ABSTRACT
Peroxisomes are essential and dynamic organelles that allow cells to rapidly adapt and cope with changing environments and/or physiological conditions by modulation of both peroxisome biogenesis and turnover. Peroxisome biogenesis involves the assembly of peroxisome membranes and the import of peroxisomal matrix proteins. The latter depends on the receptor, PEX5, which recognizes peroxisomal matrix proteins in the cytosol directly or indirectly, and transports them to the peroxisomal lumen. In this review, we discuss the role of PEX5 ubiquitination in both peroxisome biogenesis and turnover, specifically in PEX5 receptor recycling, stability and abundance, as well as its role in pexophagy (autophagic degradation of peroxisomes).
Introduction
Peroxisomes are single-membrane-bound organelles present in most eukaryotic cells, ranging from unicellular yeasts to multicellular organisms, such as plants and humans. They are involved in essential cellular metabolism, most notably β-oxidation of fatty acids, as well as the production and destruction of reactive oxygen species (ROS).Citation1 They also play crucial roles in the biosynthesis of bile acids and plasmalogens in mammals,Citation2 where they also serve as signaling hubs for antiviral innate immunityCitation3 and in modulating mTORC1 activity in response to ROS status.Citation4
Human diseases caused by peroxisome disorders underscore the vital importance of this organelle. Peroxisomal diseases fall into two categories: single-enzyme defects and peroxisome biogenesis disorders (PBDs).Citation2 PBDs are more complex in etiology in that many peroxisomal enzymes are affected, generally via lack of peroxisomal matrix protein import.Citation2 Zellweger syndrome (ZS), a prototypic PBD with the most severe phenotype exhibits an absence of functional peroxisomes. ZS patients manifest severe hypotonia from the neonatal period, psychomotor retardation, facial dysmorphism, such as high forehead and large fontanelle, hepatomegaly with prolonged jaundice and liver dysfunctions, calcific stippling of the patella, renal cortical microcysts, retinal degeneration, and congenital heart disease. These patients usually die during the first year of life, often without having made any developmental progress.Citation5
Protein ubiquitination
Ubiquitin is a highly-conserved 76 amino acid polypeptide, covalently attached to target proteins.Citation6 Protein ubiquitination is involved in almost all cellular processes.Citation7 It is accomplished by an enzymatic cascade involving an ubiquitin-activating E1 enzyme, an ubiquitin-conjugating E2 enzyme and an ubiquitin-ligating E3 enzyme.Citation8 During protein ubiquitination, ubiquitin is activated in an ATP-dependent process by the E1 enzyme, which forms a thioester with the C terminal amino acid (Gly) of ubiquitin. Next, the activated ubiquitin is transferred to the active-site cysteine of the E2 enzyme to form an E2∼Ub thioester intermediate. Finally, the E3 ligase transfers ubiquitin from the E2, generally to a lysine residue or N terminus of the substrate, but rarely also to a cysteine.Citation9 Hence E3 ligases are responsible for ubiquitination specificity by recognizing the substrates and mediating transfer of ubiquitin from the E2 to the appropriate substrate.Citation8
E3 ligases can be classified into three main families [RING (Really Interesting New Gene), HECT (homologous to the E6AP carboxyl terminus), RBR (RING-between RING)], depending on the conserved domains and on the mechanism of ubiquitin transfer to the substrate protein.Citation9 RING-family proteins represent the most abundant E3 ligases and are characterized by the presence of a zinc-binding domain called the RING domain. They function as a scaffold, binding both the E2∼Ub thioester and the substrate and catalyze the direct transfer of ubiquitin from the E2 enzyme to the substrate.Citation8,10
A single ubiquitin can be conjugated to a single or several lysine residues of the target proteins (multi-mono-ubiquitination). Alternatively, ubiquitin can be elongated through lysine (K) residues within itself, yielding a poly-ubiquitin chain. Poly-ubiquitination through K48 linkages typifies the best-known function of ubiquitination: to target protein for degradation by proteasomes.Citation11,12 Alternatively, mono- or poly-ubiquitination through other residue linkages (K63, etc) serve as non-proteolytic signals and function in intracellular localization, trafficking, DNA repair, signal transduction and organelle degradation pathways and virus budding.Citation13,14 Ubiquitination also plays crucial roles in maintaining peroxisome homeostasis in response to intracellular and extracellular cues as discussed later.
Peroxisome homeostasis
The number and activity of intracellular peroxisomes can be rapidly adjusted and tightly controlled in response to changing environments and/or physiological conditions. This homeostasis is achieved through the coordination of peroxisome biogenesis and turnover.
In yeast, peroxisomes are rapidly induced when cells are grown in the presence of specific carbon sources, like oleic acid or methanol (S. cerevisiae can utilize oleic acid while P. pastoris can use both), whose metabolism in that yeast requires peroxisomal enzymes. However, removal of these favored peroxisome proliferation stimuli results in pexophagy, the selective degradation of peroxisomes through autophagy, since these superfluous and redundant peroxisomes are no longer required.Citation15 Similar dynamic patterns of peroxisome homeostasis (balance between biogenesis and degradation) are also observed in rodents. The abundance of peroxisomes increases when the animals are administered with hypolipidemic drugs that are known peroxisome proliferatorsCitation16 and some other chemicals, such as phthalate esters.Citation17 The number of peroxisomes decreases rapidly and almost returns to the pre-proliferation state if the drugs are discontinued.Citation18,19
Peroxisome biogenesis
Work in several organisms including yeast, Chinese Hamster Ovary (CHO) cells and human cells led to the discovery of PEX genes (genes encoding peroxins*) necessary for peroxisome biogenesis.Citation20,21 So far, 35 known peroxins play crucial roles in peroxisome biogenesis and, of these, 14 have been identified in humans and linked to PBDs.
These PEX genes can be functionally categorized into the following four groups, based on their biological functions in: (1) Peroxisome membrane assembly.Citation22-24 (2) Import of peroxisomal matrix proteins.Citation25,26 Because peroxisomes don't have any genetic materials, all peroxisomal membrane and matrix proteins are encoded in the nucleus and synthesized in the cytoplasm, followed by import into peroxisomes.Citation22,23,25,26 (3) Peroxisome fission and division. Peroxisomes can proliferate through the growth and division of pre-existing peroxisomes.Citation27 Alternatively, they can arise through an endoplasmic reticulum (ER)-mediated de novo pathway.Citation24 A recent interesting finding in mammalian cells shows that mature peroxisomes are formed through a hybrid of mitochondria- and ER- derived vesicles.Citation28 (4) Peroxisome inheritance. Cells have evolved an elaborate mechanism to maintain peroxisomes in both mother and daughter cells during each round of division. This peroxisome inheritance ensures that daughter cells acquire peroxisomes from their mother cells for their faithful propagation.Citation29,30 In this review, we will discuss the mechanisms of import of peroxisomal matrix proteins and the role of PEX5 ubiquitination during this process. For detailed mechanisms regarding other steps in peroxisome biogenesis, readers are referred to other excellent reviews.Citation22-25,27,29,30
Peroxisome turnover
Autophagy is a self-digestive process to provide nutrients in response to intra- and extra- cellular stresses.Citation31,32 These stresses include lack of nutrients or growth factors, hypoxia, oxidative stress, DNA damage, protein aggregation, damaged organelles, or intracellular pathogens.Citation33 Autophagy was initially described as a non-selective bulk degradation process. However, autophagy can also target selective cargos like damaged or superfluous peroxisomes. Pexophagy, the selective degradation of peroxisomes through autophagy, is the main mechanism for peroxisome turnover in both yeast and mammalian cells.Citation34
The mechanism of pexophagy is well-established in yeast. It requires the general autophagy machinery, as well as specific elements such as the pexophagy receptors, Atg30 in P. pastorisCitation35 and Atg36 in S. cerevisiae.Citation36 These receptors tag peroxisomes for degradation: Atg30 and Atg36 are associated with the peroxisomal membrane proteins (PMPs) and then recruit the core autophagy machinery to peroxisomes by binding the scaffold proteins (Atg11 and Atg17) and the ubiquitin-like protein, Atg8.Citation35,37 For detailed mechanisms of pexophagy, readers are referred to other excellent papers.Citation15,34,38
Although the earliest description of pexophagy came from mammalian cells when peroxisomes were detected in the lysosomes,Citation39 the mechanisms of pexophagy remain largely unknown in mammalian cells. This is largely due to the challenge of detection of a small percentage of peroxisomes targeted by pexophagy in higher organisms and the absence of knowledge of signals that induce pexophagy.Citation40 Nonetheless, recent studies reveal an indispensable role of PEX5 ubiquitination in pexophagy in mammalian cells, as discussed later in this review.
