platelet (right), were counted and recorded. The data are shown as mean ± SEM (n = 5; n.s, no significant) (H).
Discussion
Since NPC is a radiosensitive tumor and its anatomic location is not fit for surgical operation therapy, radiotherapy has been the first sequence choice for NPC treatment. Early stage NPC with radiotherapy alone treatment has 5 y survival rates of more than 90 percent, but with the increase of tumor stage, 5 y survival rates of these patients dropped to 50–70 percent.Citation15 Chemotherapy exerts influence on chemosensitive subtype of NPC. In the treatment of advanced NPC, concurrent chemoradiotherapy strategy results in improved overall survival and progression-free survival. CDDP and 5-Fu are 2 conventional chemotherapeutics for NPC treatment.Citation1,4 Numerous researchers sought to further improve failure-free survival and overall survival of advanced NPC patients with an admissible toxicity and had some breakthrough progresses,Citation16 but their limitations remind us to investigate more efficient method.
Bcl-2 and Mcl-1 are the first and second discovered in the anti-apoptotic Bcl-2 family protein, respectively. Since they have critical functions in several kinds of tumorigenesis, their inhibitors, such as ABT-737, ABT-199, UMI-77 and so on, were used in tentative cancer treatments.Citation5 Oncomine cancer database reveals that NPC has higher Bcl-2 and Mcl-1 expressions compared with those of NPE tissue. These data remind us that an inhibitor of Bcl-2 or Mcl-1 has the potential of NPC drug. Bcl-2 inhibitors can free Bcl-2-binding pro-apoptotic proteins and result in Bax/Bak-dependent apoptosis, but their pro-apoptotic functions can be inhibited by high Mcl-1 level.Citation17 Although Mcl-1 inhibitor uncovers promising signs, their efficacy had dependency of high Mcl-1 expression.Citation18 Therefore, dual-target inhibitor, such as TW-37, has the specific potential application.Citation19
Both CDDP and 5-Fu decrease protein level of Bcl-2 and Mcl-1 in NPC cells. This result is comparable with several kinds of cancer.Citation20 Bcl-2 and Mcl-1 inhibitors can promote chemosensitivity of cancer through accelerating the decrease of valid level of Bcl-2 and Mcl-1.Citation21-23 Although some reports show that TW-37 mainly inhibits Mcl-1 but not Bcl-2,Citation13,24 our data suggest that both Bcl-2 and Mcl-1 play crucial role on the TW-37-induced promotion of chemotherapeutic effect of NPC cells. Bcl-2 and Mcl-1 also take part in the tolerance of cancer to radiotherapy. A study of squamous cell carcinoma suggested that TW-37 increased the antitumor effects of radiotherapy,Citation25 but the impacts of TW-37 on the radiotherapy remain unclear. Additionally, Benjamin D. Zeitlin and his colleagues reported that endothelial cells also had a high sensitivity to TW-37. Mitochondrial depolarization and activation of caspase-9 and caspase-3 mediated TW37-induced endothelial cell apoptosis. Moreover, TW37 administration brought about a decrease in the density of functional microvessels in vivo model.Citation26 Therefore, antiangiogenic effect may involve in the tumor inhibitory effect of TW-37 in NPC, but this hypothesis needs more investigation.
In summary, our findings suggest that TW-37 can enhance chemosensitivity of NPC with an acceptable toxicity for healthy tissues. Therefore, TW-37 is a promising ancillary drug for the chemotherapy of NPC. However, the reason why TW-37 has a lower sensitivity to normal cells compared with that of NPC cells still needs further exploration.
Materials and methods
Oncomine database analysis
We analyzed mRNA expression level of BCL2 and MCL1 in clinical specimens of NPC and normal nasopharyngeal tissues using the online cancer microarray database Oncomine (https://www.oncomine.org).Citation27 The Oncomine platform has 715 independent data sets with 86733 samples (Up to 04/30/2017). We used the search terms as follows: “Sample type: clinical specimen,” “Cancer type: Nasopharyngeal carcinoma,” “Analysis type: Cancer vs. Normal Analysis” and “Gene: BCL2” or “Gene: MCL1.” One data set with 10 nasopharynx samples and 31 nasopharyngeal carcinoma samples (GEO accession GSE12452)Citation28 was extracted depending on these terms. Each data was quoted from this data set which used Human Genome U133 Plus 2.0 Array platform. Reporter ID of BCL2 and MCL1 was 203685_at and 200798_x_at, respectively. The p-value was obtained from the median-ranked analysis.
