ABSTRACT
Nasopharyngeal carcinoma (NPC) is a kind of head-neck malignant tumor derived from the nasopharyngeal epithelium and is mainly prevalent in Southern China and Southeast Asia countries. Cisplatin (DDP) provides the first-line therapeutic administration in NPC patients. However, chemoresistance has been a main barrier and caused bad treatment outcome in NPC therapy. To understand the molecular mechanism of acquired resistance to DDP, multiple methods were performed to examine the morphocytology and molecular changes in DDP-resistant NPC cells. We found that drug resistance cells displayed epithelial-mesenchymal transition (EMT) characteristics. DDP-resistant NPC cells exhibited enhanced migration and invasion potential. Moreover, overexpression of TAZ, one key gene in Hippo pathway, is closely associated with the DDP resistance of NPC cells and its EMT properties. Depletion of TAZ in DDP-resistant cells reversed EMT phenotypes to MET characteristics and restored chemosensitivity of DDP-resistant cells to DDP treatment. These results suggest that inactivation of TAZ could be a promising approach for the treatment of NPC patients.
Introduction
Nasopharyngeal carcinoma (NPC) is a kind of rare non-lymphomatous squamous-cell malignant tumor. Meanwhile, NPC is an endemic cancer with a relatively high incidence in Southeast Asian countries and Southern China.Citation1 It has been reported that the annual incidence of NPC in Cantonese population in China to be over 20/100000, which counts for about 78% of the head and neck malignant tumors in recent years.Citation1,Citation2 NPC is relatively uncommon in the United States with about 1.6 per 100,000 diagnosed in 2015.Citation3 The infection of Epstein-Barr virus is thought to be the main inducing factor for NPC.Citation4,Citation5 Other factors, such as genetic susceptibility, smoking and diet habits, have also been considered to be involved in NPC initiation and development.Citation6-8 However, the detailed molecular regulatory mechanisms have not been fully understood yet.
Cisplatin is a platinum-based antineoplastic chemotherapy medication used to treat various types of solid malignancies including NPC.Citation9 Although high initial cisplatin responsiveness is obtained, the majority of NPC patients will develop acquired resistance shortly after, eventually resulting in relapse or metastases.Citation10,Citation11 The underlining mechanisms of drug resistance are still elusive. Multiple studies have implicated that epithelial-mesenchymal transition (EMT) contributes to invasion, distant metastases and acquired chemoresistance in human cancers.Citation12 EMT is characterized as a transition from the epithelial cell phenotype into a mesenchymal phenotype, which is functionally displayed by reduced cell adhesion and enhanced cell migration. At the molecular level, downregulation of epithelial cell markers (e.g. E-cadherin and β-catenin) and upregulation of stromal cell markers (e.g., Vimentin, N-cadherin, Slug, Twist and zinc finger E-box binding homeobox 1 (ZEB1) and ZEB2) were associated with EMT.Citation13,Citation14 Luo and the colleagues found that the spindle-shaped NPC cells showed obvious features of EMT.Citation15 Previous studies also revealed that blocking PI3K (Phosphoinositide 3-kinase)/Akt signaling significantly attenuated metastasis of NPC cells through reversing the process of EMT to MET (Mesenchymal to epithelial transition).Citation16 TGF-β1 (Transforming growth factor-β 1)/FMNL3 (Formin-like 3) signaling was identified to mediate EMT in NPC and closely associated with NPC metastasis.Citation17 More recently, it is reported that paclitaxel-resistant NPC cells underwent EMT, and developed multidrug resistance.Citation18 These studies highlight the clinical application potential of targeting EMT in NPC.
The Hippo pathway, which consists MST1/2 (mammalian sterile 20-like 1/2), SAV1 (Salvador), LATS1/2 (large tumor suppressor homolog 1/2), MOB1 (MOB kinase activator 1) and YAP (Yes-associated protein)/TAZ (Transcriptional co-activator with PDZ binding motif), is a highly conserved signaling cascade in mammals. This pathway has been demonstrated as a key regulator of organ size, tissue regeneration and cancer.Citation19-21 YAP and TAZ, 2 key downstream targets and effectors, are believed to mediate the biologic functions of the Hippo pathway by regulating gene transcription.Citation19 TAZ have attracted broad attention for its remarkable biologic properties in tumorigenesis.Citation22-26 It is reported that TAZ is required to maintain the self-renewal traits of breast cancer stem cells, and more importantly, activation of TAZ confers the tumor-initiation capacity on breast cancer cells.Citation27 Overexpression of TAZ induces mesenchymal marker expression and results in high-grade tumors in a murine model of glioma.Citation28 After translocation to the nucleus and interaction with TEAD, TAZ promotes cell proliferation, migration, invasion, and EMT.Citation29-35 Activated TAZ is also demonstrated to contribute to drug resistance and cancer recurrence. For instance, high YAP/TAZ activity in cultured cancer cells is responsible for resistance to drugs such as taxol, tamoxifen, and leads to tumor growth.Citation27,Citation36-38
In the present study, we developed DDP-resistant NPC cells. CNE1/DDP and CNE2/DDP cells acquired resistance to DDP and underwent EMT. We also provide evidence that high level of TAZ is closely associated with the DDP resistance of NPC cells and its EMT properties.
