ABSTRACT
Numerous studies have demonstrated that microRNAs (miRNAs) play important roles in cell growth, apoptosis and spermatogenesis. Our previous study showed that miR-638 was differentially expressed in sexually immature and mature testes of Large White boars. Here we reported that sperm-associated antigen 1 (SPAG1) was a direct target gene of miR-638. Moreover, miR-638 inhibited cell proliferation and cell cycle, and promoted apoptosis of porcine immature Sertoli cells. Key genes including phosphorylated phosphatidylinositide 3-kinases (p-PI3K) and phosphorylated serine/ threonine kinase (p-AKT) in PI3K/AKT pathway as well as cell cycle factors including c-MYC, cyclin-D1 (CCND1), cyclin-E1 (CCNE1) and cyclin-dependent kinase 4 (CDK4) were all significantly down-regulated after overexpression of miR-638 or RNAi of SPAG1. Notably, mRNA levels of SRY-related HMG-box 2 (SOX2) and POU domain, class 5, transcription factor 1 (POU5F1) essential for spermatogonia proliferation were significantly suppressed in SPAG1 siRNA- transfected ST cells. This study suggests that miR-638 regulates immature Sertoli cell growth and apoptosis by targeting SPAG1 gene which can indirectly inactivate PI3K/AKT pathway, and plays roles in pig spermatogenesis.
KEYWORDS:
Introduction
Spermatogenesis is a process that occurs in testicular seminiferous epithelium,Citation1 including spermatogonial stem cells self-renew, the meiosis of spermatocytes and the spermiogenesis of haploid spermatids. Infertility is a global reproductive health problem that affects approximate 15% of human couples, and male infertility is largely accompanied by severe spermatogenesis disorders.Citation2,3
Emerging evidence has demonstrated that microRNAs (miRNAs) induced germ cell proliferation, apoptosis, DNA damage and spermatogenesis.Citation4,5 For example, miR-16 modulates melatonin-induced cell growth in the mouse-derived spermatogonia cell line GC-1 spg cells by targeting cyclin-D1 (CCND1) gene.Citation6 Overexpression of miR-26b-5p promoted cell cycle progression by targeting cyclin-D2 (CCND2) in mouse spermatocyte- derived GC-2 cells under exposure to extremely low frequency electromagnetic fields.Citation7 miR-133b is up-regulated in Sertoli cells of Sertoli-cell-only syndrome patients and stimulates human Sertoli cell proliferation by targeting GLI family zinc finger 3 (GLI3) and by indirectly activating CCND1 and cyclin-B1 (CCNB1).Citation8
miR-638 is a primate-specific miRNA which plays important roles in cell proliferation and DNA damage repair majorly in cancer cells from gastric cancer, basal cell carcinoma and colorectal carcinoma.Citation9–11 miR-638 was differentially expressed in immature and mature testes of Large White boars through a microarray approach in our previous study, suggesting miR-638 participates in regulating spermatogenesis.Citation12 Sperm- associated antigen 1 (SPAG1) present in spermatocytes and sperm may participate in spermatogenesis and fertilization process by activating the PKC-dependent ERK1/2 signal transduction pathway.Citation13 Previous reports have showed that SPAG1 was localized to the neck and midpiece of pachytene primary spermatocytes.Citation13,14
The commercial swine testis (ST) cells have recently been identified as immature porcine Sertoli cells.Citation15 Sertoli cells are the somatic cells of the testes that are necessary for testis formation and spermatogenesis.Citation16 To elucidate the roles of miR-638 in spermatogenesis, we verified SPAG1 gene as one of its targets in ST cells. Then we demonstrated that miR-638 inhibited ST cell proliferation and promoted apoptosis by targeting SPAG1 gene, and miR-638 was involved in the modulation of cell cycle, cell proliferation and apoptosis indirectly through PI3K/AKT signal transduction pathway in ST cells. γ-tubulin (TUBG1) is a well-recognized MTOC- associated protein which guides spindle morpgogenesis. TUBG1 deletion causes spindle assembly defection, and further results in that cells are unable to complete mitosis.Citation17 SPAG1 is required for spindle morphogenesis and spermatogonia proliferation by testing TUBG1, SRY-related HMG-box 2 (SOX2) and POU domain, class 5, transcription factor 1 (POU5F) expression in knock-down experiments. This study suggests that miR-638 affect spermatogenesis by inhibiting ST cells growth and provides a potential insight into boar reproduction and male infertility.