PEX5 acts as a receptor for import of peroxisomal matrix proteins
Unlike the transport of unfolded polypeptides across the membranes of the ER, mitochondria, and chloroplast,Citation41 peroxisomes can import proteins that are already folded, cofactor-bound, and/or oligomeric.Citation42 In this respect, peroxisomal matrix protein import shows interesting parallels to protein transport via the twin-arginine translocator pathway,Citation43 as well as nuclear protein import.Citation44
Import of most peroxisomal matrix proteins depends on peroxisomal targeting signals (PTSs): PTS1, comprising a non-cleaved, C-terminal tripeptide, SKL, or its conserved variants;Citation45 or the PTS2, consisting of a nonapeptide sequence, (R/K)(L/V/I/Q)XX(L/V/I/H/Q)(L/S/G/A/K)X(H/Q)(L/A/F), localized near the N terminus of the cargo protein.Citation46 Proteins lacking the PTS signals can also be imported into peroxisomes either through binding directly to the N terminal part of PEX5 (the receptor for PTS1 proteins as described later),Citation47 or through association (piggyback import) with a protein containing the PTS.Citation48
Most peroxisomal matrix proteins have the PTS1 signal, which is recognized by the receptor, PEX5, in all the examined organisms. PEX5 protein can be functionally divided into two domains: the structurally-disordered N-terminal (NT) region that binds to membrane peroxins like PEX14 and PEX13 and mediates the docking of the receptor-cargo complex at the peroxisomes;Citation49 and a C terminal region comprising a series of tetratricopeptide repeats (TPR), which recognize the PTS1 cargos.Citation50 PTS2 proteins are recognized by their receptor PEX7. In S. cerevisiae, the Pex7 protein requires the co-receptors, including the partially-redundant Pex18/Pex21 proteins,Citation51 or Pex20 in most other yeasts and fungi,Citation52-54 as well as the longer PEX5L isoform, in plants and mammals.Citation55-57 These co-receptors share similar structural modules, with Pex7-binding and peroxisome-docking domains. These co-receptors bind to the Pex7-cargo complexes and import PTS2 cargos into peroxisomes.Citation58
After the cargos are recognized by the corresponding receptors, they dock at peroxisomes through the peroxisome-docking domain of these receptors or co-receptors, followed by translocation of these proteins across the membranes and cargo release into the peroxisome matrix. The receptors/co-receptors are then recycled back to the cytosol for the next round of protein import (, ).Citation25
Figure 1. Role of PEX5 ubiquitination in import of peroxisomal matrix proteins in yeast (A-B) and human (C-E). (A, C) Role of cysteine-mono-ubiquitination in PTS receptor recycling in yeast Pex5 (A) and human PEX5 (C). PEX5 recognizes the cargo proteins in the cytoplasm and transports them into peroxisomes after interacting with the peroxisome-associated, docking proteins, such as PEX13 and PEX14. The cargos are released from PEX5 in the matrix. PEX5 is then recycled back to the cytoplasm for subsequent rounds of import by AAA+ ATPases, PEX1 and PEX6, which are anchored to the peroxisome membranes through the PMPs, Pex15 (in yeast) and PEX26 (in humans). This depends on mono-ubiquitination of PEX5 at the C6 (in yeast) and C11 (in humans), which is achieved through an E2 enzyme (Pex4), anchored to the peroxisomal membrane by Pex22, and E3 ligases (Pex2, Pex10 and Pex12) in yeast, and through the UbcH5 (E2) and PEX2, PEX10, PEX12 (E3) proteins in humans. (B) Role of lysine poly-ubiquitination in Pex5 degradation (RADAR pathway) in yeast. When the normal recycling pathway is blocked in Δpex1, Δpex6 or Δpex15 mutant strains or due to an impairment of the mono-ubiquitination step in Δpex4 or Δpex22 strains, Pex5 is poly-ubiquitinated instead by Ubc4/5 (E2) and the Pex2, Pex10, Pex12 (E3) complex at the peroxisome membrane. This targets Pex5 for degradation via proteasomes. (D) Role of TRIM37-mediated mono-ubiquitination in PEX5 stability in humans. Mono-ubiquitination of PEX5 at K464 by UbcH5 (E2) and TRIM37 (E3) stabilizes PEX5 by inhibiting its proteasome-mediated turnover, thereby enhancing import of peroxisomal matrix proteins. (E) Role of lysine poly-ubiquitination in PEX5 degradation in humans. Multiple mutations, such as pex1, pex6, pex26 or TRIM37 deficiencies, or abnormal signals, such as high levels of ROS, relieve the negative regulation of PEX5 degradation by TRIM37, making PEX5 susceptible to poly-ubiquitination at other sites (presumably at unmapped lysines), followed by its proteasomal degradation.
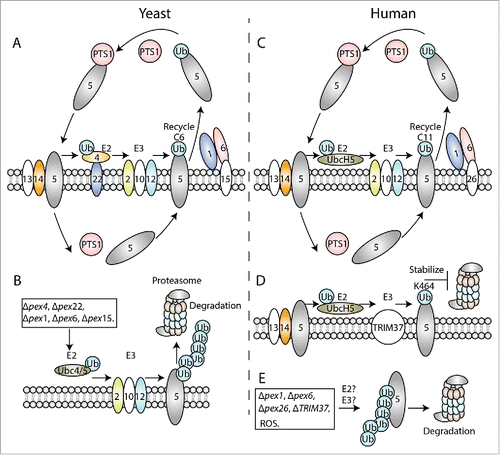
In humans, PEX5 gene mutations result in defective import of peroxisomal matrix proteins and cause PBDs. PEX5 knockout mice also display the typical biochemical abnormalities of PEX5-deficient human patients,Citation59 demonstrating the conserved function of PEX5, as well as its vital importance. The PBDs caused by PEX5 mutations include Zellweger syndrome (ZS) (the severest phenotype), neonatal adrenoleukodystrophy (NALD) and infantile Refsum disease (IRD) (the least severe phenotype).Citation60 So far, a couple of mutations have been found in human PEX5, including single amino acid substitutions, deletions and aberrant splice variants, most of which cluster around the TPR domain.Citation60-62 Based on the position and type of the mutation, these mutant proteins manifest different effects on PTS protein import. For example, some mutations affect only the import of PTS1, but not PTS2 proteins, while others impair both. These variations may contribute to the clinical heterogeneity of these patients.
