ABSTRACT
Skp2 (S-phase kinase-associated protein 2) plays an oncogenic role in a variety of human cancers. However, the function of Skp2 in osteosarcoma (OS) is elusive. Therefore, in the current study, we explore whether Skp2 exerts its oncogenic function in OS. The cell growth, apoptosis, invasion and cell cycle were measured in OS cells after Skp2 overexpression. We found that overexpression of Skp2 enhanced cell growth, and inhibited cell apoptosis in OS cells. Moreover, we observed that upregulation of Skp2 accelerated cell cycle progression in OS cells. Furthermore, the ability of migration and invasion was enhanced in Skp2 overexpressing OS cells. Mechanically, our Western blotting data suggested that Skp2 decreased the expression of E-cadherin, Foxo1, p21, and p57, but increased MMP-9 in OS cells. In conclusion, our study demonstrated that Skp2 exhibited an oncogenic function in OS cells, suggesting that inhibition of Skp2 may be a novel approach for the treatment of OS.
Introduction
Osteosarcoma (OS) has become a common primary malignant bone tumor in adolescent and young adults.Citation1,Citation2 Overall incidence accounts for one to 3 per million annually worldwide.Citation3 Despite advance in both multi-agent chemotherapy and surgery of tumor resection, the survival rate of the OS patients has been remained to 60–80%.Citation4-6 Moreover, the 5-year survival rate of the OS patients with metastasis is approximate 30%.Citation7 The effect of therapy also depends on the response rate, because the OS size may not become smaller even though it was treated with chemotherapeutic agents.Citation8,Citation9 Thus, it is highly urgent to discover the molecular mechanism of OS development and to improve the poor survival rate of OS patients.
Skp2 (S-phase kinase-associated protein 2), an oncoprotein, consists of 4 distinct domains, known as destruction domain (D-box), nuclear localization signal (NLS), F-box domain, and C-terminal LRR domain.Citation10 As a critical component of SCF (Skp, cullin, F-box containing complex), Skp2 could recognize and ubiquitinate various substrates to play its biologic role in cell cycle progression and other cellular processes.Citation11,Citation12 The defined specific substrates of Skp2 consist of p21,Citation13 p27,Citation14 p57,Citation15 p130,Citation16 Tob1 (transducer of ERBB2),Citation17 FOXO1 (Forkhead box O1),Citation18 and Myc.Citation19-21 There is no doubt that Skp2 involves in many cellular processes including cell cycle regulation, cell proliferation, apoptosis, differentiation, and survival. All the processes have an intimate relationship with tumorigenesis and these need SCFSkp2 E3 complex mediated ubiquitination and degradation.Citation12 Due to that most substrates are tumor suppressors, Skp2 is considered as an oncoprotein. In line with this notion, overexpression of Skp2 is found in multiple cancer cells like lymphomas,Citation22 prostate cancer,Citation23 melanoma,Citation24 nasopharyngeal carcinoma,Citation25 pancreatic cancer,Citation26 breast carcinomas,Citation27,Citation28 cervical cancer,Citation29 and human gastric cancer.Citation30 One report indicates that androgen signaling pathway promotes cell growth by upregulating Skp2 and targeting p27.Citation31,Citation32 More importantly, the tumorigenesis caused by the loss of p19 Arf or the PTEN (phosphatase and tensin homolog) protein can be reversed by deleting the Skp2 in mice.Citation33 Moreover, another study shows that the acetylation of Skp2 and its cytoplasmic retention enhances cell migration by degrading the E-cadherin.Citation33,Citation34 Chan et al. determines that Skp2-SCF E3 ligase regulates Akt ubiquitination, herceptin sensitivity and tumorigenesis.Citation35 The group also reports that Skp2-SCF complex restricts cancer stem cell traits and cancer progression.Citation36 SIRT2 (Sirtuin 2) inhibited cell growth via impairing Skp2-mediated p27 degradation in non-small cell lung cancer.Citation37 Moreover, phosphorylation by mTORC1 stabilized Skp2 and regulated its oncogenic function in gastric cancer.Citation38 Additionally, β-TRCP (β-transducin repeat-containing homolog protein)-FBXW2 (F-box and WD repeat domain containing 2)-Skp2 axis controlled cancer cell growth via promoting Skp2 degradation.Citation39 Another study reported that Skp2 loss destabilized EZH2 (enhancer of zeste homolog 2) through promoting TRAF6 (TNF receptor associated factor 6)-mediated ubiquitination to suppress prostate cancer.Citation40 All these studies indicate that knockdown of Skp2 may be a new way for clinical therapy.Citation41 However, the exact mechanisms of Skp2 in modulation of tumorigenesis of OS are enigmatic.
In the current study, we explore whether Skp2 exerts its oncogenic function in OS. We found that overexpression of Skp2 led to acceleration of cell growth and inhibition of cell apoptosis. Furthermore, Skp2 promoted cell cycle entry into S phase. Notably, we found that Skp2 inhibited the expression of E-cadherin, FOXO1, p21, and p57 and increased MMP-9 (Matrix metallopeptidase 9) in OS cells. Taken together, our work provides a conceptual framework that Skp2 may be a therapeutic target for OS treatment. Inhibition of Skp2 could be helpful for achieving better treatment outcomes in patients with OS.