Results
Establishment of DDP-resistant human nasopharyngeal carcinoma cell lines
DDP-resistant human nasopharyngeal carcinoma cell lines were developed by continuous stepwise selection in increasing concentrations of DDP from the parental cell lines CNE1 and CNE2 for more than 6 months. Multiple biologic changes of DDP-resistant cell lines were determined. As shown in , MTT assay revealed the reliable human nasopharyngeal carcinoma cell lines CNE1/DDP and CNE2/DDP were successfully established. DDP-resistant cells produced a resistance to 4 μM DDP. During the period of culturing in drug-free medium, the IC50 (half maximal inhibitory concentration) was measured at monthly intervals to make sure the stable resistance to DDP.
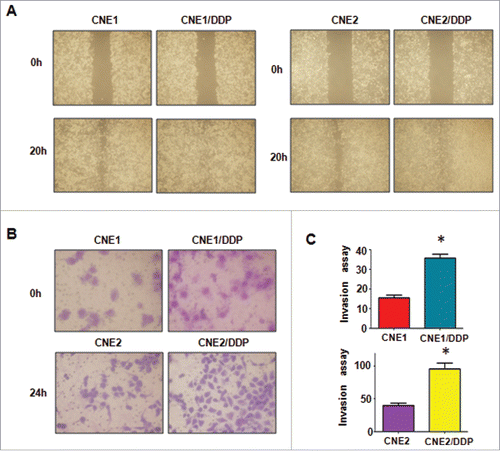
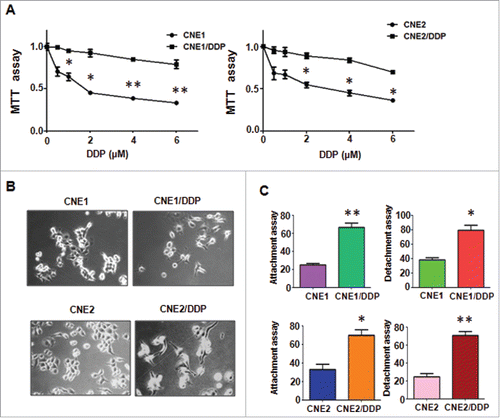
Figure 1. Cisplatin-resistant (CR) cells (CNE/DDP) exhibited EMT phenotype. A, MTT assay was performed in parental and CR NPC cells. *P < 0.05; **P < 0.01 vs control. B, Cell morphology was observed by microscopy in parental and CR cells. C, Cell attachment and attachment assays were conducted in parental and CR cells. *P < 0.05; **P < 0.01 vs control.
Morphological changes of DDP-resistant cells
It is known that drug-resistant cells exhibited EMT phenotype. Both CNE1/2 and CNE1/2 DDP-resistant cells grew adherent to the disk with a mesenchymal-like shape as polygon (). CNE1/2 cells exhibited relatively uniform cell size and shape, whereas their DDP-resistant cells displayed irregular shape and varied in size with fibroblastoid morphology.
EMT features of DDP-resistant NPC cells
As induction of EMT was documented to associate with aggressive characteristics, such as cell migration, and invasion, we detected these biologic changes of DDP-resistant cell lines. Compared with parental cells, we observed that DDP-resistant cells obtained an enhanced attachment and detachment capacity (). By wound healing assay, we detected that DDP-resistant NPC cells had acquired intensive motility activity (). Moreover, we found more DDP-resistant invaded cells through a Matrigel-coated membrane than their parental cells ( & ).
DDP-resistant NPC cells undergo EMT molecular marker changes
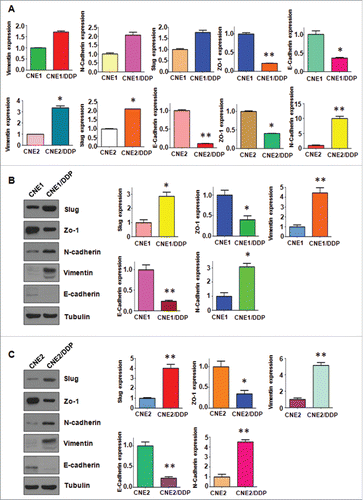
To demonstrate whether the resistant cells undergo EMT molecular marker changes, we compared the mRNA and protein levels of several EMT markers in resistant cells and their paired parental cells, respectively. As shown in , RT-PCR analysis showed the mRNA levels of epithelial molecules Zo-1 and E-cadherin were significantly decreased in DDP-resistant cells, whereas the mesenchymal markers such as Slug, Vimentin, and N-cadherin, were highly increased in DDP-resistant cells. Furthermore, our Western blotting analysis demonstrated the similar EMT markers changes in DDP-resistant cells at protein levels ( & ). Taken together, these results suggest that DDP-resistant NPC cells acquired EMT characteristics, and their mesenchymal phenotype could be responsible for the DDP resistance in NPC.
Figure 3. Cisplatin-resistant NPC cells have EMT marker changes. A, Real-time RT-PCR assay was conducted to detect the expression of EMT markers in parental and CR cells. *P < 0.05; **P < 0.01 vs control. B-C, Left panel: Western blotting analysis was used to detect the expression of E-cadherin, Zo-1, N-cadherin, E-cadherin, and Vimentin in CNE1/DDP (B) and CNE2/DDP cells (C). Right panel: Quantitative results are illustrated for left panel. *P < 0.05; **P < 0.01 vs control.