Role of PEX5 ubiquitination in peroxisome biogenesis
Role of cysteine mono-ubiquitination in PEX5 recycling
AAA+ ATPases (ATPases Associated with diverse cellular Activities) are involved in diverse processes such as DNA replication, protein degradation, membrane fusion and protein translocation, transport of targets along microtubule cables, signal transduction and transcription.Citation63 They contain one or two typical ATPase domain(s) of 200∼250 amino acids, which is characterized by domains that are important for ATP binding and hydrolysis. These include Walker A and B motifs, as well as other motifs that distinguish the AAA proteins from other P-loop (phosphate-binding) NTPases.Citation64
After cargo release into the lumen of peroxisomes, PEX5 is recycled back to cytosol in an ATP-dependent manner for further rounds of import.Citation65,66 This is accomplished by PEX1 and PEX6. They are classified as type II AAA+ ATPases, which by definition contain two, conserved ATPase domains (D1 and D2) in tandem, flanked by less conserved N- and C-terminal regions. Similar to other type II AAA+ ATPases, the two ATPase domains show different degrees of conservation. The second ATPase domain (D2) of PEX1 and PEX6 is highly conserved, compared to the first one.Citation67
PEX1 and PEX6 are conserved in all species.Citation68 They interact with each other in the presence of ATP and are both required for peroxisome biogenesis.Citation69 PEX15, PEX26, and APM9 have been identified as peroxisomal membrane anchors for PEX6 in yeast,Citation70 mammalian cells,Citation71 and plantsCitation72 respectively. These anchor proteins recruit the PEX1-PEX6 complex to peroxisome membranes (, ). As ATPases, PEX1 and PEX6 can transform the energy of ATP hydrolysis to extract PEX5 back to the cytosol from peroxisome membranes.Citation69 This, however, depends on PEX5 mono-ubiquitination at a conserved N-terminal cysteine, via a rare thioester bond in both yeast and mammalian cells (, ).Citation73-76
In yeast, this mono-ubiquitination of Pex5 is catalyzed by an E2-conjugating enzyme (Pex4) and a complex of three RING E3 ligases (Pex2, Pex10, and Pex12) (). Identification of the essential role of Pex4 in peroxisome biogenesis marked the first association of ubiquitination in the peroxisome field.Citation77 Pex4 is anchored to the peroxisome membranes through Pex22, an intrinsic membrane protein.Citation78 Its association with Pex22 stimulates the full E2 activity of Pex4.Citation79 Pex2, Pex10, and Pex12 form a sub-complex at the peroxisome membrane and regulate the stabilities of each other.Citation80,81 These three E3 ligases function cooperatively to ubiquitinate Pex5 at the conserved cysteine.Citation81,82
Mono-ubiquitination at the conserved N-terminal cysteine is also found in mammalian cells (). PEX2, PEX10, and PEX12 function as E3 ligases, of which PEX10 plays the predominant role.Citation83 However, mammalian cells lack the PEX4 and PEX22 genes. Instead, they use members of the UbcH5a/b/c family as the E2 for cysteine mono-ubiquitination.Citation84
Mono-ubiquitination on cysteine plays a conserved role in PEX5 recycling in both yeast and mammalian cells, which facilitates its extraction from peroxisomes by the AAA+ ATPases, PEX1 and PEX6 (, ).Citation73-76 Ubiquitin is attached to cysteine via a rare thioester-bond, whereas the ubiquitin-moiety is generally attached to a lysine residue via an isopeptide bond. The rationales for this have been recently examined.
Studies from both yeast and mammalian cells suggest that mono-ubiquitination on cysteine serves as a mechanism for cells to sense and cope with redox status. In the methylotrophic yeast, P. pastoris, the binding and release of PTS cargos is modulated through the conserved cysteine residue-linked, redox-sensitive oligomerization of Pex5 protein. The reduced peroxisome lumen favors a non-covalent, homo-dimeric form of Pex5, promotes cargo release from Pex5 and facilitates mono-ubiquitination. When cells are exposed to oxidative stresses, however, the reduced state of the peroxisome lumen is disrupted, interfering with the cysteine- ubiquitination and causing the delay of import of matrix proteins.Citation85 Although the cysteine involved in the redox-sensitive oligomerization of Pex5 may appear to face the cytosol in some models,Citation86 our workCitation25 shows that Pex5 can enter the peroxisome lumen where it could sense the local environment of the peroxisome lumen (). In human cells, PTS protein import is reduced during cell aging and ubiquitination of PEX5 at cysteine is inhibited under oxidative stresses.Citation87,88 Therefore, cysteine-ubiquitination serves as a redox switch to regulate PEX5-mediated PTS protein import in both yeast and human cells.
Mono-ubiquitination of PEX5 in yeast and in mammals (female rat liver and HeLa cells) is reversed via the action of deubiquitinating enzymes, Ubp15Citation89 and USP9X,Citation90 respectively, prior to the re-engagement of PEX5 in the next round of import.
Role of lysine mono-ubiquitination in maintaining PEX5 stability
Besides cysteine-ubiquitination, lysine ubiquitination (both mono- and poly-) has also been detected in PEX5.
We unveiled a novel E3 ligase TRIM37 that mono-ubiquitinates human PEX5 at K464 in the presence of the E1 enzyme, UBE1, and an E2 enzyme belonging to the UbcH5 family (UbcH5a/b/c) of proteins in human cells.Citation91 TRIM37-mediated ubiquitination stabilizes and maintains the abundance of PEX5 protein, which is required for import of peroxisomal matrix proteins ().
Role of lysine poly-ubiquitination via the RADAR pathway
In yeast, poly-ubiquitination is induced in cells lacking components of the peroxisomal AAA+ ATPases (Pex1 and Pex6) or their anchoring protein, Pex15, or the Pex4–Pex22 complex (),Citation92-94 suggesting that it occurs at a later stage of PTS protein import. Ubiquitin moieties are attached to two lysines within the N terminal of Pex5 through K48 linkages, which serve as the classical signal for degradation by proteasomes.Citation93,94 Supporting this, poly-ubiquitinated Pex5 accumulates in cells with defective 26S proteasomes.Citation94 This Pex5 poly-ubiquitination is achieved through the E2-conjugating enzyme, Ubc4/5Citation92-94 and requires the presence of the RING PEX genes (PEX2, PEX10 and PEX12).Citation82,93,94 Ubc4/5 is also involved in other cellular processes, such as catalyzing ubiquitination and degradation of proteins exposed to stressful conditions.Citation95,96 Poly-ubiquitination is not essential for peroxisomal biogenesis since the cells with mutations that diminish this process still have the functional peroxisomes. It has been proposed to serve as a quality-control mechanism, also called the RADAR (Receptor Accumulation and Degradation in Absence of Recycling), pathway that prevents accumulation of the non-functional Pex5 at the peroxisome membranes.Citation93,97
PEX5 stability is also decreased in several human PBD patient cells (deficient in PEX1, PEX6 or PEX26),Citation98,99 indicative of the existence of a similar quality-control mechanism in human cells. Our recent findings prove this possibility.Citation91 PEX5 mutant proteins that lack the site normally ubiquitinated by TRIM37 undergo a massive poly-ubiquitination and manifest robust degradation of PEX5 by proteasomes. Under oxidative stress (H2O2 treatment), a condition that disfavors peroxisomal matrix protein import,Citation88 PEX5 protein also undergoes degradation in a poly-ubiquitination-dependent manner. These results provide direct evidence that poly-ubiquitination also serves as the signal to remove nonfunctional or excessive PEX5 protein in human cells, pointing to the existence of a RADAR-like pathway also in human cells (). However, the E2 and E3 enzymes involved, as well as the site of poly-ubiquitination are not characterized and we do not understand whether this pathway occurs in the cytoplasm or on peroxisome membranes. Additionally, this TRIM37 function seems to be specific to human cells, as there is no homologous TRIM37 gene in yeast and mouse TRIM37 does not appear to play this role.