Results
SPAG1 is a direct target of miR-638 in immature porcine Sertoli cells
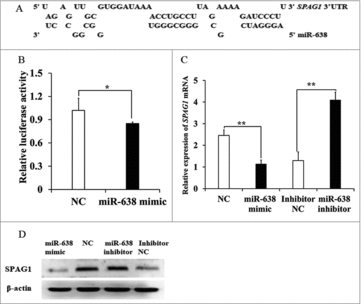
To investigate the role of miR-638 on immature porcine Sertoli cells, a putative miR-638 binding site was predicted in the SPAG1 3′ un-translated region (3′UTR) using RNAhybrid online prediction (https://bibiserv.cebitec.uni-bielefeld.de/rnahybrid?id=rnahybrid_view_submission) (). To validate this prediction, a fragment of SPAG13′UTR containing the putative miR-638 binding site was inserted into dual-luciferase reporter vector pmirGLO. MiR-638 mimics or miR-638 inhibitors were synthesized to overexpress or suppress the expression of miR-638 in ST cells (immature porcine Sertoli cells), respectively. The luciferase activity was significantly suppressed in ST cells transfected with miR-638 mimics, while increased in ST cells transfected with miR-638 inhibitors (). Furthermore, SPAG1 mRNA levels were suppressed or increased in ST cells transfected with miR-638 mimics or miR-638 inhibitors, respectively (). SPAG1 protein expression was also suppressed or increased in ST cells transfected with miR-638 mimics or miR-638 inhibitors, respectively (). These results suggested that SPAG1 was a direct target gene of miR-638.
Figure 1. SPAG1 is a direct target of miR-638 in immature porcine Sertoli cells. (A) The miR-638 binding site in the SPAG1 3′UTR was predicted using RNAhybrid. (B) pmirGLO-SPAG1-3′UTR was co-transfected into ST cells with miR-638 mimics or NC. Whole cellular lysates were obtained 24 h after transfection, and then relative luciferase activity was measured. (C) Endogenous SPAG1 mRNA levels were detected in ST cells 24 h after transfection with miR-638 mimics or NC and miR-638 inhibitors or inhibitor NC. (D) SPAG1 protein levels were also monitored using Western blot analysis for 48 h after transfection with miR-638 mimics or mimics NC and miR-638 inhibitors or inhibitor NC. Data are presented as the mean ± S. D. (three independent replicates per group). * P < 0.05, ** P < 0.01.
miR-638 inhibits immature porcine Sertoli cell growth
To test the roles of miR-638 on ST cell functions, we transfected miR-638 mimics into ST cells. Cell cycle analysis showed that miR-638 mimic-transfected ST cells were arrested at the S phase. The percentage of cells in G0/G1 phase increased while fewer cells were detected in S phase compared to controls (), suggesting that miR-638 may induce DNA synthesis phase arrest.
Figure 2. miR-638 inhibits immature Sertoli cell growth. ST cells were transfected with miR-638 mimics or NC and miR-638 inhibitors or inhibitor NC. (A) Cell cycle was analyzed 48 h after transfection by propidium iodide flow cytometry. (B) mRNA expression levels of cell cycle-related genes were determined by Q-PCR. (C) Cell cycle-related factor protein levels were detected by Western blot. (D) Cell proliferation was detected by MTT assay. (E) PCNA mRNA expression level was detected by Q-PCR. Data are presented as the mean ± S. D. (three independent replicates per group). * P < 0.05, ** P < 0.01.