Results
Overexpression of Skp2 enhanced cell proliferation
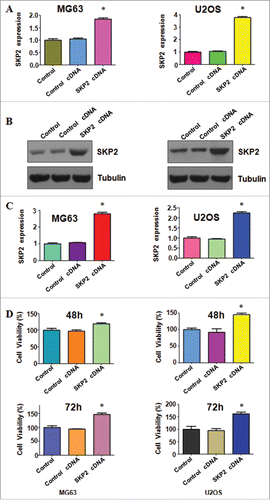
To investigate the role of Skp2 in OS cells, we transfected the Skp2 cDNA into OS cells and measured the Skp2 expression at mRNA and protein level in MG63 and U2OS cells. The real-time RT-PCR analysis results suggested that Skp2 mRNA level was increased in both MG63 and U2OS cells after Skp2 cDNA transfection (). Furthermore, our Western blotting data demonstrated that Skp2 protein level was upregulated with the Skp2 cDNA transfection in OS cells ( and ). The CTG assays were performed to measure the cell viability in both MG63 and U2OS cells transfected with Skp2 cDNA for 48 hours and 72 hours, respectively. Our result implied that overexpression of Skp2 enhanced cell proliferation in both OS cells ().
Figure 1. Overexpression of Skp2 enhanced cell proliferation. (A) The Skp2 mRNA level was detected by real-time PCR in both MG63 and U2OS cells with Skp2 cDNA transfection. (B) Western blot was performed to analyze the protein level of Skp2 in OS cells with Skp2 cDNA transfection. (C) Quantification of the Skp2 protein level was performed. *P < 0.05; ***P < 0.001. (D) Cell viability was measured by CTG solution at 48 hours and 72 hours in MG63 and U2OS cells after Skp2 overexpression.
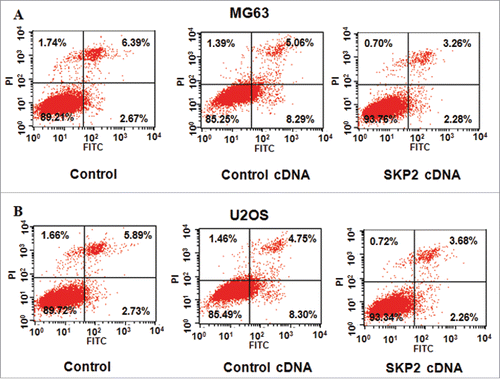
Overexpression of Skp2 inhibited cell apoptosis
To further investigate the role of Skp2 in OS cells, the PI-FITC-annexin assays were conducted to explore whether Skp2 could inhibit cell apoptosis. As we expected, the cell apoptotic death of both MG63 and U2OS cells was inhibited by Skp2 overexpression. Specifically, the percentage of cell apoptosis decreased from 13.35% in control cDNA group to 5.54% in Skp2 cDNA transfection group in MG63 cells (). Similarly, the cell apoptosis was inhibited from 13.05% to 5.94% after Skp2 overexpression in U2OS cells (). Our work indicated that Skp2 overexpression could inhibit cell apoptosis, which could partly led to cell growth promotion.
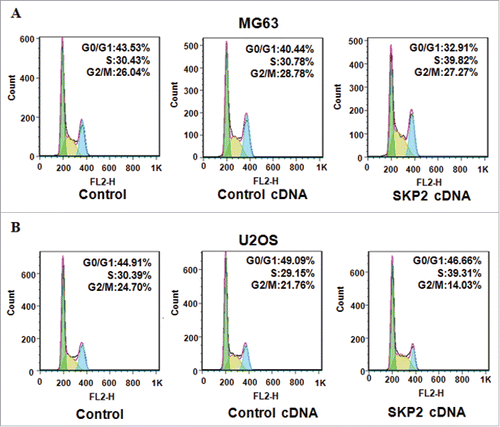
Overexpression of Skp2 enhanced cell cycle progression
To further determine how Skp2 promoted cell growth in OS cells, the cell cycle analysis was performed by PtdIns staining and flow cytometry in MG63 and U2OS cells. Our data revealed that the percentage of S phase was increased from 30.78% to 39.82% in MG63 cells after Skp2 cDNA transfection (). Meanwhile, the proportion of S phase was increased from 29.15% in control cDNA group to 39.31% in Skp2 overexpressing U2OS cells (). These data showed that Skp2 distinctly accelerated cell cycle into S phase in OS cells.
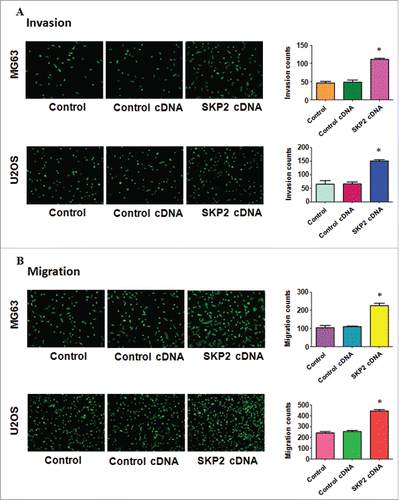
Overexpression of Skp2 promoted cell invasion and migration
The motility of OS cells with Skp2 overexpression was detected by invasion and migration assays. The Skp2 overexpressing cells increased penetration via matrigel-coated membrane compared with the control cells (). The transwell experiment was conducted to dissect the migration ability of OS cells. The number of cells was increased after Skp2 transfection in both MG63 and U2OS cells (). Taken together, Skp2 overexpression promoted the ability of cell motility in OS cell.
