Cell polarity reflects the morphological, structural and functional organization of the cell along a so-called polarity axis. The cell ability to polarize in response to its microenvironment is a prerequisite for most cell behaviors including division, differentiation and migration as well as specialized tissue functions. Loss or perturbation of cell polarity is a hall-mark of cancers suggesting that alteration of the regulatory mechanisms of cell polarity may dramatically perturb the collective cell behavior required for the maintenance of multicellular organism integrity. Accordingly, many proteins implicated in the control of cell polarity are tumor suppressors.
The cytoskeleton - mainly composed of actin microfilaments, microtubules and intermediate filaments (IFs) - is a key cellular structure that is reorganized during polarization while contributing to polarized cell functions. For instance, during migration, the cell polarizes along a front-to-rear axis which is characterized by a biased polymerization of actin toward the cell front allowing forward movement. Simultaneously, the microtubule network reorients along the same axis and allows the maintenance of the front-rear asymmetry through the polarization of intracellular trafficking and the reinforcement of polarity signaling at the cell leading edge. Due to their polar filamentous structure along with their fast and tightly regulated dynamics, actin filaments and microtubules can quickly reorganize in response to local polarity cues. In contrast, IFs lack structural polarity and undergo a much slower cycle of assembly/disassembly. They form longer lived filaments, which may appear less prone to quickly reorganize and therefore to participate in polarized functions. Nevertheless, accumulating evidence demonstrates that IFs participate in cell polarization and directed migration through a cross-talk with the other cytoskeleton networks. Vimentin IFs control actin stress fiber assemblyCitation1 and orient traction forces that drive single-cell migration.Citation2 Thanks to their low turnover Vimentin IFs serve as template and stabilize microtubule organization, promoting the maintenance of the polarity axis.Citation3 These observations infer that IFs must, like and together with actin and microtubule, be dynamically controlled during cell polarization. The apparent discrepancy between the IF inherent properties and their dynamic functions calls for a better understanding of their dynamics and regulation.
Using a scratch-induced migration assay of primary rat astrocytes to spatially and temporally control cell polarization and migration, Leduc and Etienne-MannevilleCitation4 identified key regulatory processes controlling the organization of the IF network. They first showed that the steady-state distribution of astrocyte IFs (essentially composed of vimentin, nestin, and GFAP) throughout the cytoplasm results from a perpetual movement of mature filaments. The turnover of the IF network relies on the balance between an actin-based retrograde flow and a bidirectional microtubule-mediated transport, further underlying the necessity of a coordinated regulation of all the cytoskeletal structures. The contribution of each 3 modes of IF motion was determined by FRAP (fluorescence recovery after photobleaching) and photo-conversion experiments. These experiments also showed that IF precursors or particles transport followed by fusion at the tip of mature filaments and subunit exchange along pre-existing filaments make minor contributions to the global turnover of cytoplasmic IFs. As discussed by Robert et al.,Citation5 improvement of microscopy techniques allow the discrimination between individual IF transport and filament remodeling by subunit exchange, which was not possible in the initial studies performed in the 90s. Moreover, Leduc et al. observed that the polarization of the IF network following wounding is achieved by modulating the bidirectional transport along microtubules. Transport of IFs toward the leading edge by kinesin-1 motors is increased during migration promoting the polarized extension of the network during cell movement. In contrast, dynein-driven retrograde movement of IFs back to the cell center is drastically inhibited during the first hours following wounding, during which the cell is actively polarizing toward the wound edge. Most importantly, the signaling pathway responsible for this break in the symmetry of microtubule-mediated IF transport involves the key polarity proteins Cdc42 and atypical PKC (aPKC) (). These results reveal that the small GTPase Cdc42, which is known to be a crucial player in the control of cell polarity by regulating the activity of both actin filaments and microtubules, is also directly involved in the regulation of the third cytoskeleton filament type. However, this coordinated regulation of the different cytoskeletal networks involves different downstream signaling pathways. While Cdc42/Par6/aPKC control microtubule anchoring and reorientation via APC and Dlg,Citation6 the inhibition of IF retrograde transport is independent of Dlg.