TAZ overexpression is associated with DDP-resistance in NPC cells
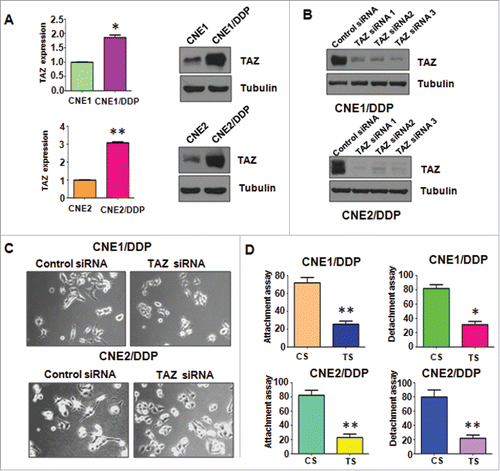
Accumulating evidence has proved that TAZ is actively involved in EMT in human cancers. In accordance with this, we found that TAZ was markedly elevated in DDP-resistant NPC cells at mRNA and protein levels, respectively (). The result suggested that TAZ is involved in DDP-induced EMT and could play a critical role in EMT in human cancers.
Figure 4. Cisplatin-resistant cells have high expression of TAZ. A, Left panel: Real-time RT-PCR assay was used to detect the expression of TAZ in parental and CR cells. *P < 0.05; **P < 0.01vs control. Right panel, Western blotting analysis was used to detect the expression of TAZ2 in parental and CR cells. B, Western blotting analysis was used to measure the efficacy of TAZ siRNA transfection. C, Cell morphology was taken by microscopy in CR cells transfected with TAZ siRNA. D, Cell attachment and detachment assays were performed in CR cells transfected with TAZ siRNA. *P < 0.05 vs control. CS: control siRNA; TS: TAZ siRNA. *P < 0.05; **P < 0.01 vs control siRNA.
Depletion of TAZ reverses EMT to MET in DDP-resistance NPC cells
TAZ was effectively knocked down in both CNE1/DDP and CNE2/DDP cells by transfection of 3 siRNAs targeting TAZ sequences (). TAZ siRNA3 was used for the subsequent transfection to further determine whether TAZ has a critical role in DDP-induced EMT. showed that knockdown of TAZ could partially reverse the EMT morphological features to mesenchymal–epithelial transition (MET) in DDP-resistant NPC cells. TAZ siRNA transfection also significantly abrogated the attachment and detachment capacity of DDP-resistant NPC cells ().
Depletion of TAZ reduces motility in DDP-resistant NPC cells
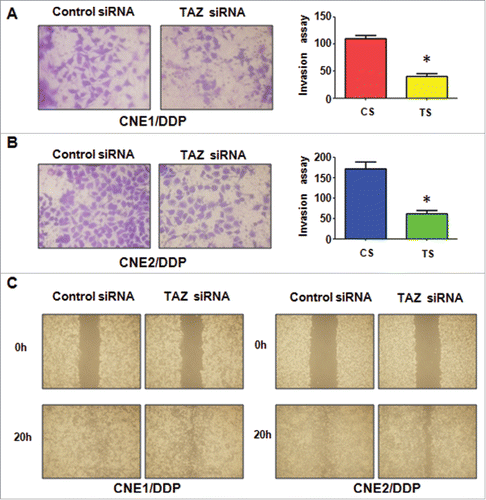
The effect of TAZ depletion on cell mobility was detected after TAZ siRNA. Transwell assay showed that the invaded DDP-resistant cells were significantly reduced by TAZ depletion ( &). In line with this, TAZ siRNA also suppressed the mobility of DDP-resistant NPC cells (). All of these results demonstrated that TAZ could play an important role in regulation of the migration and invasion characteristics in DDP-resistant NPC cells.
Figure 5. Down-regulation of TAZ inhibits motility and invasion in CR cells. A-B, Invasion assay were performed in TAZ siRNA-transfected CNE1/DDP (A) and CNE2/DDP cells (B). Right panel: Quantitative results are illustrated for left panel. *P < 0.05 vs control. C, Wound healing assays were performed to measure the motility in CR cells transfected with TAZ siRNA.
Depletion of TAZ modulates the expression of EMT markers
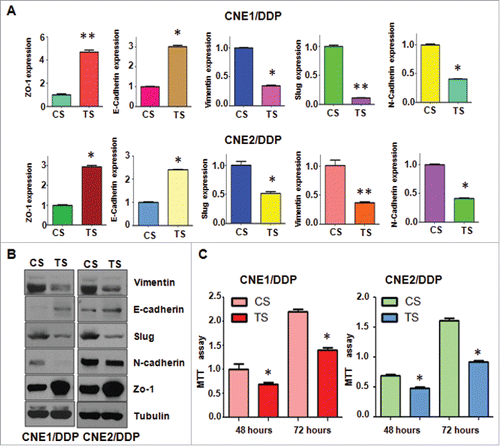
We also detected whether depletion of TAZ in DDP-resistant NPC cells could modulate the expression of EMT molecules by q–PCR and western blotting analysis, respectively. We found that depletion of TAZ in the resistant cells caused a markedly elevated expression of epithelial marker E-cadherin and Zo-1 at both mRNA and protein levels ( & ). While, the mesenchymal markers including Vimentin, Slug and N-cadherin was significantly suppressed ( & ). Taken together, these findings demonstrated that the DDP-resistant induced EMT features of NPC cells could be attenuated by TAZ depletion.
