Arsenic may be most well-known as a poison and carcinogen, but its potential for medical use is also not entirely new. Arsenic trioxide's (ATO) history has its start in traditional Chinese medicine under the name Pi-Shuang, serving as a versatile therapy through its external application.Citation1 ATO has since made its way into western medicine as an FDA-approved therapeutic for acute promyelocytic leukemia.Citation2 A cousin to ATO, inorganic sodium arsenite (iAs) also shows promise as a cell-cycle inhibitor in a variety of cell lines, including the human adenocarcinoma breast cancer cell line MCF-7.Citation3 However, the carcinogenic effects of chronic exposure to low-dose iAs remains troublesome. Understanding the dual roles of iAs and ATO as both promoters and inhibitors of cancer is critical for their successful application as therapeutics.
In this volume of Cell Cycle, Lynn A. Sheldon advances the field by unraveling the role of iAs in blocking cell proliferation.Citation4 She details the effects of iAs on key regulators of the G1/S transition—E2F family transcription factors, the retinoblastoma protein (Rb), cyclins, and cyclin-dependent kinases (Cdk). E2F1 activates genes required for entry into S-phase, including factors for DNA synthesis and positive regulators of the cell cycle ().Citation5 During quiescence and G1, E2F1 is inhibited by a hypo-phosphorylated form of Rb.Citation6 As the cell transitions to S-phase, Cdks are activated by their respective cyclins and phosphorylate Rb. This hyper-phosphorylated form of Rb releases E2F1, allowing transcription of its target genes.
Figure 1. Summary of iAs effects on regulators of G1/(S)phase. Protein interactions (shown as solid arrows) and transcriptional targets (dotted arrows) along the E2F1-Rb pathway. All components of this pathway showed decreased activity or transcription after treatment with iAs, with the exception of Rb, whose activity is increased.
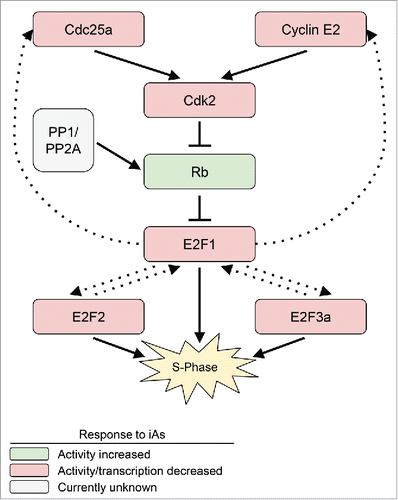
Sheldon measured the effects of iAs on MCF-7 cells released from quiescence ().Citation4 She first found that while the transcription of cyclin E2 was decreased, cyclins E1 and D1 levels remained unaffected. The study thus narrows on the pathway directly pertaining to cyclin E2, including its binding partner Cdk2 and the Cdc25a phosphatase, which activates Cdk2 by removing an inhibitory phosphate. Sheldon found that upon exposure of cells to iAs, both Cdk2 activity and Cdc25a transcription decreased. She next probed downstream components of the pathway and found that levels of activating E2F transcription factors (E2F1, E2F2, E2F3a) were also decreased. In a ChIP assay, Sheldon observed an increased presence of Rb and E2F1 at the E2F1 promoter and subsequently found that two Rb sites (T373 and S608) were less phosphorylated, which would promote dimerization with E2F1. She concludes that in cells treated with iAs, increased inhibition of E2F1 by hypo-phosphorylated Rb is the likely mechanism by which the cell cycle is blocked in G1.
Sheldon's careful study shows the profound effect of iAs on the E2F1-Rb cell cycle pathway, and it incites more exciting questions regarding the role of iAs. Which protein is the direct target of iAs, and which protein interactions or enzyme activities are affected? Sheldon postures the interesting hypothesis that it is not a lack of kinase activity that leads to Rb dephosphorylation and E2F1 inhibition, but rather an active dephosphorylation of Rb by the protein phosphatases PP1 or PP2A. PP2A dephosphoylates Rb in response to oxidative stress, a known outcome of iAs introduction.Citation7 With respect to translating iAs to the clinic, the ultimate question is whether iAs interactions can be fine-tuned to specifically harness its anti-proliferative effects. The community eagerly awaits further details that distinguish mechanisms underlying the harmful and beneficial effects of this potential anti-cancer agent.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Klaassen CD. Heavy metals and heavy-metal antagonists. In: Hardman JG, Gilman AG, Limbird LE, eds. Goodman & Gilman's The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 1996:1649-72.
- Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J, Scheinberg DA, Pandolfi PP, Warrell RP Jr. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339:1341-8. doi:10.1056/NEJM199811053391901. PMID:9801394.
- Davey JC, Bodwell JE, Gosse JA, Hamilton JW. Arsenic as an endocrine disruptor: effects of arsenic on estrogen receptor-mediated gene expression in vivo and in cell culture. Toxicol Sci. 2007;98:75-86. doi:10.1093/toxsci/kfm013. PMID:17283378.
- Sheldon LA. Inhibition of E2F1 Activity and Cell Cycle Progression by Arsenic via Retinoblastoma Protein. Cell Cycle. 2017; in press.
- Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol. 2002;3:11-20. doi:10.1038/nrm714. PMID:11823794.
- Dick FA, Rubin SM. Molecular mechanisms underlying RB protein function. Nat Rev Mol Cell Biol. 2013;14:297-306. doi:10.1038/nrm3567. PMID:23594950.
- Kolupaeva V, Janssens V. PP1 and PP2A phosphatases–cooperating partners in modulating retinoblastoma protein activation. FEBS J. 2013;280:627-43. doi:10.1111/j.1742-4658.2012.08511.x. PMID:22299668.