Figure 1. Schematics of intermediate filament (IF) organization and regulation during cell polarization. During wound-induced astrocyte polarization, IF transport is biased from the cell center toward the cell front. Such asymmetry results from a Cdc42- and atypical PKC-dependent inhibition of the dynein-mediated IF transport.
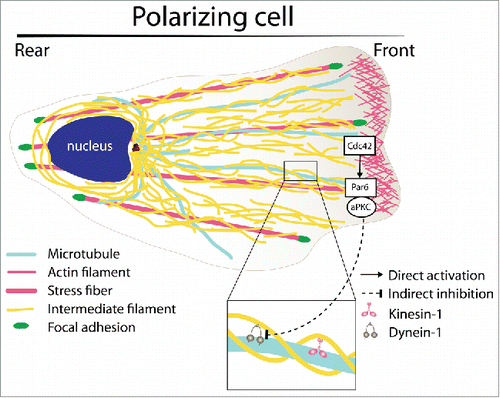
Whether other cargoes might be affected by Cdc42-mediated dynein inhibition remains unclear. Is dynein interaction with IFs specifically inhibited or is dynein activity globally decreased at the cell front? During cell polarization dynein is also involved in the anchoring of microtubules at the basal plasma membrane of the cell front, suggesting that the microtubule-associated pool of dynein although immobile is still active.Citation6 This would suggest that Cdc42 can, via aPKC, control post-translational modifications of IFs which would modulate their interaction with dynein. Although they are likely to play a key role in the regulation of IF transport, linkers coupling molecular motors to IFs still need to be identified. Finally, biochemical studies and in vitro reconstitution assays will be required to determine whether molecular motors specifically interact with certain IF proteins or indifferently bind all IF proteins. The latter would imply that changes in IF composition, such as that observed upon wounding, may modulate IF dynamics. Since dramatic changes of IF composition have been observed in cancers,Citation7 differences in IF dynamics may contribute to the alteration of cell polarity and migration of tumor cells.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Jiu Y, Peranen J, Schaible N, Cheng F, Eriksson JE, Krishnan R, Lappalainen P. Vimentin intermediate filaments control actin stress fiber assembly through GEF-H1 and RhoA. J Cell Sci 2017; 130:892-902; PMID:28096473; https://doi.org/10.1242/jcs.196881
- Costigliola N, Ding L, Burckhardt CJ, Han SJ, Gutierrez E, Mota A, Groisman A, Mitchison TJ, Danuser G. Vimentin fibers orient traction stress. Proc Natl Acad Sci U S A 2017; 114:5195-200; PMID:28465431; https://doi.org/10.1073/pnas.1614610114
- Gan Z, Ding L, Burckhardt CJ, Lowery J, Zaritsky A, Sitterley K, Mota A, Costigliola N, Starker CG, Voytas DF, Tytell J, Goldman RD, Danuser G. Vimentin intermediate filaments template microtubule networks to enhance persistence in cell polarity and directed migration. Cell Syst 2016; 3:500-01; PMID:27883892; https://doi.org/10.1016/j.cels.2016.11.011
- Leduc C, Etienne-Manneville S. Regulation of microtubule-associated motors drives intermediate filament network polarization. J Cell Biol 2017; 216(6):1689-703; PMID:28432079; https://doi.org/10.1083/jcb.201607045
- Robert A, Hookway C, Gelfand VI. Intermediate filament dynamics: What we can see now and why it matters. Bioessays 2016; 38:232-43; PMID:26763143; https://doi.org/10.1002/bies.201500142
- Manneville JB, Jehanno M, Etienne-Manneville S. Dlg1 binds GKAP to control dynein association with microtubules, centrosome positioning, and cell polarity. J Cell Biol 2010; 191:585-98; PMID:21041448; https://doi.org/10.1083/jcb.201002151
- Leduc C, Etienne-Manneville S. Intermediate filaments in cell migration and invasion: the unusual suspects. Curr Opin Cell Biol 2015; 32:102-12; PMID:25660489; https://doi.org/10.1016/j.ceb.2015.01.005