Figure 6. Depletion of TAZ regulates expression of EMT markers in PR cells. A, Real-time RT-PCR was performed to quantify mRNA expression of EMT markers in CR cells transfected with TAZ siRNA. *P< 0.05; **P< 0.01 compared with control siRNA. B, Western blotting analysis was used to measure the expression of EMT markers in CR cells transfected with TAZ siRNA. C, MTT assay was performed in CNE/DDP cells treated with TAZ siRNA. *P < 0.05 vs control siRNA.
Depletion of TAZ enhances the sensitivity of resistant NPC cells to DDP treatment
We performed MTT assay to further determine whether depletion of TAZ could affect the drug sensitivity of the DDP-resistant NPC cells. Our result showed that DDP treatment-induced cell growth inhibition in the resistant cells was antagonized by TAZ depletion (). This demonstrated that TAZ depleted NPC cells were more sensitive to DDP treatment, and TAZ could be a promising target to overcome drug resistance in human cancers.
Discussion
NPC is a kind of head and neck malignant cancer derived from nasopharyngeal epithelium, with the highest incidence in Southern China.Citation39 DDP is one of the most widely used antineoplastic drugs with good outcomes to treat various types of human malignancies, including NPC, lung, breast, mesothelium.Citation40,Citation41 However, development of cisplatin resistance, either acquired or intrinsic, is the major cause of treatment failure in NPC patients.Citation42 Therefore, exploration of the mechanism of drug resistance in NPC and development of new therapeutic strategies to prolong survival of patients is of importance.
It was reported that EMT contributes to invasion, distant metastases and acquired chemoresistance in human cancers.Citation12 In line with this, we observed that the DDP-resistant NPC cells developed EMT phenotype. At the molecular level, the resistant cells showed decreased expression of epithelial cell markers (E-cadherin and Zo-1) and increased mesenchymal cell markers (Slug, N-cadherin, and Vimentin). Moreover, we also provide evidence that overexprssion of TAZ is closely associated with the DDP resistance of NPC cells and its EMT properties. Depletion of TAZ reversed the EMT features to MET and restored the DDP sensitivity in resistant NPC cells. It has been reported that TAZ could regulate E-cadherin expression in human cancers.Citation26,Citation43 E-cadherin is an important factor to be involved in triggering EMT process.Citation44 Therefore, overexpression of TAZ induced EMT partly due to inhibition of E-cadherin in NPC cells. Taken together, our results suggested that TAZ could be a novel therapeutic target to overcome drug resistance in NPC patients.
Accumulating data have demonstrated that EMT is closely associated with cancer cell chemoresistance. It was reported that gemcitabine-resistant pancreatic cancer cells and hepatocellular carcinoma cells underwent EMT progression.Citation45,Citation46 Paclitaxel-resistant in breast cancer cells and NPC cells also exhibited EMT phenotypes.Citation18,Citation47 Cisplatin treatment inhibited the expression of E-cadherin and promoted the expression of vimentin in bladder cancer cell, and the cisplatin sensitivity was remarkably enhanced after EMT blockage.Citation48 It was also showed that EMT was necessary for acquired resistance to cisplatin and increased the metastatic potential of NPC cells.Citation49 Corresponded with these reports, our results showed that DDP-resistant NPC cells acquired EMT features and enhanced migration and invasion potential. It has been reported that TAZ was found to associate with EMT progression and drug resistance.Citation50 Chan and colleagues provide evidence that TAZ regulates cell proliferation, migration and EMT in ovarian cancer cells, and MET could be induced by TAZ-knockdown.Citation29 In breast cancer stem cells, TAZ is required for metastatic activity and chemoresistance, conversely, loss of TAZ severely impaired metastatic colonization and chemoresistance.Citation51,Citation52 Overexpression of TAZ promotes temozolomide (TMZ) resistance by inhibiting TMZ-induced apoptosis in glioma cells, and depletion of TAZ restores TMZ sensitivity of TMZ-resistant glioma cells.Citation53 Indeed, we observed high level of TAZ in DDP-resistant NPC cells which expressed EMT marker molecules and displayed EMT phenotype. Interestingly, depletion of TAZ reversed EMT to MET phenotype and restored the DDP sensitivity of DDP-resistant cells. All these results together indicated that inhibition of TAZ will not only suppress cancer initiation and progression, but also potentially sensitize cancer cells to chemotherapies and prevent cancer relapse.Citation19
In conclusion, the present study showed that activated TAZ was involved at least in part in DDP-induced EMT-like properties and drug resistance of NPC cells. Moreover, depletion of TAZ with siRNA oligos inhibited the migration and invasion capacity of DDP-resistant NPC cells and enhanced chemosensitivity of resistant cells to DDP treatment. These results suggested that activation of TAZ signaling may be a novel mechanism regulating EMT in NPC, which is closely relevant to NPC metastasis. Thus, inactivation of TAZ could be a promising approach for the treatment of NPC patients.
