Oncogenic activation underlies cellular transformation and initiates tumorigenesis. Cellular transformation is driven by the ability of oncogenes to deregulate cell proliferation, apoptosis and metabolism. As transformed cells exhibit enhanced growth rates and consequently overactive biosynthetic pathways, they require high nutrient levels (especially glucose and glutamine) to maintain sufficient ATP production and to preserve redox balance. These render transformed cells particularly sensitive to reduced nutrient levels.Citation1 Cancer cells are indeed intermittently exposed to acute severe nutrient deprivation (ND) within the tumor microenvironment, as the tumor vasculature is often abnormal and suboptimal for nutrient delivery.Citation2 These environmental conditions are detrimental to tumors if they are unable to evolve mechanisms of adaptation to ND.
A key mechanism supporting tumor adaptation to ND is through the tight control of protein synthesis. As protein synthesis is a highly energy-consuming process, cells must rapidly inhibit this activity under ND to preserve tumor cell survival.Citation3 We previously reported that under acute ND, cancer cells promptly inhibit mRNA translation at the elongation step to save energy and prevent cell death. This occurs via activation of eukaryotic elongation factor 2 kinase (eEF2K) by AMP kinase and other nutrient-responsive pathways, leading to phosphorylation and inactivation of the translation elongation factor eEF2, a rate-limiting enzyme in translation elongation.Citation4
The MYC family of oncogenes, which contribute to more than 50% of all human cancer types, has profound effects on mRNA translation by increasing ribosome biogenesis and promoting the translation of many cellular mRNAs.Citation5 Consequently, MYC overexpression renders cells hypersensitive to death under acute ND,Citation1 suggesting that specific mechanisms must be in place concomitantly with MYC activation to balance the increased sensitivity to ND. The MYC family member MYCN is amplified and overexpressed in about 20% of pediatric neuroblastomas (NBs). This genetic alteration is a major poor prognostic factor in NB, independently characterizing a highly aggressive and therapy resistant subset of these tumors. We recently reported that eEF2K is overexpressed and hyperactive in MYCN amplified NB patient specimens compared with non-MYCN amplified tumors.Citation6 Moreover, both eEF2K mRNA expression and enzymatic activity are poor prognostic factors in NB.Citation6 We found that MYCN amplified NB cells require eEF2K to adapt and survive under ND, both in vitro and in vivo (), in contrast to non-MYCN amplified NB cells, which are not reliant on eEF2K for survival under these conditions. In particular, we observed that genetic inactivation of eEF2K leads to massive geographic necrosis in MYCN amplified subcutaneous NB xenografts in mice, particularly when animals were placed under caloric restriction (i.e. receiving ∼70% of normal caloric intake).Citation6 These results argue that eEF2K, which limits energy-demanding mRNA translation under ND, is necessary for cells to be tolerant of MYCN overexpression when energy sources are limiting.
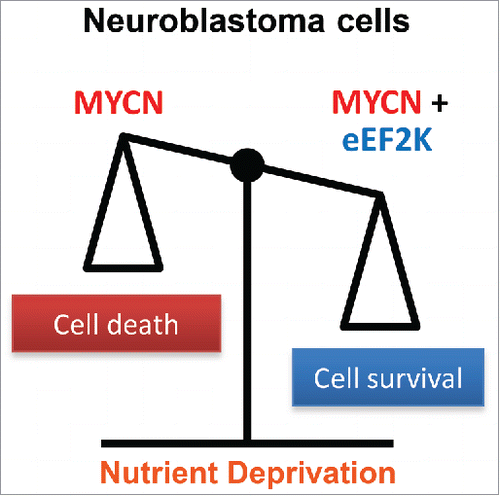
Figure 1. Schematic diagram of the effects of MYCN and eEF2K activity on neuroblastoma cell survival under nutrient deprivation, whereby high MYCN activity leads to cell death unless cells are protected through increased eEF2K activity.
