Schwann cells (SCs), the glial cells of the peripheral nervous system (PNS), protect axons and enwrap them with multilayered myelin sheaths that facilitate saltatory nerve conduction.Citation1 Myelin synthesis by SCs is one of the most spectacular examples for the interplay between cellular anabolism and cell-cell interactions. Unraveling of the underlying mechanisms has implications for the development of treatments of a wide spectrum of conditions associated with defective PNS myelination such as diabetic neuropathy or Charcot-Marie-Tooth disease.
As a prerequisite for myelination, SC precursors undergo extensive proliferation upon contact with developing axons. They then become progressively quiescent to adopt a series of differentiation steps necessary for myelinogenesis. SC proliferation is regulated by multiple growth factors, as well as extracellular matrix signals. However, only little is known about the downstream intracellular signaling pathways that quench the initially vigorous SC proliferation to allow cell cycle exit, SC differentiation and finally myelin investment of axons.
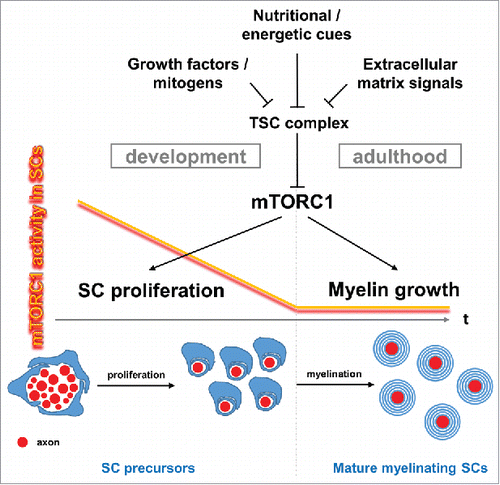
We have recently proposed a novel model in which a gradual downregulation of mammalian target of rapamycin complex 1 (mTORC1) signaling, observed in nascent SCs during nerve development, is responsible to curtail SC proliferation, thus allowing the transition to differentiationCitation2 (). mTORC1, which constitutes the better characterized branch of the mTOR pathway aside from mTORC2, is a central signaling hub assimilating a multitude of cell growth and proliferation signals including growth factors and information about nutrient, energy, and oxygen levels.Citation3 With respect to SC myelination it is thought that mTORC1 is a major executioner of phosphatidylinositol 3′ kinase (PI3K)/AKT signaling which is viewed as central driver of myelinogenesis.Citation1 The downstream effectors of mTORC1 are often studied in context of protein and lipid biosynthesis, regulation of bioenergetics, and autophagy. Perturbations of this signaling nexus are implicated in cancer, aging, neurodegeneration, and a variety of metabolic conditions.Citation3 This makes mTORC1 an interesting molecular candidate for aberrant myelination in peripheral neuropathies oftentimes accompanied by supernumerary SCs. To probe the role of mTORC1 on SC development, we uncoupled mTORC1 activity from upstream growth factors and nutritional cues by engineering mutant mice with different levels of sustained mTORC1 hyperactivity in SC precursors.Citation2 This was achieved through disruption of the negative mTORC1 regulators TSC2 or TSC1, together forming the TSC complex, or upstream PTEN (phosphatase and tensin homolog). We found that excessive mTORC1 hyperactivity following TSC2 ablation rendered SCs unable to exit the cell cycle, differentiate, and myelinate axons. Less intense mTORC1 hyperactivity was observed in SCs lacking TSC1 or PTEN. However, these mutant SCs escaped the differentiation blockage and eventually myelinated axons, supporting the model that mTORC1 hyperactivity above a certain threshold during development is incompatible with myelination. In support, the mTORC1 inhibitor rapamycin normalized SC proliferation and rescued myelination in SCs lacking TSC2.
Figure 1. Context-dependent functions of mTORC1 signaling in Schwann cells (SCs). Schematic graph showing developmental downregulation of SC mTORC1 activity to basal levels in adulthood. The mTORC1 decline is necessary for the differentiation of SC precursors to mature SCs that myelinate axons. While mTORC1 activity promotes proliferation of SC precursors during development, it stimulates myelin growth in adulthood. mTORC1 activity in SCs is adjusted by the TSC complex, which is negatively regulated by various upstream pathways.
