Epithelial ovarian cancer (EOC) remains the most lethal gynecological malignancy in the developed world. EOC is composed of multiple separate diseases. Ovarian clear cell carcinoma (OCCC) and ovarian endometrioid carcinoma (OEC) are the second and third deadliest subtypes of EOC. Notably, OCCC is associated with the worst prognosis compared to other EOC subtypes when diagnosed at advanced stages. OCCC typically has a low response rate to platinum-based standard of care for EOC, and there is currently no effective therapy for this disease.
The past few years have brought unprecedented progress in understanding the genetics of EOC, but cures remain elusive due to the lack of insights into mechanisms that can be targeted to develop new therapies. The discovery of somatic ARID1 A mutations in over 50% OCCC and 30% OEC represents a major advance in these histosubtypes.Citation1,Citation2 Over 90% of the ARID1 A mutations observed in EOC are frame-shift or nonsense mutations that result in loss of ARID1 A expression. Notably, loss of ARID1 A correlates with late-stage disease and predicts early recurrence. Thus, there is an even greater need for targeted therapy that is selective for ARID1 A-mutated EOC. Mutations in ARID1 A and TP53 are typically mutually exclusive in EOC.Citation3 However, therapeutic strategies that harness this genetic characteristic have not been explored. A recent study demonstrates that ARID1 A-mutated EOC depend on HDAC6 activity, and inhibition of HDAC6 promotes apoptosis in ARID1 A-mutated cells in a p53-dependent manner.Citation4 This work explains, at least in part, the mutual exclusivity between ARID1 A and TP53 mutations because it indicates that ARID1 A mutation contributes to inactivation of p53's apoptosis-promoting function. In addition, these studies suggest potential translation of these findings by repurposing clinically applicable HDAC6 inhibitors for ARID1 A-mutated EOC.
ARID1 A epigenetically regulates gene expression via the SWI/SNF chromatin-remodeling complex by controlling gene accessibility. SWI/SNF complexes contribute to both gene activation and repression in a context-dependent manner. Citation5 Previous studies demonstrated that SWI/SNF promoted transcriptional activation of tumor suppressor genes that are repressed by EZH2.Citation6 In contrast, ARID1 A reduced the promoter activity of HDAC6 gene, thus acting as a direct transcriptional repressor. Mechanistically, it was shown that lysine 120 acetylated p53 (p53K120 Ac), a pro-apoptotic posttranslational modification, is a direct target for HDAC6 deacetylase activity. Thus, ARID1 A-mutated tumors have elevated levels of HDAC6, which in turn represses apoptotic function of p53 by reducing p53K120 Ac levels. The apoptosis induced by HDAC6 inhibition correlates with an increase of mitochondria localization of p53K120 Ac and a decrease in mitochondria membrane potential (). In both xenografts and genetically engineered mouse models of OCCC, pharmacological inhibition of HDAC6 using a clinically applicable drug ACY1215 induced apoptosis selectively in ARID1 A-mutated, but not wildtype, OCCC tumors. Indeed, ACY1215 improved the survival of mice bearing ARID1 A-mutated OCCCs.
Figure 1. Schematics of molecular mechanism by which HDAC6 inhibition is selectively against ARID1 A mutation in a p53-dependent manner.
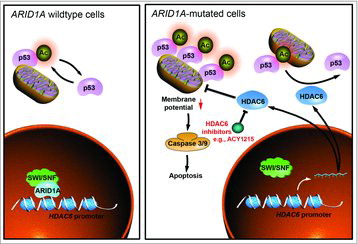
Beyond EOC, the mutual exclusivity between ARID1 A and TP53 mutations was observed in a number of other major cancer types such as prostate, stomach, colon and uterine. It will be interesting to determine whether HDAC6 inhibition is equally effective in these cancers with mutated ARID1 A and wildtype TP53. Interestingly, in contrast to ARID1 A inactivation, knockdown of BRG1, the catalytic subunit of the SWI/SNF, did not affect sensitivity to HDAC6 inhibition. This is consistent with the notion that ARID1 A targets the SWI/SNF complex to specific genomic loci.Citation5 ARID1 A exists in multiple different complexes based on mutual exclusivity among different subunits including the catalytic subunits.Citation5 Thus, the observed differences between ARID1 A and BRG1 inactivation may reflect the unique ARID1 A-dependent genomic distribution of the SWI/SNF complex and the fact that BRG1 loss can be compensated by the mutual exclusive catalytic subunit BRM.