Role of PEX5 ubiquitination in peroxisome turnover
In yeast, the mechanism for pexophagy is well established and Pex5 ubiquitination plays exclusive roles only in peroxisome biogenesis and is not required for pexophagy.Citation100
In mammals, however, several lines of evidence show that pexophagy depends on ubiquitination. Pexophagy is triggered by over-expressing PMP proteins, like PMP34 and PEX3, fused with a single ubiquitin moiety in their cytosolic domains, via a process that requires the ubiquitin-binding autophagy adaptor, p62. Although this is an artificial ubiquitin fusion protein not reflecting the physiological pexophagy process, it reveals a critical role of ubiquitination in regulation of pexophagy, similar to those in other types of selective autophagy (mitophagy, etc).Citation101 Later, another ubiquitin-binding, autophagic receptor, NBR1, was found to be required for pexophagy, functioning cooperatively with p62.Citation102 Supporting this, PEX3 or PEX2 over-expression triggers ubiquitination of peroxisomes and its degradation in lysosomes, which depends on NBR1.Citation103,104 More importantly, recent studies suggest that PEX5 serves as the potential pexophagy receptor in mammalian cells.Citation105
The protein kinase ataxia-telangiectasia mutated (ATM) is a serine/threonine protein kinase. Its best-known function is to maintain genomic stability in response to double strand breaks.Citation106 Besides its nuclear role, the cytoplasmic portion of ATM is recruited to peroxisome membranes in a PEX5-dependent manner in response to ROS such as H2O2.Citation107,108 On the one hand, ATM signaling inhibits mTOR activity and induces general autophagy in response to H2O2; on the other hand, peroxisome-localized ATM phosphorylates PEX5 at S141, which promotes the ubiquitination of the adjacent K209. The autophagy receptor, p62, recognizes the ubiquitinated PEX5 and targets it for pexophagy ().Citation108
Figure 2. Role of PEX5 ubiquitination in mammalian pexophagy in different conditions. (A) Excessive ROS in the peroxisomal lumen activates ATM, which associates with PEX5 at the peroxisome membranes and phosphorylates PEX5 at S141. This facilitates PEX5 mono-ubiquitination, by the PEX2, PEX10, PEX12 (E3) complex, at K209, which is recognized by the autophagy receptor, p62, and induces pexophagy to eliminate the damaged peroxisomes in response to oxidative stresses. (B) In normal growth condition, PEX5 fused to a C terminal bulky tag is mono-ubiquitinated at C11 by UbcH5 (E2) and the PEX2, PEX10, PEX12 (E3) complex. However, this PEX5 fusion protein cannot be recycled back to cytosol by PEX1/PEX6, and is trapped at the peroxisome membrane because of the bulky tag. The accumulation of PEX5 mono-ubiquitination serves as a signal for pexophagy to remove the abnormal peroxisomes, but this process is independent of the usual pexophagy receptors, p62 and NBR1, and is also cell-type specific.Citation109
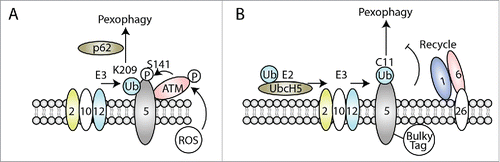
Besides ubiquitination at K209, however, other residues (C11, K464, and other known and unknown lysine residues) are also ubiquitinated within PEX5 protein, as discussed above. It is unclear if these have any role in pexophagy. Another study showed that pexophagy depends on C11 mono-ubiquitination. When PEX5 proteins are fused to a bulky C-terminal tag, it triggers pexophagy in SV40 large T antigen-transformed mouse embryonic fibroblastsCitation109 Mechanistic studies reveal that this PEX5 fusion protein can be normally mono-ubiquitinated at C11, but fails to be exported from peroxisome membranes. The trapped and accumulated mono-ubiquitinated PEX5 protein serves as the peoxophagy signal (). Interestingly, C11-dependent pexophagy is cell-type specific and does not require any known autophagy receptors, such as p62 and NBR1.
This study not only shows a role for cysteine ubiquitination in pexophagy, but also suggests that defective recycling of PEX5 protein can induce pexophagy. Indeed, loss of the AAA+ ATPases causes accumulation of ubiquitinated PEX5 on peroxisome membranes and induces pexophagy in patient cells, which serves as a quality control mechanism to reduce these dysfunctional peroxisomes in these patients.Citation110,111 However, accumulation of Pex5 ubiquitination due to lack of exportomer components (Pex1, Pex6, Pex15), is not required for pexophagy in yeast.Citation100
Supporting this, PEX5 (and PMP70) ubiquitination are found in amino acid starvation-induced pexophagy conditions, in a process that depends only on PEX2 (as the E3 ligase), rather than on PEX10 and PEX12.Citation104 However, the site of PEX5 ubiquitination for this starvation-induced pexophagy is not mapped.
Though these studies show that PEX5 ubiquitination plays a crucial role in pexophagy, the mechanisms and ubiquitination sites are distinct and incompletely mapped under different physiological conditions. A possible explanation for the complexity might be that pexophagy is context dependent, varying with the cell type as well as the particular stress conditions. It would be very interesting to investigate how cells integrate these different signals impacting PEX5 ubiquitination and respond to these detrimental conditions by modulating peroxisome physiology.
Concluding remarks
Our understanding of mechanisms of peroxisome biogenesis largely depends on the findings in yeast because the peroxisome is a very conserved organelle with similar functions across evolutionarily-diverse organisms and many homologous PEX genes have been found among different species. One such example is the well-established role of the cysteine-mono-ubiquitination of PEX5 in receptor recycling, as discussed earlier.
Substantial progress has been made in the role of PEX5 ubiquitination, however, there are still lots of questions remaining to be explored especially in human cells. For example, how do cells sense and respond to changing intra- and extra-cellular conditions with respect to PEX5 ubiquitination and peroxisome biogenesis? How are ubiquitination pathways with opposing physiological outcomes, such as peroxisome biogenesis versus pexophagy, or PEX5 stabilization versus degradation, sensed intra-cellularly and regulated? Are there human patients, in which mutation of these ubiquitination sites is directly responsible for the PBD and/or turnover defect, although none are reported to date? Are there other E2 and/or E3 enzymes responsible for PEX5 ubiquitination in response to different cellular conditions, especially in human cells? Can we interpret and translate these PEX5 ubiquitination data to treat the PBDs or other peroxisome-related diseases, such as aging and cancer? Future work investigating these unanswered questions will help to decode the mysteries of peroxisomes.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by NIH grant (5RO1 DK41737) to SS.
References
- Smith JJ, Aitchison JD. Peroxisomes take shape. Nat Rev Mol Cell Biol. 2013;14:803-17. doi:10.1038/nrm3700. PMID:24263361
- Waterham HR, Ferdinandusse S, Wanders RJ. Human disorders of peroxisome metabolism and biogenesis. Biochimica et biophysica acta. 2016;1863:922-33. doi:10.1016/j.bbamcr.2015.11.015. PMID:26611709.
- Dixit E, Boulant S, Zhang Y, Lee AS, Odendall C, Shum B, Hacohen N, Chen ZJ, Whelan SP, Fransen M, et al. Peroxisomes are signaling platforms for antiviral innate immunity. Cell. 2010;141:668-81. doi:10.1016/j.cell.2010.04.018. PMID:20451243.