Figure 4. Overexpression of Skp2 promoted cell invasion and migration. (A) Left panel: The ability of cell invasion was measured via transwell assay in OS cells after Skp2 cDNA transfection. Right panel: Quantitative results are illustrated for left panel. (B) Left panel: The ability of cell migration was determined via transwell assay without Matrigel in OS cells after Skp2 cDNA transfection. Right panel: Quantitative results are illustrated for left panel.
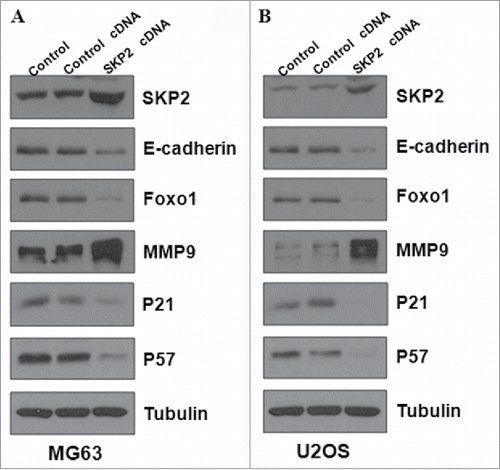
Overexpression of Skp2 regulated its downstream genes in OS cells
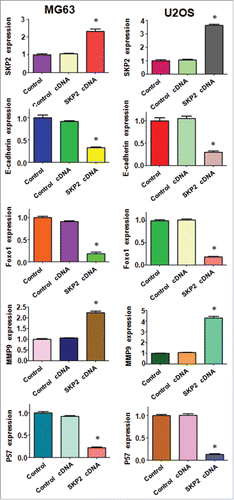
To further investigate the molecular mechanisms of Skp2-induced cell growth and invasion in OS cells, we performed Western blotting to detect the expression of Skp2 downstream genes in MG63 and U2OS cells. It has been reported that E-cadherin, FOXO1, p21 and p57 are the substrates of Skp2.Citation41 In line with this, we found that expression of E-cadherin and Foxo1 was significantly decreased in both MG63 and U2OS cells after Skp2 overexpression ( and ). Importantly, the protein level of cell cycle related genes p21 and p57 was markedly reduced, indicating that Skp2 was involved in cell cycle in part through targeting p21 and p57 in OS cells ( and ). Several studies have revealed that Skp2 enhanced MMP-9 expression in cancer cells.Citation42,Citation43 Therefore, we also measured the expression of MMP-9 in OS cells after Skp2 overexpression. We observed that MMP9 protein was increased markedly after Skp2 cDNA transfection ( and ). Altogether, these data implied that Skp2 exerted its oncogenic function partly through regulation of its downstream proteins in OS cells.
Figure 5. Overexpression of Skp2 regulated the related genes in OS cells. (A) The expression of downstream targets of Skp2 was detected by Western blotting in MG63 cells after Skp2 cDNA transfection. (B) Western blotting was performed to measure the expression of Skp2 downstream targets in U2OS cells with Skp2 overexpression.
Discussion
A wealth of evidence has demonstrated that Skp2 was critically involved in tumorigenesis including OS. It has been reported that Skp2 was highly expressed in OS tissue samples. Moreover, Skp2 expression was correlated with the relapse, metastasis, and survival rate in OS.Citation44 This finding implied that Skp2 could be a key oncoprotein in the occurrence and development of OS, and might be a prognostic indicator in OS.Citation44 One study has shown that knockdown of GLI2, one key driver in Hedgehog pathway, enhanced cell cycle arrest via reduction of Skp2 in OS cells.Citation45 Overexpression of GLI2 promoted cell proliferation and accelerated cell cycle progression via overexpression of Skp2 in OS cells, indicating that Skp2 played a pivotal role in regulation of cell growth in OS cells.Citation45 Another study found that Forkhead box M1 controlled the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase.Citation46 Consistent with the oncogenic role of Skp2 in OS cells, we found that overexpression of Skp2 enhanced cell growth and invasion, inhibited apoptosis and accelerated cell cycle progression in OS cells. Our study provided the direct evidence for oncogenic function of Skp2 in OS cells.
Considering the oncogenic role of Skp2 in various cancer cells, it may be a good alternative to target Skp2 or to find Skp2 inhibitor for clinical cancer therapy. The MG132, a normal proteasome inhibitor, could inhibit cell proliferation and promote cell apoptosis though downregulating Skp2 in lymphoma cells.Citation47 However, the patients treated with proteasome inhibitor have many side effects.Citation48 It is urgent to develop small molecular inhibitors without side effects to suppress Skp2. Cyclopamine, a specific inhibitor of SMO, slowed the cell growth and promoted cell cycle arrest via reducing the expression of Skp2 and subsequent induction of p21 in OS cells.Citation49 Moreover, inhibition of Notch pathway by its gamma secretase inhibitor prevents OS cell growth by cell cycle regulation via reduction of Skp2 expression.Citation50 One study reports that CpdA (compound A) could block Skp2 binding to the SCF complex and suppress cell proliferation by inhibiting cell cycle and promoting apoptosis in myeloma cells.Citation51 Moreover, CpdA is intensive to chemotherapeutic agents such as dexamethasone, doxorubicin, and melphalan, as well as proteasome inhibitor bortezomib in multiple myeloma.Citation51 Interestingly, SMIP0004, one chemical compound, downregulates Skp2 in prostate cancer cells and accumulates protein p27.Citation52 Recently, a new Skp2 inhibitor, compound 25, was found to restrict cancer stem cell traits and cancer progression.Citation36 There is limitation to use chemical compounds to suppress Skp2 due to the inappropriate in vivo for human cancer.