Despite the great progress in exploring checkpoint blockade immunotherapy, biomarkers of response to immune checkpoint blockade remain to be fully explored. Of proposed biomarkers, microsatellite instability (MSI) is particularly interesting in the context of ARID1 A-mutated cancers, because ARID1 A loss is often associated with MSI in endometrial and gastric cancers. In addition, a recently study showed that OCCCs with MSI had higher levels of CD8+ and PD-1+ tumor infiltrating lymphocytes compared to MSI-negative OCCCs.Citation7 Thus, ARID1 A-mutated cancers may be susceptible to anti-PD-1/PD-L1 immunotherapies. HDAC6 has been reported to positively regulate PD-L1 expression, enhance T-cell activation and improve function of antigen-presenting cells. These data raise the possibility that HDAC6 inhibition in combination with PD-1/PD-L1 blockade is potentially a promising strategy for ARID1 A-mutated cancers. This strategy will not only eliminate ARID1 A-mutated tumor cells in a p53-dependent manner but also restore anti-tumor immunity. This synergistic combination could maximize the therapeutic efficacy, minimize the toxicity and possibility of developing resistance.
In summary, these findings demonstrate that targeting HDAC6 activity represents a novel therapeutic strategy for ARID1 A-mutated EOC. HDAC6 inhibitor ACY1215 displayed minimal toxicities in clinical studies, supporting high translational potential of our study. This approach takes advantage of ARID1 A mutational status and mutual exclusivity between ARID1 A and TP53 mutations. Considering that ARID1 A shows one of the highest mutation rates among epigenetic regulators across all human cancers, these findings should have far reaching implications for the future development of epigenetic therapeutic strategies. Combinational approaches and, in particular, synergistically utilizing epigenetic and immune therapies warrant further exploration for ARID1 A-mutated cancers.
Abbreviations
| EOC | = | epithelial ovarian cancer |
| MSI | = | microsatellite instability |
| OCCC | = | ovarian clear cell carcinoma |
| OEC | = | ovarian endometrioid carcinoma |
| p53K120 Ac | = | lysine 120 acetylated p53 |
References
- Jones S, Wang TL, Shih IeM, Mao TL, Nakayama K, Roden R, Glas R, Slamon D, Diaz LA Jr, Vogelstein B, Kinzler KW, Velculescu VE, et al. Frequent mutations of chromatin remodeling gene ARID1 A in ovarian clear cell carcinoma. Science. 2010;330:228-31. doi: 10.1126/science.1196333. PMID: 20826764
- Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, Yang W, Heravi-Moussavi A, Giuliany R, et al. ARID1 A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532-43. doi: 10.1056/NEJMoa1008433. PMID: 20942669
- Guan B, Wang TL, Shih IeM. ARID1 A, a factor that promotes formation of SWI/SNF-mediated chromatin remodeling, is a tumor suppressor in gynecologic cancers. Cancer Res. 2011;71:6718-27. doi:10.1158/0008-5472.CAN-11-1562. PMID: 21900401
- Bitler BG, Wu S, Park PH, Hai Y, Aird KM, Wang Y, Zhai Y, Kossenkov AV, Vara-Ailor A, Rauscher Iii FJ, et al. ARID1 A-mutated ovarian cancers depend on HDAC6 activity. Nat Cell Biol. 2017;19:962-73. doi: 10.1038/ncb3582. PMID: 28737768
- Wilson BG, Roberts CW. SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer. 2011;11:481-92. doi: 10.1038/nrc3068. PMID: 21654818
- Bitler BG, Aird KM, Garipov A, Li H, Amatangelo M, Kossenkov AV, Schultz DC, Liu Q, Shih IeM, Conejo-Garcia JR, Speicher DW, et al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1 A-mutated cancers. Nat Med. 2015;21:231-8. doi: 10.1038/nm.3799. PMID: 25686104
- Howitt BE, Strickland KC, Sholl LM, Rodig S, Ritterhouse LL, Chowdhury D, D'Andrea AD, Matulonis UA, Konstantinopoulos PA. Clear cell ovarian cancers with microsatellite instability: A unique subset of ovarian cancers with increased tumor-infiltrating lymphocytes and PD-1/PD-L1 expression. Oncoimmunology. 2017;6:e1277308. doi:10.1080/2162402X.2016.1277308. PMID: 28344892