Cell lines and regents
Human NPC cell lines HNE1 and CNE1-LMP1 were provided by the Cancer Research Institute of Central South University (Changsha, China). The human immortalized NPE cell line NP69 was provided by Sun Yat-sen University (Guangzhou, China). Both NPC cell lines were cultured in RPMI-1640 medium (Gibco, Cat. No.11875085) supplemented with 10% FBS (Gibco, Cat. No.10099141). NP69 was cultured in Defined Keratinocyte-SFM medium (Gibco, Cat. No.10744019) supplemented with bovine pituitary extract (50 μg/mL, Gibco, Cat. No.13028014) and epidermal growth factor (5 ng/mL, Gibco, Cat. No.PHG0311L). All cell lines were grown in a 5% CO2 atmosphere at 37°C.
All of TW-37 (Cat. No.S1121), CDDP (Cat. No.S1166) and 5-Fu (Cat. No.S1209) were purchased from Selleck Chemicals. The concentration of stock solution (dissolved in DMSO) of TW-37, CDDP and 5-Fu was 100 mM, 30 mM and 150 mM, respectively.
Western blotting
The protein of the indicated cells was isolated using M-PER™ Mammalian Protein Extraction Reagent (Thermo Fisher Scientific, Cat. No.78501) and was quantified using BCA Protein Assay Kit (Thermo Fisher Scientific, Cat. No.23227). Samples of equal protein concentration were subjected to SDS-PAGE and transferred onto nitrocellulose membrane (Whatman, Cat. No.10401396). Protein blots were incubated with primary antibodies against Bcl-2 (Cat. No.15071S), Mcl-1 (Cat. No.39224S) and β-actin (Cat. No. 3700S) (all from Cell Signaling Technology) and then with IRDye® 680LT Donkey anti-Rabbit IgG (H + L) (Cat. No. 926–68023) or IRDye® 800CW Goat anti-Mouse IgG (H + L) (Cat. No. 926–32210) (both from LI-COR Biosciences). The protein lanes were detected with an Odyssey® CLx Imaging System (LI-COR Biosciences). β-actin was used for normalization. The value of specific protein band was showed as fold change relative to corresponding β-actin.
Detection of cell viability
Cells were cultured at a density of 4, 000 cells per well in 96-well plates for 12 hours and then pretreated with the indicated concentration of TW-37 for 24 hours. Finally, cells received chemotherapeutics treatment. CDDP and 5-Fu were used at different concentrations (CDDP, 0, 1, 2 and 3 μM; 5-Fu, 0, 0.5, 1, 2, 4, 8, 16, 32, 64 μM). After 48 hours, cell viabilities of the indicated cells were measured with CCK8 (Dojindo Laboratories, Cat. No.CK04) according to the manufacturer's protocol. The cell viability of 5-Fu-treated cells was used to determine the IC50 value.
Recombinant adenoviral vectors infection
Recombinant adenovirus containing human Bcl-2 (Cat. No.GOCA3141051483) or Mcl-1 (Cat. No.GOCA3451015872) were obtained from Shanghai GeneChem. CNE-LMP1 cells were infected with Adv-Bcl-2 or Adv-Mcl-1 at MOI 10 concentration. After 48 hours, infected cells were detected Bcl-2 or Mcl-1 expression by western blot.
siRNA transfection
siRNA transfection was performed by siRNA Reagent System (Santa Cruz Biotechnology, Cat. No.sc-45064) according to the manufacturer's protocols. The following siRNAs were used: Control siRNA-A (Cat. No.sc-37007), Bcl-2 siRNA (h) (Cat. No.sc-29214) and Mcl-1 siRNA (h) (Cat. No. sc-35877) (all from Santa Cruz Biotechnology). At 72 hours post transfection, transfected CNE1-LMP1 cells were detected Bcl-2 or Mcl-1 expression by western blotting.
Annexin V-FITC/PtdIns double staining apoptosis detection
The indicated cells were stained with Annexin V Alexa fluor ®488 and PtdIns (Thermo Fisher Scientific, Cat. No.V13241) according to the manufacturer's protocol. 104 cells per sample were analyzed by a FACS Calibur (BD Biosciences) and apoptosis cells (Annexin V positive) were counted.