- Zhang J, Kim J, Alexander A, Cai S, Tripathi DN, Dere R, Tee AR, Tait-Mulder J, Di Nardo A, Han JM, et al. A tuberous sclerosis complex signalling node at the peroxisome regulates mTORC1 and autophagy in response to ROS. Nat Cell Biol. 2013;15:1186-96. doi:10.1038/ncb2822. PMID:23955302.
- Suzuki Y, Shimozawa N, Orii T, Tsukamoto T, Osumi T, Fujiki Y, Kondo N. Genetic and molecular bases of peroxisome biogenesis disorders. Genetics in medicine: official journal of the American College of Medical Genetics. 2001;3:372-6. doi:10.1097/00125817-200109000-00007. PMID:11545691.
- Goldstein G, Scheid M, Hammerling U, Schlesinger DH, Niall HD, Boyse EA. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proceedings of the National Academy of Sciences of the United States of America 1975;72:11-5. doi:10.1073/pnas.72.1.11. PMID:1078892.
- Metzger MB, Hristova VA, Weissman AM. HECT and RING finger families of E3 ubiquitin ligases at a glance. Journal of cell science. 2012;125:531-7. doi:10.1242/jcs.091777. PMID:22389392.
- Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem. 2009;78:399-434. doi:10.1146/annurev.biochem.78.101807.093809. PMID:19489725.
- Berndsen CE, Wolberger C. New insights into ubiquitin E3 ligase mechanism. Nat Struct Mol Biol. 2014;21:301-7. doi:10.1038/nsmb.2780.
- Budhidarmo R, Nakatani Y, Day CL. RINGs hold the key to ubiquitin transfer. Trends Biochem Sci. 2012;37:58-65. doi:10.1016/j.tibs.2011.11.001. PMID:22154517.
- Chau V, Tobias JW, Bachmair A, Marriott D, Ecker DJ, Gonda DK, Varshavsky A. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science 1989;243:1576-83. doi:10.1126/science.2538923. PMID:2538923.
- Thrower JS, Hoffman L, Rechsteiner M, Pickart CM. Recognition of the polyubiquitin proteolytic signal. EMBO J. 2000;19:94-102. doi:10.1093/emboj/19.1.94. PMID:10619848.
- Haglund K, Dikic I. Ubiquitylation and cell signaling. EMBO J. 2005;24:3353-9. doi:10.1038/sj.emboj.7600808. PMID:16148945.
- Hicke L. Protein regulation by monoubiquitin. Nat Rev Mol Cell Biol. 2001;2:195-201. doi:10.1038/35056583. PMID:11265249.
- Farre JC, Subramani S. Mechanistic insights into selective autophagy pathways: lessons from yeast. Nat Rev Mol Cell Biol. 2016;17:537-52. doi:10.1038/nrm.2016.74. PMID:27381245.
- Fahimi HD, Reinicke A, Sujatta M, Yokota S, Ozel M, Hartig F, Stegmeier K. The short- and long-term effects of bezafibrate in the rat. Annals of the New York Academy of Sciences 1982;386:111-35. doi:10.1111/j.1749-6632.1982.tb21410.x. PMID:6953842.
- Yokota S. Quantitative immunocytochemical studies on differential induction of serine:pyruvate aminotransferase in mitochondria and peroxisomes of rat liver cells by administration of glucagon or di-(2-ethylhexyl)phthalate. Histochemistry 1986;85:145-55. doi:10.1007/BF00491762. PMID:3744897.
- Yokota S. Formation of autophagosomes during degradation of excess peroxisomes induced by administration of dioctyl phthalate. European journal of cell biology 1993;61:67-80. PMID:8223709.
- Ezaki J, Komatsu M, Yokota S, Ueno T, Kominami E. Method for monitoring pexophagy in mammalian cells. Methods in enzymology. 2009;452:215-26. doi:10.1016/S0076-6879(08)03614-8. PMID:19200885.
- Erdmann R. Assembly, maintenance and dynamics of peroxisomes. Biochimica et biophysica acta. 2016;1863:787-9. doi:10.1016/j.bbamcr.2016.01.020. PMID:26851075.
- Honsho M, Yamashita S, Fujiki Y. Peroxisome homeostasis: Mechanisms of division and selective degradation of peroxisomes in mammals. Biochimica et biophysica acta. 2016;1863:984-91. doi:10.1016/j.bbamcr.2015.09.032. PMID:26434997.
- Hettema EH, Erdmann R, van der Klei I, Veenhuis M. Evolving models for peroxisome biogenesis. Current opinion in cell biology. 2014;29:25-30. doi:10.1016/j.ceb.2014.02.002. PMID:24681485.
- Mayerhofer PU. Targeting and insertion of peroxisomal membrane proteins: ER trafficking versus direct delivery to peroxisomes. Biochimica et biophysica acta. 2016;1863:870-80. doi:10.1016/j.bbamcr.2015.09.021. PMID:26392202.
- Agrawal G, Subramani S. De novo peroxisome biogenesis: Evolving concepts and conundrums. Biochimica et biophysica acta. 2016;1863:892-901. doi:10.1016/j.bbamcr.2015.09.014. PMID:26381541.
- Ma C, Agrawal G, Subramani S. Peroxisome assembly: matrix and membrane protein biogenesis. J Cell Biol. 2011;193:7-16. doi:10.1083/jcb.201010022. PMID:21464226.
- Hasan S, Platta HW, Erdmann R. Import of proteins into the peroxisomal matrix. Frontiers in physiology. 2013;4:261. doi:10.3389/fphys.2013.00261. PMID:24069002.
- Schrader M, Costello JL, Godinho LF, Azadi AS, Islinger M. Proliferation and fission of peroxisomes – An update. Biochimica et biophysica acta. 2016;1863:971-83. doi:10.1016/j.bbamcr.2015.09.024. PMID:26409486.
- Sugiura A, Mattie S, Prudent J, McBride HM. Newly born peroxisomes are a hybrid of mitochondrial and ER-derived pre-peroxisomes. Nature. 2017;542:251-4. doi:10.1038/nature21375. PMID:28146471.
- Fagarasanu A, Mast FD, Knoblach B, Rachubinski RA. Molecular mechanisms of organelle inheritance: lessons from peroxisomes in yeast. Nat Rev Mol Cell Biol. 2010;11:644-54. doi:10.1038/nrm2960. PMID:20717147.
- Knoblach B, Rachubinski RA. Sharing with your children: Mechanisms of peroxisome inheritance. Biochimica et biophysica acta. 2016;1863:1014-8. doi:10.1016/j.bbamcr.2015.11.023. PMID:26620799.
- Mizushima N. Autophagy: process and function. Genes & development. 2007;21:2861-73. doi:10.1101/gad.1599207.
- Kaur J, Debnath J. Autophagy at the crossroads of catabolism and anabolism. Nat Rev Mol Cell Biol. 2015;16:461-72. doi:10.1038/nrm4024. PMID:26177004.
- Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280-93. doi:10.1016/j.molcel.2010.09.023. PMID:20965422.
- Till A, Lakhani R, Burnett SF, Subramani S. Pexophagy: the selective degradation of peroxisomes. International journal of cell biology. 2012;2012:512721. doi:10.1155/2012/512721. PMID:22536249.
- Farre JC, Manjithaya R, Mathewson RD, Subramani S. PpAtg30 tags peroxisomes for turnover by selective autophagy. Developmental cell. 2008;14:365-76. doi:10.1016/j.devcel.2007.12.011. PMID:18331717.