Materials and methods
Cell culture and reagents
Human nasopharyngeal carcinoma cell lines CNE1 and CNE2 were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 U/ml). The reagents DDP and MTT [3-(4,5-dimethythi-azol- 2-yl)-2,5-diphenyl tetrazolium bromide] were purchased from Sigma (St Louis, MO, USA). Matrigel was purchased from BD Biosciences (Bedford, MA, USA). Lipofectamine 2000 was purchased from Invitrogen (Waltham, MA USA). Primary antibodies against Slug, Zo-1, N-cadherin, E-cadherin, Vimentin, TAZ and Tubulin were bought from Santa Cruz Biotechnology (Santa Cruz, CA, USA). To generate DDP-resistant cell lines, CNE1 and CNE2 cells were exposed to increasing concentrations of DDP for more than 6 months.Citation54,Citation55 The morphological changes of DDP-resistant cells were observed and photographed.
Cell proliferation assay
Both NPC cells and DDP-resistant cells were seeded in 96-well plates and incubated overnight. Then, different concentrations of DDP were added. The cells were treated for 2 or 3 d. MTT assay was performed for cell viability measurement by a spectrophotometer at 570 nm.Citation56
Cell attachment and detachment
For attachment assay, 5 × 104 pretreated cells were seeded in each well of 24-well plates and incubated for 1 hour. Then the unattached cells were removed and the attached cells were counted. Cells were seeded and incubated for 24h for cell detachment assay. The cells underwent 0.05% trypsinization for 3min were counted as detached cells.
Wound healing assay
The NPC and DDP-resistant cells were cultured in 6-well plate. After the cells reach to almost 100% confluence, the supernatant was absorbed and the scratch wound was generated by scraping the surface cells with a pipette tip. The cells were rinsed carefully with PBS and then supplemented with medium. The wound healing images at 0 h and 20 h were photographed, respectively.Citation57
Invasion assay
The cells invasion capacities were determined by Matrigel precoated 24-well Transwell inserts according to the manufacturer protocol. The NPC cells, DDP-resident cells and TAZ siRNA transfected cells were cultured in the upper-chamber of the inserts with 200 μl serum-free RPMI-1640 medium. Medium with 10% FBS was added to the under chamber. After about 24 h, the invading cells on the bottom surface of the membrane were stained with Giemsa and photographed under a microscope.
Real-time quantitative RT-PCR
Total RNAs of NPC cells, DDP-resident cells and TAZ siRNA transfected cells were extracted and transcribed into cDNA according to the manufacturer's protocol. The mRNA expression levels of TAZ and EMT associated markers, including Vimentin, Slug, Zo-1, E-cadherin and N-cadherin, were detected using SYBR green assay kit on applied biosystems 7500.Citation58,Citation59 The mRNA levels were normalized with GAPDH.
Transfection
Cells were seeded in 60mm dishes and transfected with TAZ siRNAs (GenePharma, Shanghai, China) using Lipofectamine 2000 following the manufacture's instruments.Citation60
Western blotting analysis
The harvested cells were washed with PBS and then resuspended in proteinlysis buffer. The bicinchoninic acid (BCA, Thermo Scientific, Haverhill, MA) protein assay was performed to measure the protein concentrations. 40 µg of proteins samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred onto membranes. The membranes were immunoblotted with proper primary antibodies at 4˚C overnight. After membranes were washed with TBST and probed with second antibody at room temperature for 1 hour. The expression of proteins was detected with gel imaging equipment (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Statistical analysis was evaluated using GraphPad Prism 4.0 (Graph pad Software, La Jolla, CA, USA). The statistical significance was analyzed using the 2-tailed Student's t-test. Data are presented with means ± SEM.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by grants from the Knowledge Innovation Project of Shenzhen (JCYJ20150402152130190), the Science and Technology Foundation of Zhuhai (PC20081088 and 20161027F060002), and the Science and Technology Foundation of Guangdong Province, China (2016A020215030, 2014A030313101, and 2017A020215180).