Detection of LDH release
The LDH release of the indicated cells was examined with a LDH Cytotoxicity Detection Kit (GenWay Biotech. Inc., Cat. No.GWB-787DA8) according to the manufacturer's Manual. The percentage LDH cytotoxicity is determined by the following equation: LDH cytotoxicity (%) = (exp. value - low control)/(high control - low control) × 100%. All experiments were repeated 3 times.
Clonogenic assay
The indicated NPC cells were cultured at 2, 000 cells per well in 6-well plates for 12 hours and then treated as indicated. After 10 or 14 days, the colonies were fixed with 4% paraformaldehyde (Cat. No.P0098) and stained with crystal violet (Cat. No.C0121) (both from Beyotime). Finally, the wells were captured with an IX73 microscope (Olympus Corporation) and the visible colonies (cell numbers > 50) were recorded.
In vivo experiments
All mice were obtained from Guangxi Medical University Laboratory Animal Center (Nanning, Guangxi, China) and kept in a specified pathogen-free animal facility. The care and use of laboratory animals were in accordance with the National Institutes of Health guide. All animal protocols were approved by the Animal Care Committee of Guangxi Medical University.
Male BALB/c nude mice (6 to 8 weeks old, weighing 15–18 g) were injected subcutaneously with 2 × 105 CNE1-LMP1 or HNE1 cells, respectively. Tumor volume was monitored by measuring the longest diameter (‘a’) and the shortest diameter (‘b’) of the tumor with electronic calipers and calculated with the formula: volume = 0.5*a*b*b. When tumor volume became about 200 mm3, mice were randomly divided into 4 groups: control group, TW-37 group, chemotherapeutics (CDDP or 5-Fu) group and TW-37 + chemotherapeutics (CDDP or 5-Fu) group. Each group had 8 mice, and there was no significant difference in tumor volume among 4 groups.
Then, TW-37-treated mice were received TW-37 (15 mg/kg, drug weight/body weight) by intraperitoneal injection daily for 10 days, and chemotherapeutics-treated mice were received CDDP (5 mg/kg) or 5-Fu (30 mg/kg) by intraperitoneal injection 3 times (once every 3 days) from the second day of TW-37 administration. Parts of mice (n = 3) were killed 24 hours post the last TW-37 administration and were used to detect tumor volumes and tumor cell apoptosis in each groups. Tumors were soaked in 4% paraformaldehyde for paraffin embedding. Tunel staining kit (Merck Millipore, Calbiochem, Cat. No. QIA39–1EACN) was used to evaluate the apoptosis level of tumor tissue sections according to the manufacturer's protocol. Meanwhile, tumor volume of chemotherapeutics group and TW-37 + chemotherapeutics group were measured daily to determine the time of treatment failure.
Male BALB/c mice (6 to 8 weeks old, weighing 15–18 g) were also randomly divided into 4 groups: CDDP group, TW-37+CDDP group, 5-Fu group and TW-37+5-Fu group. Each group had 5 mice, and there was no significant difference in body weight among 4 groups. Chemotherapeutics and TW-37 were administered to the mice in corresponding groups as described in the NPC tumor model. All mice were killed 24 hours post the last TW-37 administration. The weights of the whole body, liver, kidneys (left and right), spleen, thymus and (axillary, brachial and inguinal) lymph nodes were detected and recorded. Blood cell counts were measured with a COULTER LH 750 haematology analyzer (Beckman).
Statistical analysis
Differences were analyzed by Student's t-test and one-way ANOVA analysis. Data were expressed as mean ± SEM from at least 3 independent experiments. P< 0.05 was considered to be significant. GraphPad Prism 6.04 software (Graphpad Software) was used for all statistical analysis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
Funding
This work was supported by Guangxi Zhuang Autonomous Region Natural Science Foundation project (Grant #2011GXZSFA018236); Liuzhou City Science and technology research projects (Grant #2016G020204).