It is thought that “natural agents” may overcome these limitations and side effects. Recently, researchers found that curcumin, quercetin, lycopene, silibinin, epigallocatechin-3 gallate, could inhibit cell cycle progression and decrease the level of Skp2 in human cancers.Citation53-56 Saurolactam, a natural compound isolated from the aerial portions of Saururus chinensis, was reported to inhibit proliferation, migration, and invasion via reduction of Skp2 expression in human OS cells.Citation57 Additionally, 15,16-dihydrotanshinone I (DHTI), a lipophilic tanshinone extracted from Danshen root, was found to induce apoptosis and inhibit the cell proliferation, migration via suppression of Skp2 expression in OS cells.Citation58 Recently, rottlerin was found to exert its antitumor activity through inhibition of Skp2 in human cancer cells.Citation59,Citation60 Matrine derivative YF-18 inhibited cell proliferation and migration via downregulation of Skp2 in lung cancer.Citation61 It is required to discover new Skp2 inhibitors for the treatment of OS. In conclusion, our work validated the oncogenic role of Skp2 in OS, suggesting that targeting Skp2 could be an effective approach to treat OS.
Materials and methods
Cell culture and agents
The MG63 and U2OS cells were cultured in DMEM medium supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin in 5% CO2 and 37°C atmosphere. Primary antibodies for Skp2 and p57 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The E-cadherin was bought form Abcam. The antibodies for FOXO1 and p21 were obtained from Cell Signaling Technology (Danvers, MA, USA). The second antibodies used were purchased from Thermo Scientific. Lipofectamine 2000 was obtained from Invitrogen. Anti-tubulin and CTG were bought from Sigma-Aldrich (St. Louis, MO).
Transfection
Cells were seeded in 96-well or 6-well plates for overnight and transfected with control cDNA and Skp2 cDNA via lipofectamine 2000 following the instruction's protocol.Citation62 After the transfection, cells were used to further analysis as described under the results sections.
Cell viability assay
The cells were seeded in 96 wells for overnight. Skp2 cDNA and empty cDNA plasmid were transfected into cells. After 48 hours and 72 hours, 20ul of CTG were added to the wells and incubated for 10 min at 37°C. Then the reaction mixture was detected by the microplateau at 490 nm.
Cell apoptosis assay
Cells were seeded in 6-well plates and transfected with empty cDNA and Skp2 cDNA for 48 hours. Then the cells were collected and washed with 1 x PBS. Subsequently, cells were suspended in 500μl binding buffer consisting of 5 μl Propidium iodide (PtdIns) and 5 μl FITC-conjugated anti-Annexin V antibody. Apoptosis was measured by a FACScalibur flow cytometer (BD, USA) as described before.Citation63
Cell cycle analysis
Exponentially growing cells were seeded in a 6-well plate for overnight and then transfected with Skp2 cDNA and control cDNA. After 48 hours, cells were harvested and washed with cold PBS. The cells were resuspended in 70% cold alcohol and kept at 4°C overnight. On the next day, cells were washed with cold PBS and suspended in 1 × 106 cells/ml in PBS. Subsequently, cells were incubated with 0.1mg/ml RNase I and 50 mg/ml PtdIns at 37°C for 30 min. Cell cycle was further detected with a FACScalibur flow cytometer (BD, USA).
Cell migration assay
Cells were seeded and transfected with control cDNA and Skp2 cDNA. The transfected cells were seeded on the upper chamber without the matrigel-coated membrane in 200 μl serum-free medium. After 24h, the migrated cells were stained with 4 ug/mL calcein AM in Hanks’ buffered saline at 37°C for 1 h. Migration was assessed by calculating the migrating cells with a microscope.
Cell invasion assay
Trans-well chamber was conducted to measure tumor cell invasion. MG63 and U2OS were transfected with control cDNA and Skp2 cDNA. The transfected cells were seeded on the upper chamber with the matrigel-coated membrane in 200 μl serum-free medium. After 24h, the invaded cells were stained with 4 ug/mL calcein AM at 37°C for 1 h. Invasiveness was photographed by a microscope.
Quantitative real-time reverse transcription-PCR analysis
Total RNA was extracted from OS cells. Then the cDNA was generated by reverse transcription (RT) using RevertAid TM First Strand cDNA Synthesis Kits (Fermentas Life Sciences, Chicago, IL, USA) and oligo (dT) primers. PCR were performed using Power SYBR Green PCR Master Mix and the results were calculated by 2-ΔΔCt method as described previously.Citation62 The primers used in the PCR reaction are: Skp2, forward primer (5′– GCT GCT AAAGGT CTC TGG TGT -3′) and reverse primer (5′- AGG CTT AGA TTC TGC AAC TTG -3′); GAPDH, forward primer (5′- ACC CAG AAG ACT GTG GAT GG -3′) and reverse primer (5′- CAG TGA GCT TCC CGT TCA G- 3′).