- Motley AM, Nuttall JM, Hettema EH. Pex3-anchored Atg36 tags peroxisomes for degradation in Saccharomyces cerevisiae. EMBO J. 2012;31:2852-68. doi:10.1038/emboj.2012.151. PMID:22643220.
- Farre JC, Burkenroad A, Burnett SF, Subramani S. Phosphorylation of mitophagy and pexophagy receptors coordinates their interaction with Atg8 and Atg11. EMBO reports. 2013;14:441-9. doi:10.1038/embor.2013.40. PMID:23559066.
- Anding AL, Baehrecke EH. Cleaning House: Selective Autophagy of Organelles. Developmental cell. 2017;41:10-22. doi:10.1016/j.devcel.2017.02.016. PMID:28399394.
- De Duve C, Baudhuin P. Peroxisomes (microbodies and related particles). Physiological reviews 1966;46:323-57. PMID:5325972.
- Wang W, Subramani S. Assays to Monitor Pexophagy in Yeast. Methods in enzymology. 2017;588:413-27. doi:10.1016/bs.mie.2016.09.088. PMID:28237113.
- Schnell DJ, Hebert DN. Protein translocons: multifunctional mediators of protein translocation across membranes. Cell. 2003;112:491-505. doi:10.1016/S0092-8674(03)00110-7. PMID:12600313.
- Leon S, Goodman JM, Subramani S. Uniqueness of the mechanism of protein import into the peroxisome matrix: transport of folded, co-factor-bound and oligomeric proteins by shuttling receptors. Biochimica et biophysica acta. 2006;1763:1552-64. doi:10.1016/j.bbamcr.2006.08.037. PMID:17011644.
- Gutensohn M, Fan E, Frielingsdorf S, Hanner P, Hou B, Hust B, Klosgen RB. Toc, Tic, Tat et al.: structure and function of protein transport machineries in chloroplasts. Journal of plant physiology. 2006;163:333-47. doi:10.1016/j.jplph.2005.11.009. PMID:16386331.
- Gorlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annual review of cell and developmental biology 1999;15:607-60. doi:10.1146/annurev.cellbio.15.1.607. PMID:10611974.
- Gould SJ, Keller GA, Hosken N, Wilkinson J, Subramani S. A conserved tripeptide sorts proteins to peroxisomes. J Cell Biol 1989;108:1657-64. doi:10.1083/jcb.108.5.1657. PMID:2654139.
- Swinkels BW, Gould SJ, Bodnar AG, Rachubinski RA, Subramani S. A novel, cleavable peroxisomal targeting signal at the amino-terminus of the rat 3-ketoacyl-CoA thiolase. EMBO J 1991;10:3255-62. PMID:1680677.
- Klein AT, van den Berg M, Bottger G, Tabak HF, Distel B. Saccharomyces cerevisiae acyl-CoA oxidase follows a novel, non-PTS1, import pathway into peroxisomes that is dependent on Pex5p. J Biol Chem. 2002;277:25011-9.
- Effelsberg D, Cruz-Zaragoza LD, Tonillo J, Schliebs W, Erdmann R. Role of Pex21p for Piggyback Import of Gpd1p and Pnc1p into Peroxisomes of Saccharomyces cerevisiae. J Biol Chem. 2015;290:25333-42. doi:10.1074/jbc.M115.653451. PMID:26276932.
- Saidowsky J, Dodt G, Kirchberg K, Wegner A, Nastainczyk W, Kunau WH, Schliebs W. The di-aromatic pentapeptide repeats of the human peroxisome import receptor PEX5 are separate high affinity binding sites for the peroxisomal membrane protein PEX14. J Biol Chem. 2001;276:34524-9. doi:10.1074/jbc.M104647200. PMID:11438541.
- Gatto GJ, Jr., Geisbrecht BV, Gould SJ, Berg JM. Peroxisomal targeting signal-1 recognition by the TPR domains of human PEX5. Nat Struct Biol. 2000;7:1091-5. doi:10.1038/81930. PMID:11101887.
- Purdue PE, Yang X, Lazarow PB. Pex18p and Pex21p, a novel pair of related peroxins essential for peroxisomal targeting by the PTS2 pathway. J Cell Biol 1998;143:1859-69. doi:10.1083/jcb.143.7.1859. PMID:9864360.
- Einwachter H, Sowinski S, Kunau WH, Schliebs W. Yarrowia lipolytica Pex20p, Saccharomyces cerevisiae Pex18p/Pex21p and mammalian Pex5pL fulfil a common function in the early steps of the peroxisomal PTS2 import pathway. EMBO Rep. 2001;2:1035-9. doi:10.1093/embo-reports/kve228. PMID:11606420.
- Sichting M, Schell-Steven A, Prokisch H, Erdmann R, Rottensteiner H. Pex7p and Pex20p of Neurospora crassa function together in PTS2-dependent protein import into peroxisomes. Mol Biol Cell. 2003;14:810-21. doi:10.1091/mbc.E02-08-0539. PMID:12589072.
- Elgersma Y, Elgersma-Hooisma M, Wenzel T, McCaffery JM, Farquhar MG, Subramani S. A mobile PTS2 receptor for peroxisomal protein import in Pichia pastoris. J Cell Biol 1998;140:807-20. doi:10.1083/jcb.140.4.807. PMID:9472033.
- Braverman N, Dodt G, Gould SJ, Valle D. An isoform of pex5p, the human PTS1 receptor, is required for the import of PTS2 proteins into peroxisomes. Hum Mol Genet. 1998;7:1195-205. doi:10.1093/hmg/7.8.1195. PMID:9668159.
- Otera H, Harano T, Honsho M, Ghaedi K, Mukai S, Tanaka A, Kawai A, Shimizu N, Fujiki Y. The mammalian peroxin Pex5pL, the longer isoform of the mobile peroxisome targeting signal (PTS) type 1 transporter, translocates the Pex7p.PTS2 protein complex into peroxisomes via its initial docking site, Pex14p. J Biol Chem. 2000;275:21703-14. doi:10.1074/jbc.M000720200. PMID:10767286.
- Lee JR, Jang HH, Park JH, Jung JH, Lee SS, Park SK, Chi YH, Moon JC, Lee YM, Kim SY, et al. Cloning of two splice variants of the rice PTS1 receptor, OsPex5pL and OsPex5pS, and their functional characterization using pex5-deficient yeast and Arabidopsis. The Plant journal: for cell and molecular biology. 2006;47:457-66. doi:10.1111/j.1365-313X.2006.02797.x. PMID:16792693.
- Schliebs W, Kunau WH. PTS2 co-receptors: diverse proteins with common features. Biochimica et biophysica acta. 2006;1763:1605-12. doi:10.1016/j.bbamcr.2006.08.051. PMID:17028014.
- Baes M, Gressens P, Baumgart E, Carmeliet P, Casteels M, Fransen M, Evrard P, Fahimi D, Declercq PE, Collen D, et al. A mouse model for Zellweger syndrome. Nat Genet. 1997;17:49-57. doi:10.1038/ng0997-49. PMID:9288097.
- Shimozawa N, Zhang Z, Suzuki Y, Imamura A, Tsukamoto T, Osumi T, Fujiki Y, Orii T, Barth PG, Wanders RJ, et al. Functional heterogeneity of C-terminal peroxisome targeting signal 1 in PEX5-defective patients. Biochem Biophys Res Commun. 1999;262:504-8. doi:10.1006/bbrc.1999.1232. PMID:10462504.