References
- Chua ML, Wee JT, Hui EP, Chan AT. Nasopharyngeal carcinoma. Lancet 2016; 387:1012-24; PMID:26321262; https://doi.org/10.1016/S0140-6736(15)00055-0
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136:E359-86; PMID:25220842; https://doi.org/10.1002/ijc.29210
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China. 2015. CA Cancer J Clin 2016; 66:115-32; PMID:26808342; https://doi.org/10.3322/caac.21338
- Xu C, Chen YP, Ma J. Clinical trials in nasopharyngeal carcinoma-past, present and future. Chin Clin Oncol 2016; 5:20; PMID:27121880; https://doi.org/10.21037/cco.2016.03.12
- Vogler M. Targeting BCL2-Proteins for the treatment of solid tumours. Adv Med 2014; 2014:943648; PMID:26556430; https://doi.org/10.1155/2014/943648
- Mohammad RM, Goustin AS, Aboukameel A, Chen B, Banerjee S, Wang G, Nikolovska-Coleska Z, Wang S, Al-Katib A. Preclinical studies of TW-37, a new nonpeptidic small-molecule inhibitor of Bcl-2, in diffuse large cell lymphoma xenograft model reveal drug action on both Bcl-2 and Mcl-1. Clin Cancer Res 2007; 13:2226-35; PMID:17404107; https://doi.org/10.1158/1078-0432.CCR-06-1574
- Verhaegen M, Bauer JA, Martin de la Vega C, Wang G, Wolter KG, Brenner JC, Nikolovska-Coleska Z, Bengtson A, Nair R, Elder JT, et al. A novel BH3 mimetic reveals a mitogen-activated protein kinase-dependent mechanism of melanoma cell death controlled by p53 and reactive oxygen species. Cancer Res 2006; 66:11348-59; PMID:17145881; https://doi.org/10.1158/0008-5472.CAN-06-1748
- Wang Z, Azmi AS, Ahmad A, Banerjee S, Wang S, Sarkar FH, Mohammad RM. TW-37, a small-molecule inhibitor of Bcl-2, inhibits cell growth and induces apoptosis in pancreatic cancer: Involvement of Notch-1 signaling pathway. Cancer Res 2009; 69:2757-65; PMID:19318573; https://doi.org/10.1158/0008-5472.CAN-08-3060
- Ouchi K, Kuwahara Y, Iehara T, Miyachi M, Katsumi Y, Tsuchiya K, Konishi E, Yanagisawa A, Hosoi H. A NOXA/MCL-1 imbalance underlies chemoresistance of malignant rhabdoid tumor cells. J Cell Physiol 2016; 231:1932-40; PMID:26680268; https://doi.org/10.1002/jcp.25293
- Wang H, Zhang Z, Wei X, Dai R. Small-molecule inhibitor of Bcl-2 (TW-37) suppresses growth and enhances cisplatin-induced apoptosis in ovarian cancer cells. J Ovarian Res 2015; 8:3; PMID:25823945; https://doi.org/10.1186/s13048-015-0130-x
- Lin X, Liu S, Luo X, Ma X, Guo L, Li L, Li Z, Tao Y, Cao Y. EBV-encoded LMP1 regulates Op18/stathmin signaling pathway by cdc2 mediation in nasopharyngeal carcinoma cells. Int J Cancer 2009; 124:1020-7; PMID:19048596; https://doi.org/10.1002/ijc.23767
- Du CW, Wen BG, Li DR, Lin YC, Zheng YW, Chen L, Chen JY, Lin W, Wu MY. Latent membrane protein-1 of Epstein - Barr virus increases sensitivity to arsenic trioxide-induced apoptosis in nasopharyngeal carcinoma cell. Exp Oncol 2005; 27:267-72; PMID:16404345
- Varadarajan S, Vogler M, Butterworth M, Dinsdale D, Walensky LD, Cohen GM. Evaluation and critical assessment of putative MCL-1 inhibitors. Cell Death Differ 2013; 20:1475-84; PMID:23832116; https://doi.org/10.1038/cdd.2013.79
- Brumatti G, Ekert PG. Seeking a MCL-1 inhibitor. Cell Death Differ 2013; 20:1440-1; PMID:24096932; https://doi.org/10.1038/cdd.2013.114
- Lee AW, Sze WM, Au JS, Leung SF, Leung TW, Chua DT, Zee BC, Law SC, Teo PM, Tung SY, et al. Treatment results for nasopharyngeal carcinoma in the modern era: The Hong Kong experience. Int J Radiat Oncol Biol Phys 2005; 61:1107-16; PMID:15752890; https://doi.org/10.1016/j.ijrobp.2004.07.702
- Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, Sun Y, Chen XZ, Li JG, Zhu XD, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase 3, multicentre, randomised controlled trial. Lancet Oncol 2016; 17:1509-20; PMID:27686945; https://doi.org/10.1016/S1470-2045(16)30410-7
- Hann CL, Daniel VC, Sugar EA, Dobromilskaya I, Murphy SC, Cope L, Lin X, Hierman JS, Wilburn DL, Watkins DN, et al. Therapeutic efficacy of ABT-737, a selective inhibitor of BCL-2, in small cell lung cancer. Cancer Res 2008; 68:2321-8; PMID:18381439; https://doi.org/10.1158/0008-5472.CAN-07-5031
- Kotschy A, Szlavik Z, Murray J, Davidson J, Maragno AL, Le Toumelin-Braizat G, Chanrion M, Kelly GL, Gong JN, Moujalled DM, et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 2016; 538:477-82; PMID:27760111; https://doi.org/10.1038/nature19830
- Renault TT, Elkholi R, Bharti A, Chipuk JE. B cell lymphoma-2 (BCL-2) homology domain 3 (BH3) mimetics demonstrate differential activities dependent upon the functional repertoire of pro- and anti-apoptotic BCL-2 family proteins. J Biol Chem 2014; 289:26481-91; PMID:25096574; https://doi.org/10.1074/jbc.M114.569632
- Vela L, Marzo I. Bcl-2 family of proteins as drug targets for cancer chemotherapy: The long way of BH3 mimetics from bench to bedside. Curr Opin Pharmacol 2015; 23:74-81; PMID:26079328; https://doi.org/10.1016/j.coph.2015.05.014
- Paik PK, Rudin CM, Pietanza MC, Brown A, Rizvi NA, Takebe N, Travis W, James L, Ginsberg MS, Juergens R, et al. A phase II study of obatoclax mesylate, a Bcl-2 antagonist, plus topotecan in relapsed small cell lung cancer. Lung Cancer 2011; 74:481-5; PMID:21620511; https://doi.org/10.1016/j.lungcan.2011.05.005
- Baggstrom MQ, Qi Y, Koczywas M, Argiris A, Johnson EA, Millward MJ, Murphy SC, Erlichman C, Rudin CM, Govindan R, et al. A phase II study of AT-101 (Gossypol) in chemotherapy-sensitive recurrent extensive-stage small cell lung cancer. J Thorac Oncol 2011; 6:1757-60; PMID:21918390; https://doi.org/10.1097/JTO.0b013e31822e2941
- Varin E, Denoyelle C, Brotin E, Meryet-Figuiere M, Giffard F, Abeilard E, Goux D, Gauduchon P, Icard P, Poulain L. Downregulation of Bcl-xL and Mcl-1 is sufficient to induce cell death in mesothelioma cells highly refractory to conventional chemotherapy. Carcinogenesis 2010; 31:984-93; PMID:20142415; https://doi.org/10.1093/carcin/bgq026
- Wang G, Nikolovska-Coleska Z, Yang CY, Wang R, Tang G, Guo J, Shangary S, Qiu S, Gao W, Yang D, et al. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. J Med Chem 2006; 49:6139-42; PMID:17034116; https://doi.org/10.1021/jm060460o
- Zeitlin BD, Spalding AC, Campos MS, Ashimori N, Dong Z, Wang S, Lawrence TS, Nor JE. Metronomic small molecule inhibitor of Bcl-2 (TW-37) is antiangiogenic and potentiates the antitumor effect of ionizing radiation. Int J Radiat Oncol Biol Phys 2010; 78:879-87; PMID:20675079; https://doi.org/10.1016/j.ijrobp.2010.04.024
- Zeitlin BD, Joo E, Dong Z, Warner K, Wang G, Nikolovska-Coleska Z, Wang S, Nor JE. Antiangiogenic effect of TW37, a small-molecule inhibitor of Bcl-2. Cancer Res 2006; 66:8698-706; PMID:16951185; https://doi.org/10.1158/0008-5472.CAN-05-3691
- Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM. ONCOMINE: A cancer microarray database and integrated data-mining platform. Neoplasia 2004; 6:1-6; PMID:15068665
- Sengupta S, den Boon JA, Chen IH, Newton MA, Dahl DB, Chen M, Cheng YJ, Westra WH, Chen CJ, Hildesheim A, et al. Genome-wide expression profiling reveals EBV-associated inhibition of MHC class I expression in nasopharyngeal carcinoma. Cancer Res 2006; 66:7999-8006; PMID:16912175; https://doi.org/10.1158/0008-5472.CAN-05-4399