Western Blotting analysis
Proteins were extracted and the cell lysates were loaded into each lane in equal amounts and resolved by SDS-PAGE transferred to nitrocellulose membranes. Then the membranes were incubated with the primary antibodies (anti-Skp2: 1:2000; anti-E-cadherin: 1:1000; anti-FOXO1: 1:1000; anti-MMP-9: 1:500; anti-p21: 1:1000; anti-p57: 1:1000; anti-tubulin: 1:5000) at 4°C overnight. On the second day, the primaries antibodies were washed and then second antibodies were added to the membranes for 1 hour. Subsequently, the expression of protein was measured by electrochemiluminescence assay.
Statistic analysis
All statistical data were conducted by GraphPad Prism 5.0 (Graph Pad Software, La Jolla, CA). Student t test was used to evaluate significance. The results were presented as means ± SD. P < 0.05 was considered as statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank our colleagues for their critical comments and help.
Funding
This work is supported by the program for graduate research innovation of Xinjiang Medical University (CXCY2017033) and the research innovation program of Xinjiang Medical University (XYDCX201695).
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017; 67:7-30; https://doi.org/10.3322/caac.21387
- Gianferante DM, Mirabello L, Savage SA. Germline and somatic genetics of osteosarcoma - connecting aetiology, biology and therapy. Nat Rev Endocrinol 2017; 13(8):480-491; PMID:28338660; https://doi.org/10.1038/nrendo.2017.16
- Chaiyawat P, Settakorn J, Sangsin A, Teeyakasem P, Klangjorhor J, Soongkhaw A, Pruksakorn D. Exploring targeted therapy of osteosarcoma using proteomics data. Onco Targets Ther 2017; 10:565-77; PMID:28203090; https://doi.org/10.2147/OTT.S119993
- Wu PK, Chen WM, Chen CF, Lee OK, Haung CK, Chen TH. Primary osteogenic sarcoma with pulmonary metastasis: clinical results and prognostic factors in 91 patients. Jpn J Clin Oncol 2009; 39:514-22; PMID:19525290; https://doi.org/10.1093/jjco/hyp057
- Iwamoto Y, Tanaka K, Isu K, Kawai A, Tatezaki S, Ishii T, Kushida K, Beppu Y, Usui M, Tateishi A, et al. Multiinstitutional phase II study of neoadjuvant chemotherapy for osteosarcoma (NECO study) in Japan: NECO-93J and NECO-95J. J Orthop Sci 2009; 14:397-404; PMID:19662473; https://doi.org/10.1007/s00776-009-1347-6
- Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D, Bernstein ML, Conrad E, Ferguson W, Gebhardt M, Goorin AM, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol 2005; 23:2004-11; PMID:15774791; https://doi.org/10.1200/JCO.2005.06.031
- Allison DC, Carney SC, Ahlmann ER, Hendifar A, Chawla S, Fedenko A, Angeles C, Menendez LR. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma 2012; 2012:704872; PMID:22550423; https://doi.org/10.1155/2012/704872
- Moore DD, Luu HH. Osteosarcoma. Cancer Treat Res 2014; 162:65-92; PMID:25070231
- Le Deley MC, Guinebretiere JM, Gentet JC, Pacquement H, Pichon F, Marec-Berard P, Entz-Werle N, Schmitt C, Brugieres L, Vanel D, et al. SFOP OS94: a randomised trial comparing preoperative high-dose methotrexate plus doxorubicin to high-dose methotrexate plus etoposide and ifosfamide in osteosarcoma patients. Eur J Cancer 2007; 43:752-61; PMID:17267204; https://doi.org/10.1016/j.ejca.2006.10.023
- Zheng N, Huo Z, Zhang B, Meng M, Cao Z, Wang Z, Zhou Q. Thrombomodulin reduces tumorigenic and metastatic potential of lung cancer cells by up-regulation of E-cadherin and down-regulation of N-cadherin expression. Biochem Biophys Res Commun 2016; 476:252-9; PMID:27223053; https://doi.org/10.1016/j.bbrc.2016.05.105
- Wang Z, Fukushima H, Inuzuka H, Wan L, Liu P, Gao D, Sarkar FH, Wei W. Skp2 is a promising therapeutic target in breast cancer. Front Oncol 2012; 1; PMID:22279619; https://doi.org/10.3389/fonc.2011.00057
- Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer 2008; 8:438-49; PMID:18500245; https://doi.org/10.1038/nrc2396
- Yu ZK, Gervais JL, Zhang H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21(CIP1/WAF1) and cyclin D proteins. Proc Natl Acad Sci U S A 1998; 95:11324-9; PMID:9736735; https://doi.org/10.1073/pnas.95.19.11324