- Ebberink MS, Mooyer PA, Koster J, Dekker CJ, Eyskens FJ, Dionisi-Vici C, Clayton PT, Barth PG, Wanders RJ, Waterham HR. Genotype-phenotype correlation in PEX5-deficient peroxisome biogenesis defective cell lines. Hum Mutat. 2009;30:93-8. doi:10.1002/humu.20833. PMID:18712838.
- Williams CP, Stanley WA. Peroxin 5: a cycling receptor for protein translocation into peroxisomes. The international journal of biochemistry & cell biology. 2010;42:1771-4. doi:10.1016/j.biocel.2010.07.004.
- White SR, Lauring B. AAA+ ATPases: achieving diversity of function with conserved machinery. Traffic. 2007;8:1657-67. doi:10.1111/j.1600-0854.2007.00642.x. PMID:17897320.
- Lupas AN, Martin J. AAA proteins. Curr Opin Struct Biol. 2002;12:746-53. doi:10.1016/S0959-440X(02)00388-3. PMID:12504679.
- Gouveia AM, Guimaraes CP, Oliveira ME, Reguenga C, Sa-Miranda C, Azevedo JE. Characterization of the peroxisomal cycling receptor, Pex5p, using a cell-free in vitro import system. J Biol Chem. 2003;278:226-32. doi:10.1074/jbc.M209498200. PMID:12411433.
- Oliveira ME, Gouveia AM, Pinto RA, Sa-Miranda C, Azevedo JE. The energetics of Pex5p-mediated peroxisomal protein import. J Biol Chem. 2003;278:39483-8. doi:10.1074/jbc.M305089200. PMID:12885776.
- Ciniawsky S, Grimm I, Saffian D, Girzalsky W, Erdmann R, Wendler P. Molecular snapshots of the Pex1/6 AAA+ complex in action. Nat Commun. 2015;6:7331. doi:10.1038/ncomms8331. PMID:26066397.
- Kiel JA, Veenhuis M, van der Klei IJ. PEX genes in fungal genomes: common, rare or redundant. Traffic. 2006;7:1291-303. doi:10.1111/j.1600-0854.2006.00479.x. PMID:16978390.
- Grimm I, Erdmann R, Girzalsky W. Role of AAA(+)-proteins in peroxisome biogenesis and function. Biochimica et biophysica acta. 2016;1863:828-37. doi:10.1016/j.bbamcr.2015.10.001. PMID:26453804.
- Birschmann I, Stroobants AK, van den Berg M, Schafer A, Rosenkranz K, Kunau WH, Tabak HF. Pex15p of Saccharomyces cerevisiae provides a molecular basis for recruitment of the AAA peroxin Pex6p to peroxisomal membranes. Mol Biol Cell. 2003;14:2226-36. doi:10.1091/mbc.E02-11-0752. PMID:12808025.
- Matsumoto N, Tamura S, Fujiki Y. The pathogenic peroxin Pex26p recruits the Pex1p-Pex6p AAA ATPase complexes to peroxisomes. Nat Cell Biol. 2003;5:454-60. doi:10.1038/ncb982. PMID:12717447.
- Goto S, Mano S, Nakamori C, Nishimura M. Arabidopsis ABERRANT PEROXISOME MORPHOLOGY9 is a peroxin that recruits the PEX1-PEX6 complex to peroxisomes. Plant Cell. 2011;23:1573-87. doi:10.1105/tpc.110.080770. PMID:21487094.
- Williams C, van den Berg M, Sprenger RR, Distel B. A conserved cysteine is essential for Pex4p-dependent ubiquitination of the peroxisomal import receptor Pex5p. J Biol Chem. 2007;282:22534-43. doi:10.1074/jbc.M702038200. PMID:17550898.
- Platta HW, El Magraoui F, Schlee D, Grunau S, Girzalsky W, Erdmann R. Ubiquitination of the peroxisomal import receptor Pex5p is required for its recycling. J Cell Biol. 2007;177:197-204. doi:10.1083/jcb.200611012. PMID:17452527.
- Carvalho AF, Pinto MP, Grou CP, Alencastre IS, Fransen M, Sa-Miranda C, Azevedo JE. Ubiquitination of mammalian Pex5p, the peroxisomal import receptor. J Biol Chem. 2007;282:31267-72. doi:10.1074/jbc.M706325200. PMID:17726030.
- Okumoto K, Misono S, Miyata N, Matsumoto Y, Mukai S, Fujiki Y. Cysteine ubiquitination of PTS1 receptor Pex5p regulates Pex5p recycling. Traffic. 2011;12:1067-83. doi:10.1111/j.1600-0854.2011.01217.x. PMID:21554508.
- Wiebel FF, Kunau WH. The Pas2 protein essential for peroxisome biogenesis is related to ubiquitin-conjugating enzymes. Nature 1992;359:73-6. doi:10.1038/359073a0. PMID:1326082.
- Koller A, Snyder WB, Faber KN, Wenzel TJ, Rangell L, Keller GA, Subramani S. Pex22p of Pichia pastoris, essential for peroxisomal matrix protein import, anchors the ubiquitin-conjugating enzyme, Pex4p, on the peroxisomal membrane. J Cell Biol 1999;146:99-112. PMID:10402463.
- El Magraoui F, Schrotter A, Brinkmeier R, Kunst L, Mastalski T, Muller T, Marcus K, Meyer HE, Girzalsky W, Erdmann R, et al. The cytosolic domain of Pex22p stimulates the Pex4p-dependent ubiquitination of the PTS1-receptor. PloS one. 2014;9:e105894. doi:10.1371/journal.pone.0105894. PMID:25162638.
- Agne B, Meindl NM, Niederhoff K, Einwachter H, Rehling P, Sickmann A, Meyer HE, Girzalsky W, Kunau WH. Pex8p: an intraperoxisomal organizer of the peroxisomal import machinery. Mol Cell. 2003;11:635-46. doi:10.1016/S1097-2765(03)00062-5. PMID:12667447.
- El Magraoui F, Baumer BE, Platta HW, Baumann JS, Girzalsky W, Erdmann R. The RING-type ubiquitin ligases Pex2p, Pex10p and Pex12p form a heteromeric complex that displays enhanced activity in an ubiquitin conjugating enzyme-selective manner. FEBS J. 2012;279:2060-70. doi:10.1111/j.1742-4658.2012.08591.x. PMID:22471590.
- Platta HW, El Magraoui F, Baumer BE, Schlee D, Girzalsky W, Erdmann R. Pex2 and Pex12 function as protein-ubiquitin ligases in peroxisomal protein import. Mol Cell Biol. 2009;29:5505-16. doi:10.1128/MCB.00388-09. PMID:19687296.
- Okumoto K, Noda H, Fujiki Y. Distinct modes of ubiquitination of peroxisome-targeting signal type 1 (PTS1) receptor Pex5p regulate PTS1 protein import. J Biol Chem. 2014;289:14089-108. doi:10.1074/jbc.M113.527937. PMID:24662292.
- Grou CP, Carvalho AF, Pinto MP, Wiese S, Piechura H, Meyer HE, Warscheid B, Sa-Miranda C, Azevedo JE. Members of the E2D (UbcH5) family mediate the ubiquitination of the conserved cysteine of Pex5p, the peroxisomal import receptor. J Biol Chem. 2008;283:14190-7. doi:10.1074/jbc.M800402200. PMID:18359941.
- Ma C, Hagstrom D, Polley SG, Subramani S. Redox-regulated cargo binding and release by the peroxisomal targeting signal receptor, Pex5. J Biol Chem. 2013;288:27220-31. doi:10.1074/jbc.M113.492694. PMID:23902771.