References
- Wei KR, Zheng RS, Zhang SW, Liang ZH, Ou ZX, Chen WQ. Nasopharyngeal carcinoma incidence and mortality in China in 2010. Chin J Cancer 2014; 33:381-7. PMID:25096544
- Sun R, Wang X, Li X. Correlation Analysis of Nasopharyngeal Carcinoma TNM Staging with Serum EA IgA and VCA IgA in EBV and VEGF–C and –D. Med Sci Monit 2015; 21:2105-9. doi:10.12659/MSM.893415. PMID:26191775
- Wu EL, Riley CA, Hsieh MC, Marino MJ, Wu XC, McCoul ED. Chronic sinonasal tract inflammation as a precursor to nasopharyngeal carcinoma and sinonasal malignancy in the United States. Int Forum Allergy Rhinol 2017. doi:10.1002/alr.21956
- Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet 2005; 365:2041-54. doi:10.1016/S0140-6736(05)66698-6. PMID:15950718
- Feng FT, Cui Q, Liu WS, Guo YM, Feng QS, Chen LZ, Xu M, Luo B, Li DJ, Hu LF, et al. A single nucleotide polymorphism in the Epstein–Barr virus genome is strongly associated with a high risk of nasopharyngeal carcinoma. Chin J Cancer 2015; 34:563-72. doi:10.1186/s40880-015-0073-z. PMID:26675171
- Liu Z, Chang ET, Liu Q, Cai Y, Zhang Z, Chen G, Huang QH, Xie SH, Cao SM, Shao JY, et al. Quantification of familial risk of nasopharyngeal carcinoma in a high–incidence area. Cancer 2017; 123(14):2716-2725. doi:10.1002/cncr.30643. PMID:28241094
- Wang C, Lin XL, Fan YY, Liu YT, Zhang XL, Lu YK, Xu CH, Chen YM. Diet quality scores and risk of Nasopharyngeal Carcinoma in Chinese adults: a case–control study. Nutrients 2016; 8:112. doi:10.3390/nu8030112. PMID:26927167
- Lin JH, Jiang CQ, Ho SY, Zhang WS, Mai ZM, Xu L, Lo CM, Lam TH. Smoking and nasopharyngeal carcinoma mortality: a cohort study of 101,823 adults in Guangzhou, China. BMC Cancer 2015; 15:906. doi:10.1186/s12885-015-1902-9. PMID:26573573
- Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, Sun Y, Chen XZ, Li JG, Zhu XD, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 2016; 17:1509-20. doi:10.1016/S1470-2045(16)30410-7. PMID:27686945
- Hsu CH, Chen CL, Hong RL, Chen KL, Lin JF, Cheng AL. Prognostic value of multidrug resistance 1, glutathione–S–transferase–pi and p53 in advanced nasopharyngeal carcinoma treated with systemic chemotherapy. Oncology 2002; 62:305-12. doi:10.1159/000065061. PMID:12138237
- Wang X, Masters JR, Wong YC, Lo AK, Tsao SW. Mechanism of differential sensitivity to cisplatin in nasopharyngeal carcinoma cells. Anticancer Res 2001; 21:403-8. PMID:11299769
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 2009; 9:265-73. doi:10.1038/nrc2620. PMID:19262571
- Ksiazkiewicz M, Markiewicz A, Zaczek AJ. Epithelial–mesenchymal transition: a hallmark in metastasis formation linking circulating tumor cells and cancer stem cells. Pathobiology 2012; 79:195-208. doi:10.1159/000337106. PMID:22488297
- Samatov TR, Tonevitsky AG, Schumacher U. Epithelial–mesenchymal transition: focus on metastatic cascade, alternative splicing, non–coding RNAs and modulating compounds. Mol Cancer 2013; 12:107. doi:10.1186/1476-4598-12-107. PMID:24053443
- Luo WR, Chen XY, Li SY, Wu AB, Yao KT. Neoplastic spindle cells in nasopharyngeal carcinoma show features of epithelial–mesenchymal transition. Histopathology 2012; 61:113-22. doi:10.1111/j.1365-2559.2012.04205.x. PMID:22486228
- Jiang H, Gao M, Shen Z, Luo B, Li R, Jiang X, Ding R, Ha Y, Wang Z, Jie W. Blocking PI3K/Akt signaling attenuates metastasis of nasopharyngeal carcinoma cells through induction of mesenchymal–epithelial reverting transition. Oncol Rep 2014; 32:559-66. PMID:24889918
- Wu Y, Shen Z, Wang K, Ha Y, Lei H, Jia Y, Ding R, Wu D, Gan S, Li R, et al. High FMNL3 expression promotes nasopharyngeal carcinoma cell metastasis: role in TGF–beta1–induced epithelia–to–mesenchymal transition. Sci Rep 2017; 7:42507. doi:10.1038/srep42507. PMID:28198387
- Hou Y, Zhu Q, Li Z, Peng Y, Yu X, Yuan B, Liu Y, Liu Y, Yin L, Peng Y, et al. The FOXM1–ABCC5 axis contributes to paclitaxel resistance in nasopharyngeal carcinoma cells. Cell Death Dis 2017; 8:e2659. doi:10.1038/cddis.2017.53. PMID:28277541
- Yu FX, Zhao B, Guan KL. Hippo pathway in organ Size control, tissue homeostasis, and cancer. Cell 2015; 163:811-28. doi:10.1016/j.cell.2015.10.044. PMID:26544935
- Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer 2013; 13:246-57. doi:10.1038/nrc3458. PMID:23467301
- Deng J, Lei W, Xiang X, Zhang L, Lei J, Gong Y, Song M, Wang Y, Fang Z, Yu F, et al. Cullin 4A (CUL4A), a direct target of miR–9 and miR–137, promotes gastric cancer proliferation and invasion by regulating the Hippo signaling pathway. Oncotarget 2016; 7:10037-50. PMID:26840256