- Tsvetkov LM, Yeh KH, Lee SJ, Sun H, Zhang H. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol 1999; 9:661-4; PMID:10375532; https://doi.org/10.1016/S0960-9822(99)80290-5
- Kamura T, Hara T, Kotoshiba S, Yada M, Ishida N, Imaki H, Hatakeyama S, Nakayama K, Nakayama KI. Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc Natl Acad Sci U S A 2003; 100:10231-6; PMID:12925736; https://doi.org/10.1073/pnas.1831009100
- Tedesco D, Lukas J, Reed SI. The pRb-related protein p130 is regulated by phosphorylation-dependent proteolysis via the protein-ubiquitin ligase SCF(Skp2). Genes Dev 2002; 16:2946-57; PMID:12435635; https://doi.org/10.1101/gad.1011202
- Hiramatsu Y, Kitagawa K, Suzuki T, Uchida C, Hattori T, Kikuchi H, Oda T, Hatakeyama S, Nakayama KI, Yamamoto T, et al. Degradation of Tob1 mediated by SCFSkp2-dependent ubiquitination. Cancer Res 2006; 66:8477-83; PMID:16951159; https://doi.org/10.1158/0008-5472.CAN-06-1603
- Huang H, Regan KM, Wang F, Wang D, Smith DI, van Deursen JM, Tindall DJ. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc Natl Acad Sci U S A 2005; 102:1649-54; PMID:15668399; https://doi.org/10.1073/pnas.0406789102
- Song MS, Song SJ, Kim SJ, Nakayama K, Nakayama KI, Lim DS. Skp2 regulates the antiproliferative function of the tumor suppressor RASSF1A via ubiquitin-mediated degradation at the G1-S transition. Oncogene 2008; 27:3176-85; PMID:18071316; https://doi.org/10.1038/sj.onc.1210971
- von der Lehr N, Johansson S, Wu S, Bahram F, Castell A, Cetinkaya C, Hydbring P, Weidung I, Nakayama K, Nakayama KI, et al. The F-box protein Skp2 participates in c-Myc proteosomal degradation and acts as a cofactor for c-Myc-regulated transcription. Mol Cell 2003; 11:1189-200; PMID:12769844; https://doi.org/10.1016/S1097-2765(03)00193-X
- Kim SY, Herbst A, Tworkowski KA, Salghetti SE, Tansey WP. Skp2 regulates Myc protein stability and activity. Mol Cell 2003; 11:1177-88; PMID:12769843; https://doi.org/10.1016/S1097-2765(03)00173-4
- Lim MS, Adamson A, Lin Z, Perez-Ordonez B, Jordan RC, Tripp S, Perkins SL, Elenitoba-Johnson KS. Expression of Skp2, a p27(Kip1) ubiquitin ligase, in malignant lymphoma: correlation with p27(Kip1) and proliferation index. Blood 2002; 100:2950-6; PMID:12351407; https://doi.org/10.1182/blood.V100.8.2950
- Yang G, Ayala G, De Marzo A, Tian W, Frolov A, Wheeler TM, Thompson TC, Harper JW. Elevated Skp2 protein expression in human prostate cancer: association with loss of the cyclin-dependent kinase inhibitor p27 and PTEN and with reduced recurrence-free survival. Clin Cancer Res 2002; 8:3419-26; PMID:12429629.
- Rose AE, Wang G, Hanniford D, Monni S, Tu T, Shapiro RL, Berman RS, Pavlick AC, Pagano M, Darvishian F, et al. Clinical relevance of SKP2 alterations in metastatic melanoma. Pigment Cell Melanoma Res 2011; 24:197-206; PMID:20883453; https://doi.org/10.1111/j.1755-148X.2010.00784.x
- Xu HM, Liang Y, Chen Q, Wu QN, Guo YM, Shen GP, Zhang RH, He ZW, Zeng YX, Xie FY, et al. Correlation of Skp2 overexpression to prognosis of patients with nasopharyngeal carcinoma from South China. Chin J Cancer 2011; 30:204-12; PMID:21352698; https://doi.org/10.5732/cjc.010.10403
- Schuler S, Diersch S, Hamacher R, Schmid RM, Saur D, Schneider G. SKP2 confers resistance of pancreatic cancer cells towards TRAIL-induced apoptosis. Int J Oncol 2011; 38:219-25; PMID:21109943
- Radke S, Pirkmaier A, Germain D. Differential expression of the F-box proteins Skp2 and Skp2B in breast cancer. Oncogene 2005; 24:3448-58; PMID:15782142; https://doi.org/10.1038/sj.onc.1208328
- Zheng WQ, Zheng JM, Ma R, Meng FF, Ni CR. Relationship between levels of Skp2 and P27 in breast carcinomas and possible role of Skp2 as targeted therapy. Steroids 2005; 70:770-4; PMID:16024059; https://doi.org/10.1016/j.steroids.2005.04.012
- Fu HC, Yang YC, Chen YJ, Lin H, Ou YC, Chien CC, Huang EY, Huang HY, Lan J, Chi HP, et al. Increased expression of SKP2 is an independent predictor of locoregional recurrence in cervical cancer via promoting DNA-damage response after irradiation. Oncotarget 2016; 7:44047-61; PMID:27317767.