- Alencastre IS, Rodrigues TA, Grou CP, Fransen M, Sa-Miranda C, Azevedo JE. Mapping the cargo protein membrane translocation step into the PEX5 cycling pathway. J Biol Chem. 2009;284:27243-51. doi:10.1074/jbc.M109.032565. PMID:19632994.
- Legakis JE, Koepke JI, Jedeszko C, Barlaskar F, Terlecky LJ, Edwards HJ, Walton PA, Terlecky SR. Peroxisome senescence in human fibroblasts. Mol Biol Cell. 2002;13:4243-55. doi:10.1091/mbc.E02-06-0322. PMID:12475949.
- Apanasets O, Grou CP, Van Veldhoven PP, Brees C, Wang B, Nordgren M, Dodt G, Azevedo JE, Fransen M. PEX5, the shuttling import receptor for peroxisomal matrix proteins, is a redox-sensitive protein. Traffic. 2014;15:94-103. doi:10.1111/tra.12129. PMID:24118911.
- Debelyy MO, Platta HW, Saffian D, Hensel A, Thoms S, Meyer HE, Warscheid B, Girzalsky W, Erdmann R. Ubp15p, a ubiquitin hydrolase associated with the peroxisomal export machinery. J Biol Chem. 2011;286:28223-34. doi:10.1074/jbc.M111.238600. PMID:21665945.
- Grou CP, Francisco T, Rodrigues TA, Freitas MO, Pinto MP, Carvalho AF, Domingues P, Wood SA, Rodriguez-Borges JE, Sa-Miranda C, et al. Identification of ubiquitin-specific protease 9X (USP9X) as a deubiquitinase acting on ubiquitin-peroxin 5 (PEX5) thioester conjugate. J Biol Chem. 2012;287:12815-27. doi:10.1074/jbc.M112.340158. PMID:22371489.
- Wang W, Xia ZJ, Farre JC, Subramani S. TRIM37, a novel E3 ligase for PEX5-mediated peroxisomal matrix protein import. J Cell Biol. 2017. doi:10.1083/jcb.201611170.
- Kragt A, Voorn-Brouwer T, van den Berg M, Distel B. The Saccharomyces cerevisiae peroxisomal import receptor Pex5p is monoubiquitinated in wild type cells. J Biol Chem. 2005;280:7867-74. doi:10.1074/jbc.M413553200. PMID:15632140.
- Kiel JA, Emmrich K, Meyer HE, Kunau WH. Ubiquitination of the peroxisomal targeting signal type 1 receptor, Pex5p, suggests the presence of a quality control mechanism during peroxisomal matrix protein import. J Biol Chem. 2005;280:1921-30. doi:10.1074/jbc.M403632200. PMID:15536088.
- Platta HW, Girzalsky W, Erdmann R. Ubiquitination of the peroxisomal import receptor Pex5p. The Biochemical journal. 2004;384:37-45. doi:10.1042/BJ20040572. PMID:15283676.
- Seufert W, McGrath JP, Jentsch S. UBC1 encodes a novel member of an essential subfamily of yeast ubiquitin-conjugating enzymes involved in protein degradation. EMBO J 1990;9:4535-41. PMID:2265617.
- Seufert W, Jentsch S. Ubiquitin-conjugating enzymes UBC4 and UBC5 mediate selective degradation of short-lived and abnormal proteins. EMBO J 1990;9:543-50. PMID:2154373.
- Leon S, Zhang L, McDonald WH, Yates J, 3rd, Cregg JM, Subramani S. Dynamics of the peroxisomal import cycle of PpPex20p: ubiquitin-dependent localization and regulation. J Cell Biol. 2006;172:67-78. doi:10.1083/jcb.200508096. PMID:16390998.
- Dodt G, Gould SJ. Multiple PEX genes are required for proper subcellular distribution and stability of Pex5p, the PTS1 receptor: evidence that PTS1 protein import is mediated by a cycling receptor. J Cell Biol 1996;135:1763-74. doi:10.1083/jcb.135.6.1763. PMID:8991089.
- Yahraus T, Braverman N, Dodt G, Kalish JE, Morrell JC, Moser HW, Valle D, Gould SJ. The peroxisome biogenesis disorder group 4 gene, PXAAA1, encodes a cytoplasmic ATPase required for stability of the PTS1 receptor. EMBO J 1996;15:2914-23. PMID:8670792.
- Nuttall JM, Motley AM, Hettema EH. Deficiency of the exportomer components Pex1, Pex6, and Pex15 causes enhanced pexophagy in Saccharomyces cerevisiae. Autophagy. 2014;10:835-45. doi:10.4161/auto.28259. PMID:24657987.
- Kraft C, Peter M, Hofmann K. Selective autophagy: ubiquitin-mediated recognition and beyond. Nat Cell Biol. 2010;12:836-41. doi:10.1038/ncb0910-836. PMID:20811356.
- Deosaran E, Larsen KB, Hua R, Sargent G, Wang Y, Kim S, Lamark T, Jauregui M, Law K, Lippincott-Schwartz J, et al. NBR1 acts as an autophagy receptor for peroxisomes. Journal of cell science. 2013;126:939-52. doi:10.1242/jcs.114819. PMID:23239026.
- Yamashita S, Abe K, Tatemichi Y, Fujiki Y. The membrane peroxin PEX3 induces peroxisome-ubiquitination-linked pexophagy. Autophagy. 2014;10:1549-64. doi:10.4161/auto.29329. PMID:25007327.
- Sargent G, van Zutphen T, Shatseva T, Zhang L, Di Giovanni V, Bandsma R, Kim PK. PEX2 is the E3 ubiquitin ligase required for pexophagy during starvation. J Cell Biol. 2016;214:677-90. doi:10.1083/jcb.201511034. PMID:27597759.
- Subramani S. A mammalian pexophagy target. Nat Cell Biol. 2015;17:1371-3. doi:10.1038/ncb3253. PMID:26458245.
- Shiloh Y, Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol. 2013;14:197-210. doi:10.1038/nrm3546.
- Watters D, Kedar P, Spring K, Bjorkman J, Chen P, Gatei M, Birrell G, Garrone B, Srinivasa P, Crane DI, et al. Localization of a portion of extranuclear ATM to peroxisomes. J Biol Chem 1999;274:34277-82. doi:10.1074/jbc.274.48.34277. PMID:10567403.
- Zhang J, Tripathi DN, Jing J, Alexander A, Kim J, Powell RT, Dere R, Tait-Mulder J, Lee JH, Paull TT, et al. ATM functions at the peroxisome to induce pexophagy in response to ROS. Nat Cell Biol. 2015;17:1259-69. doi:10.1038/ncb3230. PMID:26344566.
- Nordgren M, Francisco T, Lismont C, Hennebel L, Brees C, Wang B, Van Veldhoven PP, Azevedo JE, Fransen M. Export-deficient monoubiquitinated PEX5 triggers peroxisome removal in SV40 large T antigen-transformed mouse embryonic fibroblasts. Autophagy. 2015;11:1326-40. doi:10.1080/15548627.2015.1061846. PMID:26086376.
- Law KB, Bronte-Tinkew D, Di Pietro E, Snowden A, Jones RO, Moser A, Brumell JH, Braverman N, Kim PK. The peroxisomal AAA ATPase complex prevents pexophagy and development of peroxisome biogenesis disorders. Autophagy. 2017;13:868-84. doi:10.1080/15548627.2017.1291470. PMID:28521612.
- Nazarko TY. Pexophagy is responsible for 65% of cases of peroxisome biogenesis disorders. Autophagy. 2017;13:991-4. doi:10.1080/15548627.2017.1291480. PMID:28318378.