- Piccolo S, Cordenonsi M, Dupont S. Molecular pathways: YAP and TAZ take center stage in organ growth and tumorigenesis. Clin Cancer Res 2013; 19:4925-30. doi:10.1158/1078-0432.CCR-12-3172. PMID:23797907
- Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov 2014; 13:63-79. doi:10.1038/nrd4161. PMID:24336504
- Li W, Dong S, Wei W, Wang G, Zhang A, Pu P, Jia Z. The role of transcriptional coactivator TAZ in gliomas. Oncotarget 2016; 7:82686-99. PMID:27764783
- Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell 2016; 29:783-803. doi:10.1016/j.ccell.2016.05.005. PMID:27300434
- Brusgard JL, Choe M, Chumsri S, Renoud K, MacKerell AD, Jr, Sudol M, Passaniti A. RUNX2 and TAZ–dependent signaling pathways regulate soluble E–Cadherin levels and tumorsphere formation in breast cancer cells. Oncotarget 2015; 6:28132-50. doi:10.18632/oncotarget.4654. PMID:26320173
- Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, Poletti A, et al. The Hippo transducer TAZ confers cancer stem cell–related traits on breast cancer cells. Cell 2011; 147:759-72. doi:10.1016/j.cell.2011.09.048. PMID:22078877
- Bhat KP, Salazar KL, Balasubramaniyan V, Wani K, Heathcock L, Hollingsworth F, James JD, Gumin J, Diefes KL, Kim SH, et al. The transcriptional coactivator TAZ regulates mesenchymal differentiation in malignant glioma. Genes Dev 2011; 25:2594-609. doi:10.1101/gad.176800.111. PMID:22190458
- Chen G, Xie J, Huang P, Yang Z. Overexpression of TAZ promotes cell proliferation, migration and epithelial–mesenchymal transition in ovarian cancer. Oncol Lett 2016; 12:1821-5. PMID:27588129
- Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, Zhao S, Xiong Y, Guan KL. TAZ promotes cell proliferation and epithelial–mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol 2008; 28:2426-36. doi:10.1128/MCB.01874-07. PMID:18227151
- Zhang H, Liu CY, Zha ZY, Zhao B, Yao J, Zhao S, Xiong Y, Lei QY, Guan KL. TEAD transcription factors mediate the function of TAZ in cell growth and epithelial–mesenchymal transition. J Biol Chem 2009; 284:13355-62. doi:10.1074/jbc.M900843200. PMID:19324877
- Xie D, Cui J, Xia T, Jia Z, Wang L, Wei W, Zhu A, Gao Y, Xie K, Quan M. Hippo transducer TAZ promotes epithelial mesenchymal transition and supports pancreatic cancer progression. Oncotarget 2015; 6:35949-63. PMID:26416426
- Li Z, Wang Y, Zhu Y, Yuan C, Wang D, Zhang W, Qi B, Qiu J, Song X, Ye J, et al. The Hippo transducer TAZ promotes epithelial to mesenchymal transition and cancer stem cell maintenance in oral cancer. Mol Oncol 2015; 9:1091-105. doi:10.1016/j.molonc.2015.01.007. PMID:25704916
- Xiao H, Jiang N, Zhou B, Liu Q, Du C. TAZ regulates cell proliferation and epithelial–mesenchymal transition of human hepatocellular carcinoma. Cancer Sci 2015; 106:151-9. doi:10.1111/cas.12587. PMID:25495189
- Wang Q, Xu Z, An Q, Jiang D, Wang L, Liang B, Li Z. TAZ promotes epithelial to mesenchymal transition via the upregulation of connective tissue growth factor expression in neuroblastoma cells. Mol Med Rep 2015; 11:982-8. PMID:25354978
- Lai D, Ho KC, Hao Y, Yang X. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res 2011; 71:2728-38. doi:10.1158/0008-5472.CAN-10-2711. PMID:21349946
- Zhou X, Wang S, Wang Z, Feng X, Liu P, Lv XB, Li F, Yu FX, Sun Y, Yuan H, et al. Estrogen regulates Hippo signaling via GPER in breast cancer. J Clin Invest 2015; 125:2123-35. doi:10.1172/JCI79573. PMID:25893606
- Fujimoto D, Ueda Y, Hirono Y, Goi T, Yamaguchi A. PAR1 participates in the ability of multidrug resistance and tumorigenesis by controlling Hippo–YAP pathway. Oncotarget 2015; 6:34788-99. PMID:26431277
- Chan JW, Parvathaneni U, Yom SS. Reducing radiation–related morbidity in the treatment of nasopharyngeal carcinoma. Future Oncol 2017; 13:425-31. doi:10.2217/fon-2016-0410. PMID:27875901
- Townsend DM, Tew KD, He L, King JB, Hanigan MH. Role of glutathione S–transferase Pi in cisplatin–induced nephrotoxicity. Biomed Pharmacother 2009; 63:79-85. doi:10.1016/j.biopha.2008.08.004. PMID:18819770
- Dugbartey GJ, Peppone LJ, de Graaf IA. An integrative view of cisplatin–induced renal and cardiac toxicities: Molecular mechanisms, current treatment challenges and potential protective measures. Toxicology 2016; 371:58-66. doi:10.1016/j.tox.2016.10.001. PMID:27717837
- Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin resistance: a cellular self–defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev 2012; 64:706-21. doi:10.1124/pr.111.005637. PMID:22659329
- Matteucci E, Maroni P, Luzzati A, Perrucchini G, Bendinelli P, Desiderio MA. Bone metastatic process of breast cancer involves methylation state affecting E–cadherin expression through TAZ and WWOX nuclear effectors. Eur J Cancer 2013; 49:231-44. doi:10.1016/j.ejca.2012.05.006. PMID:22717556
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol 2014; 15:178-96. doi:10.1038/nrm3758. PMID:24556840
- Wang Z, Li Y, Kong D, Banerjee S, Ahmad A, Azmi AS, Ali S, Abbruzzese JL, Gallick GE, Sarkar FH. Acquisition of epithelial–mesenchymal transition phenotype of gemcitabine–resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res 2009; 69:2400-7. doi:10.1158/0008-5472.CAN-08-4312. PMID:19276344
- Wu Q, Wang R, Yang Q, Hou X, Chen S, Hou Y, Chen C, Yang Y, Miele L, Sarkar FH, et al. Chemoresistance to gemcitabine in hepatoma cells induces epithelial–mesenchymal transition and involves activation of PDGF–D pathway. Oncotarget 2013; 4:1999-2009. doi:10.18632/oncotarget.1471. PMID:24158561
- Yang Q, Huang J, Wu Q, Cai Y, Zhu L, Lu X, Chen S, Chen C, Wang Z. Acquisition of epithelial–mesenchymal transition is associated with Skp2 expression in paclitaxel–resistant breast cancer cells. Br J Cancer 2014; 110:1958-67. doi:10.1038/bjc.2014.136. PMID:24642627
- Chen DJ, Chen W, Jiang H, Yang H, Wang YC, Chen JH. Downregulation of DOCK1 sensitizes bladder cancer cells to cisplatin through preventing epithelial–mesenchymal transition. Drug Des Devel Ther 2016; 10:2845-53. doi:10.2147/DDDT.S101998. PMID:27660415
- Zhang P, Liu H, Xia F, Zhang QW, Zhang YY, Zhao Q, Chao ZH, Jiang ZW, Jiang CC. Epithelial–mesenchymal transition is necessary for acquired resistance to cisplatin and increases the metastatic potential of nasopharyngeal carcinoma cells. Int J Mol Med 2014; 33:151-9. PMID:24173500
- Zhou X, Lei QY. Regulation of TAZ in cancer. Protein Cell 2016; 7:548-61. doi:10.1007/s13238-016-0288-z. PMID:27412635
- Bartucci M, Dattilo R, Moriconi C, Pagliuca A, Mottolese M, Federici G, Benedetto AD, Todaro M, Stassi G, Sperati F, et al. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene 2015; 34:681-90. doi:10.1038/onc.2014.5. PMID:24531710
- Zhao Y, Yang X. Regulation of sensitivity of tumor cells to antitubulin drugs by Cdk1–TAZ signalling. Oncotarget 2015; 6:21906-17. doi:10.18632/oncotarget.4259. PMID:26183396
- Tian T, Li A, Lu H, Luo R, Zhang M, Li Z. TAZ promotes temozolomide resistance by upregulating MCL–1 in human glioma cells. Biochem Biophys Res Commun 2015; 463:638-43. doi:10.1016/j.bbrc.2015.05.115. PMID:26043698
- Wang R, Li Y, Hou Y, Yang Q, Chen S, Wang X, Wang Z, Yang Y, Chen C, Wu Q. The PDGF–D/miR–106a/Twist1 pathway orchestrates epithelial–mesenchymal transition in gemcitabine resistance hepatoma cells. Oncotarget 2015; 6:7000-10. doi:10.18632/oncotarget.3193. PMID:25760076
- Yang Q, Huang J, Wu Q, Cai Y, Zhu L, Lu X, Chen S, Chen C, Wang Z. Acquisition of epithelial–mesenchymal transition is associated with Skp2 expression in paclitaxel–resistant breast cancer cells. Br J Cancer 2014; 110:1958-67. doi:10.1038/bjc.2014.136. PMID:24642627
- Wang L, Ye X, Cai X, Su J, Ma R, Yin X, Zhou X, Li H, Wang Z. Curcumin suppresses cell growth and invasion and induces apoptosis by down–regulation of Skp2 pathway in glioma cells. Oncotarget 2015; 6:18027-37. doi:10.18632/oncotarget.4090. PMID:26046466
- Zhou X, Su J, Feng S, Wang L, Yin X, Yan J, Wang Z. Antitumor activity of curcumin is involved in down–regulation of YAP/TAZ expression in pancreatic cancer cells. Oncotarget 2016; 7:79076-88. PMID:27738325
- Ma J, Zeng F, Ma C, Pang H, Fang B, Lian C, Yin B, Zhang X, Wang Z, Xia J. Synergistic reversal effect of epithelial–to–mesenchymal transition by miR–223 inhibitor and genistein in gemcitabine–resistant pancreatic cancer cells. Am J Cancer Res 2016; 6:1384-95. PMID:27429851
- Yang Q, Wang Y, Lu X, Zhao Z, Zhu L, Chen S, Wu Q, Chen C, Wang Z. MiR–125b regulates epithelial–mesenchymal transition via targeting Sema4C in paclitaxel–resistant breast cancer cells. Oncotarget 2015; 6:3268-79. doi:10.18632/oncotarget.3065. PMID:25605244
- Wang L, Hou Y, Yin X, Su J, Zhao Z, Ye X, Zhou X, Zhou L, Wang Z. Rottlerin inhibits cell growth and invasion via down–regulation of Cdc20 in glioma cells. Oncotarget 2016; 7:69770-82. PMID:27626499