- Masuda TA, Inoue H, Sonoda H, Mine S, Yoshikawa Y, Nakayama K, Nakayama K, Mori M. Clinical and biological significance of S-phase kinase-associated protein 2 (Skp2) gene expression in gastric carcinoma: modulation of malignant phenotype by Skp2 overexpression, possibly via p27 proteolysis. Cancer Res 2002; 62:3819-25; PMID:12097295
- Waltregny D, Leav I, Signoretti S, Soung P, Lin D, Merk F, Adams JY, Bhattacharya N, Cirenei N, Loda M. Androgen-driven prostate epithelial cell proliferation and differentiation in vivo involve the regulation of p27. Mol Endocrinol 2001; 15:765-82; PMID:11328857; https://doi.org/10.1210/mend.15.5.0640
- Lu L, Schulz H, Wolf DA. The F-box protein SKP2 mediates androgen control of p27 stability in LNCaP human prostate cancer cells. BMC Cell Biol 2002; 3:22; PMID:12188931; https://doi.org/10.1186/1471-2121-3-22
- Lin HK, Chen Z, Wang G, Nardella C, Lee SW, Chan CH, Yang WL, Wang J, Egia A, Nakayama KI, et al. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature 2010; 464:374-9; PMID:20237562; https://doi.org/10.1038/nature08815
- Inuzuka H, Gao D, Finley LW, Yang W, Wan L, Fukushima H, Chin YR, Zhai B, Shaik S, Lau AW, et al. Acetylation-dependent regulation of Skp2 function. Cell 2012; 150:179-93; PMID:22770219; https://doi.org/10.1016/j.cell.2012.05.038
- Chan CH, Li CF, Yang WL, Gao Y, Lee SW, Feng Z, Huang HY, Tsai KK, Flores LG, Shao Y, et al. The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, herceptin sensitivity, and tumorigenesis. Cell 2012; 149:1098-111; PMID:22632973; https://doi.org/10.1016/j.cell.2012.02.065
- Chan CH, Morrow JK, Li CF, Gao Y, Jin G, Moten A, Stagg LJ, Ladbury JE, Cai Z, Xu D, et al. Pharmacological inactivation of Skp2 SCF ubiquitin ligase restricts cancer stem cell traits and cancer progression. Cell 2013; 154:556-68; PMID:23911321; https://doi.org/10.1016/j.cell.2013.06.048
- Li Z, Huang J, Yuan H, Chen Z, Luo Q, Lu S. SIRT2 inhibits non-small cell lung cancer cell growth through impairing Skp2-mediated p27 degradation. Oncotarget 2016; 7:18927-39; PMID:26942878.
- Geng Q, Liu J, Gong Z, Chen S, Li X, Lu Y, Zhu X, Lin HK, Xu D. Phosphorylation by mTORC1 stablizes Skp2 and regulates its oncogenic function in gastric cancer. Mol Cancer 2017; 16:83; PMID:28446188; https://doi.org/10.1186/s12943-017-0649-0
- Xu J, Zhou W, Yang F, Chen G, Li H, Zhao Y, Liu P, Tan M, Xiong X, Sun Y. The beta-TrCP-FBXW2-SKP2 axis regulates lung cancer cell growth with FBXW2 acting as a tumour suppressor. Nat Commun 2017; 8:14002; PMID:28090088; https://doi.org/10.1038/ncomms14002
- Lu W, Liu S, Li B, Xie Y, Izban MG, Ballard BR, Sathyanarayana SA, Adunyah SE, Matusik RJ, Chen Z. SKP2 loss destabilizes EZH2 by promoting TRAF6-mediated ubiquitination to suppress prostate cancer. Oncogene 2017; 36:1364-73; PMID:27869166; https://doi.org/10.1038/onc.2016.300
- Wang Z, Gao D, Fukushima H, Inuzuka H, Liu P, Wan L, Sarkar FH, Wei W. Skp2: a novel potential therapeutic target for prostate cancer. Biochim Biophys Acta 2012; 1825:11-7; PMID:21963805.
- Hung WC, Tseng WL, Shiea J, Chang HC. Skp2 overexpression increases the expression of MMP-2 and MMP-9 and invasion of lung cancer cells. Cancer Lett 2010; 288:156-61; PMID:19625121; https://doi.org/10.1016/j.canlet.2009.06.032
- Yamada S, Yanamoto S, Naruse T, Matsushita Y, Takahashi H, Umeda M, Nemoto TK, Kurita H. Skp2 Regulates the Expression of MMP-2 and MMP-9, and Enhances the Invasion Potential of Oral Squamous Cell Carcinoma. Pathol Oncol Res 2016; 22:625-32; PMID:26874697; https://doi.org/10.1007/s12253-016-0049-6
- Liao QD, Zhong D, Chen Q. Protein expression of Skp2 in osteosarcoma and its relation with prognosis. Zhong nan da xue xue bao Yi xue ban 2008; 33:606-11; PMID:18667774.