Our studies do not support a direct (or indirect) role for MYCN in transcriptionally activating eEF2K expression, nor does MYCN increase eEF2K protein stability,Citation6 raising several intriguing questions. First, what is the mechanism by which MYCN amplified cells upregulate eEF2K expression? One possibility we have not ruled out is that MYCN increases eEF2K translation, although that would not explain why expression of eEF2K and MYCN mRNAs are so tightly correlated when the latter is amplified.Citation6 Another interesting possibility is that high eEF2K-expressing cells are somehow selected for in MYCN amplified clones, for example because high MYCN levels would otherwise be lethal to NB when nutrients become scarce. Second, since the eEF2K locus is not apparently amplified in NB,Citation6 what is the role of epigenetic regulation of eEF2K expression in NB, and does this link to selection of high-expressing eEF2K cells in the context of MYCN amplified NB? Third, do other MYC family overexpressing tumors, such as Group 3 and 4 medulloblastoma or glioblastoma, also require eEF2K for survival under ND?
Our findings further support the critical role of reduced mRNA translation in tumor cell adaptation to ND, and specifically highlight the importance of regulating the elongation step of translation in this process. Whereas control of mRNA translation has historically been linked mainly to the initiation step, emerging evidence now emphasize the regulatory role of elongation.Citation7 Given that MYC family members ramp up protein synthesis, it is tempting to speculate that, on the one hand, eEF2K is required to balance this function to keep the rate of protein synthesis under control, to prevent energy crisis and cell death under acute ND (). On the other hand, eEF2K activation may selectively promote the translation of other mRNAs with pro-survival and/or catabolic functions. Such preferentially translated mRNAs might be essential for the development of MYC family driven tumors, particularly when exposed to ND. Further and more specific studies are necessary to elucidate how loss of eEF2K activity may influence cellular transformation by MYC family proteins.
Finally, our observation that eEF2K inhibition and caloric restriction act synergistically to block MYCN amplified tumor growth in vivo opens the possibility for future therapeutic intervention. These should aim at combining eEF2K pharmacological inhibitors with caloric restriction regimen or caloric restriction mimetic drugs, some of which are already safely used in a clinical setting for pathologies such as diabetes. Such combinatory approaches might be particularly relevant for pediatric tumors where MYC family proteins play a major oncogenic role, such as in NB and aggressive brain tumors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by a Canadian Cancer Society Research Institute (CCSRI) Impact Grant to PHS (grant #703205).
References
- Shim H, Chun YS, Lewis BC, Dang CV. A unique glucose-dependent apoptotic pathway induced by c-Myc. Proc Natl Acad Sci U S A 1998; 95(4):1511-6; PMID:9465046; https://doi.org/10.1073/pnas.95.4.1511
- Wek RC, Staschke KA. How do tumours adapt to nutrient stress? EMBO J 2010; 29(12):1946-7; PMID:20551969; https://doi.org/10.1038/emboj.2010.110
- Leprivier G, Rotblat B, Khan D, Jan E, Sorensen PH. Stress-mediated translational control in cancer cells. Biochim Biophys Acta 2015; 1849(7):845-60; PMID:25464034; https://doi.org/10.1016/j.bbagrm.2014.11.002
- Leprivier G, Remke M, Rotblat B, Dubuc A, Mateo AR, Kool M, et al. The eEF2 kinase confers resistance to nutrient deprivation by blocking translation elongation. Cell 2013; 153(5):1064-79; PMID: 23706743; https://doi.org/10.1016/j.cell.2013.04.055
- Cole MD, Cowling VH. Transcription-independent functions of MYC: regulation of translation and DNA replication. Nat Rev Mol Cell Biol 2008; 9(10):810-5; PMID:18698328; https://doi.org/10.1038/nrm2467
- Delaidelli A, Negri GL, Jan A, Jansonius B, El-Naggar A, Lim JKM, et al. MYCN amplified neuroblastoma requires the mRNA translation regulator eEF2 kinase to adapt to nutrient deprivation. Cell Death Differ 2017; 24(9):1564–1576; PMID:28574509; https://doi.org/10.1038/cdd.2017.79
- Richter JD, Coller J. Pausing on Polyribosomes: Make way for elongation in translational control. Cell 2015; 163(2):292-300; PMID:26451481; https://doi.org/10.1016/j.cell.2015.09.041