How does mTORC1 hyperactivity lead to aberrant SC proliferation on molecular level? Our work demonstrates that TSC2-deficient SCs contain drastically decreased levels of the key CDK inhibitor p27Kip1. Interestingly, it has been proposed that TSC2 binds to and promotes the stability of nuclear p27Kip1 by preventing its cytoplasmic localization, where it is rapidly degraded.Citation4 However, we consider that the diminished p27Kip1 levels are rather a consequence of mTORC1 hyperactivity which is thought to regulate cell cycling through modulation of RNA translation.Citation5 For example, it has been proposed that mTORC1 regulates the expression of positive regulators of cell cycle progression via the eukaryotic translation initiation factor 4E-binding proteins (4E-BPs).Citation6 Thus, these proteins could suppress p27Kip1 levels in TSC2-deficient cells.Citation5 Indeed, we documented increased levels of cyclin D1 and B1 and CDK2 in TSC2-depleted nerves.Citation2 Additionally in line with our interpretation, rapamycin administration, that has no influence on TSC2 levels, is known to induce p27Kip1 expression.Citation7 Notwithstanding, in TSC2-deficient SCs the amount of p27Kip1 bound to CDKs is diminished leading to enhanced phosphorylation of the Retinoblastoma tumor suppressor protein (Rb). This relieves the inhibitory effects of Rb on the transcription factor E2F, and promotes S-phase progression and uncontrolled proliferation.
In marked contrast to the role of mTORC1 during SC development, we uncovered that in differentiated SCs increased mTORC1 activity promotes the growth of myelin rather than stimulating SC divisionCitation2 (). In all mutants studied we observed that increased mTORC1 activity caused overgrowth of myelin sheaths once their formation was initiated or already completed in adulthood. Still, experiments are underway in our laboratory to test whether or not strongly induced mTORC1 activity can release mature SCs from quiescence.
In summary, our data indicate that mTORC1 signaling, restrained by the upstream TSC complex, has discrete functions in developing and mature SCs. It is conceivable that mTORC1 dysregulation contributes to abnormal myelination and SC hyperplasia in peripheral neuropathies. Hence, manipulating mTORC1 activity with a growing repertoire of recently developed drugsCitation3 could be therapeutically valuable. Future work will be needed to elucidate how the multitude of modifications of the TSC complex by pathways such as AMP-activated kinase (AMPK), AKT and mitogen-activated protein kinase (MAPK) impact on mTORC1 activity and its downstream targets in the SC lineage. It is also important to understand how this can be integrated with key upstream components known to orchestrate SC proliferation and myelination.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468:244-52. doi:10.1038/nature09614. PMID:21068833.
- Beirowski B, Wong KM, Babetto E, Milbrandt J. mTORC1 promotes proliferation of immature Schwann cells and myelin growth of differentiated Schwann cells. Proc Natl Acad Sci U S A. 2017;114:E4261-70. doi:10.1073/pnas.1620761114. PMID:28484008.
- Laplante M, Sabatini DM. mTOR Signaling in Growth Control and Disease. Cell. 2012;149:274-93. doi:10.1016/j.cell.2012.03.017. PMID:22500797.
- Rosner M, Freilinger A, Hanneder M, Fujita N, Lubec G, Tsuruo T, Hengstschläger M. p27Kip1 localization depends on the tumor suppressor protein tuberin. Hum Mol Genet. 2007;16:1541-56. doi:10.1093/hmg/ddm103. PMID:17470459.
- Wang X, Proud CG. Nutrient control of TORC1, a cell-cycle regulator. Trends Cell Biol. 2009;19:260-7. doi:10.1016/j.tcb.2009.03.005. PMID:19419870.
- Dowling RJ, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E, Wang X, Larsson O, Selvaraj A, Liu Y, Kozma SC, Thomas G, Sonenberg N. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science. 2010;328:1172-76. doi:10.1126/science.1187532. PMID:20508131.
- Luo Y, Marx SO, Kiyokawa H, Koff A, Massagué J, Marks AR. Rapamycin resistance tied to defective regulation of p27Kip1. Mol Cell Biol. 1996;16:6744-51. PMID:8943329.