- Nagao H, Ijiri K, Hirotsu M, Ishidou Y, Yamamoto T, Nagano S, Takizawa T, Nakashima K, Komiya S, Setoguchi T. Role of GLI2 in the growth of human osteosarcoma. J Pathol 2011; 224:169-79; PMID:21506130; https://doi.org/10.1002/path.2880
- Wang IC, Chen YJ, Hughes D, Petrovic V, Major ML, Park HJ, Tan Y, Ackerson T, Costa RH. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol Cell Biol 2005; 25:10875-94; PMID:16314512; https://doi.org/10.1128/MCB.25.24.10875-10894.2005
- Hussain AR, Ahmed M, Ahmed SO, Al-Thari S, Khan AS, Razack S, Platanias LC, Al-Kuraya KS, Uddin S. Proteasome inhibitor MG-132 mediated expression of p27Kip1 via S-phase kinase protein 2 degradation induces cell cycle coupled apoptosis in primary effusion lymphoma cells. Leuk Lymphoma 2009; 50:1204-13; PMID:19557642; https://doi.org/10.1080/10428190902951799
- Palumbo A, Gay F, Bringhen S, Falcone A, Pescosta N, Callea V, Caravita T, Morabito F, Magarotto V, Ruggeri M, et al. Bortezomib, doxorubicin and dexamethasone in advanced multiple myeloma. Ann Oncol 2008; 19:1160-5; PMID:18326520; https://doi.org/10.1093/annonc/mdn018
- Hirotsu M, Setoguchi T, Sasaki H, Matsunoshita Y, Gao H, Nagao H, Kunigou O, Komiya S. Smoothened as a new therapeutic target for human osteosarcoma. Mol Cancer 2010; 9:5; PMID:20067614; https://doi.org/10.1186/1476-4598-9-5
- Tanaka M, Setoguchi T, Hirotsu M, Gao H, Sasaki H, Matsunoshita Y, Komiya S. Inhibition of Notch pathway prevents osteosarcoma growth by cell cycle regulation. Br J Cancer 2009; 100:1957-65; PMID:19455146; https://doi.org/10.1038/sj.bjc.6605060
- Chen Q, Xie W, Kuhn DJ, Voorhees PM, Lopez-Girona A, Mendy D, Corral LG, Krenitsky VP, Xu W, Moutouh-de Parseval L, et al. Targeting the p27 E3 ligase SCF(Skp2) results in p27- and Skp2-mediated cell-cycle arrest and activation of autophagy. Blood 2008; 111:4690-9; PMID:18305219; https://doi.org/10.1182/blood-2007-09-112904
- Rico-Bautista E, Yang CC, Lu L, Roth GP, Wolf DA. Chemical genetics approach to restoring p27Kip1 reveals novel compounds with antiproliferative activity in prostate cancer cells. BMC Biol 2010; 8:153; PMID:21182779; https://doi.org/10.1186/1741-7007-8-153
- Roy S, Kaur M, Agarwal C, Tecklenburg M, Sclafani RA, Agarwal R. p21 and p27 induction by silibinin is essential for its cell cycle arrest effect in prostate carcinoma cells. Mol Cancer Ther 2007; 6:2696-707; PMID:17938263; https://doi.org/10.1158/1535-7163.MCT-07-0104
- Yang ES, Burnstein KL. Vitamin D inhibits G1 to S progression in LNCaP prostate cancer cells through p27Kip1 stabilization and Cdk2 mislocalization to the cytoplasm. J Biol Chem 2003; 278:46862-8; PMID:12954644; https://doi.org/10.1074/jbc.M306340200
- Huang HC, Lin CL, Lin JK. 1,2,3,4,6-penta-O-galloyl-beta-D-glucose, quercetin, curcumin and lycopene induce cell-cycle arrest in MDA-MB-231 and BT474 cells through downregulation of Skp2 protein. J Agric Food Chem 2011; 59:6765-75; PMID:21598989; https://doi.org/10.1021/jf201096v
- Huang HC, Way TD, Lin CL, Lin JK. EGCG stabilizes p27kip1 in E2-stimulated MCF-7 cells through down-regulation of the Skp2 protein. Endocrinology 2008; 149:5972-83; PMID:18719023; https://doi.org/10.1210/en.2008-0408
- Li Z, Liu H, Li B, Zhang Y, Piao C. Saurolactam Inhibits Proliferation, Migration, and Invasion of Human Osteosarcoma Cells. Cell Biochem Biophys 2015; 72:719-26; PMID:25627547; https://doi.org/10.1007/s12013-015-0523-x
- Chen X, Li Q, He Y, Du H, Zhan Z, Zhao H, Shi J, Ye Q, Hu J. 15,16-dihydrotanshinone I induces apoptosis and inhibits the proliferation, migration of human osteosarcoma cell line 143B in vitro. Anticancer Agents Med Chem 2015; https://doi.org/10.2174/1871520615666151019092919
- Yin X, Zhang Y, Su J, Hou Y, Wang L, Ye X, Zhao Z, Zhou X, Li Y, Wang Z. Rottlerin exerts its anti-tumor activity through inhibition of Skp2 in breast cancer cells. Oncotarget 2016; 7:66512-24; PMID:27582552
- Su J, Wang L, Yin X, Zhao Z, Hou Y, Ye X, Zhou X, Wang Z. Rottlerin exhibits anti-cancer effect through inactivation of S phase kinase-associated protein 2 in pancreatic cancer cells. Am J Cancer Res 2016; 6:2178-91; PMID:27822410
- Wu L, Wang G, Wei J, Huang N, Zhang S, Yang F, Li M, Zhou G, Wang L. Matrine derivative YF-18 inhibits lung cancer cell proliferation and migration through down-regulating Skp2. Oncotarget 2017; 8:11729-38; PMID:28036296.
- Ma J, Fang B, Zeng F, Ma C, Pang H, Cheng L, Shi Y, Wang H, Yin B, Xia J, et al. Down-regulation of miR-223 reverses epithelial-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Oncotarget 2015; 6:1740-9; PMID:25638153; https://doi.org/10.18632/oncotarget.2714
- Wang L, Ye X, Cai X, Su J, Ma R, Yin X, Zhou X, Li H, Wang Z. Curcumin suppresses cell growth and invasion and induces apoptosis by down-regulation of Skp2 pathway in glioma cells. Oncotarget 2015; 6:18027-37; PMID:26046466; https://doi.org/10.18632/oncotarget.4090