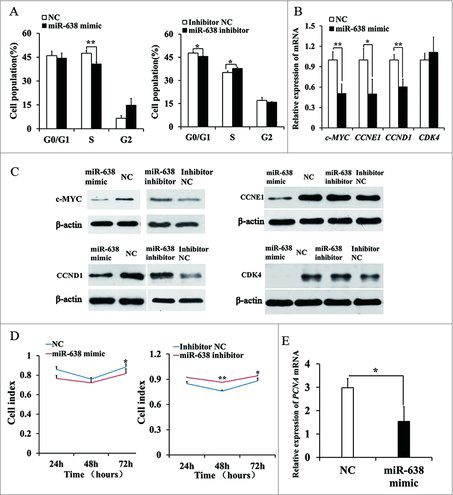
Cell cycle G1/S phase is mainly regulated by c-MYC which modulates the expression of important factors that promote cell cycle progression to S phase, including cyclins, cyclin dependent kinases (CDK), CDK inhibitors and the pRb-binding transcription factor E2F.Citation18 We examined the effect of miR-638 mimics on c-MYC and cell cycle-related gene expression. The expressions of c-MYC, CCNE1 and CCND1 were significantly suppressed in miR-638 mimic-transfected ST cells at mRNA and protein level (, ), whereas the expression of these proteins was increased by miR-638 inhibitors (). CDK4 protein expression was also decreased in miR-638 mimic group, but CDK4 mRNA expression did not change (, ).
Furthermore, MTT (Methylthiazolyldiphenyl-tetrazolium bromide) assay confirmed that cell proliferation rate was decreased compared with the controls (). Consistent with the result of cell proliferation, miR-638 overexpression also suppressed the mRNA level of proliferating cell nuclear antigen (PCNA), a marker gene for cell proliferation (). The effect of miR-638 inhibitors on cell cycle and proliferation was opposite to that of miR-638 mimics (, ). These data clearly showed that miR-638 suppressed the expression of c-MYC, and thus induced ST cell cycle arrest.
miR-638 inhibits immature Sertoli cell growth partly through suppressing SPAG1
So we assumed that miR-638 was crucial for the porcine Sertoli cell destiny and spermatogenesis probably via its target SPAG1. Then we transfected ST cells with SPAG1 siRNA to knock down SPAG1. Cell cycle analysis showed that the percentage of cells in S phase was significantly decreased in SPAG1 siRNA- transfected cells while more cells stayed in G0/G1 phase compared to the control (), suggesting that SPAG1 inhibited G1/S transition.
Figure 3. miR-638 inhibits immature Sertoli cell growth partly through suppressing SPAG1. ST cells were treated with SPAG1 siRNA or siRNA NC. (A) Cell cycle was analyzed 48 h after transfection by propidium iodide flow cytometry. (B) mRNA expression levels of cell cycle-related genes was detected by Q-PCR. (C) Cell cycle-related factors protein levels were detected by Western blot. (D) PCNA mRNA expression level was detected by Q-PCR. (E) When the cell index reached 1.0, cell growth dynamics were then continuously monitored using the xCELLigence system. Data are presented as the mean ± S. D. (three independent replicates per group). ** P < 0.01.
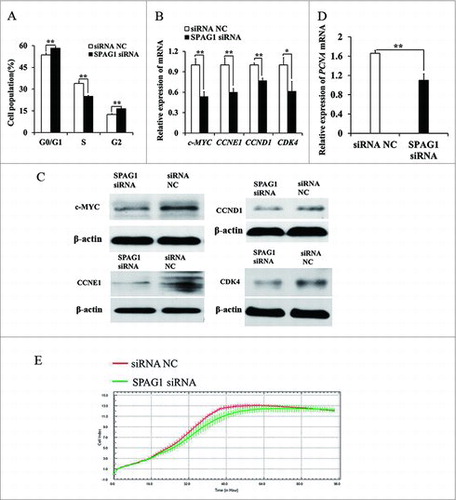
To investigate whether miR-638 reduced c-MYC expression by suppressing SPAG1, we examined c-MYC expression in SPAG1 siRNA-transfected ST cells. The expressions of c-MYC, CCNE1, CCND1 and CDK4 were dramatically reduced in SPAG1 siRNA group at mRNA and protein level (, ).
Similarly, SPAG1 siRNA-transfected cells displayed a reduced growth rate and suppressed PCNA mRNA level (, ). These results indicated that miR-638 prevented ST cell growth by inhibiting its target gene SPAG1.
miR-638 promotes immature Sertoli cells apoptosis through inactivating PI3K/AKT signaling pathway
Intracellular adenosine triphosphate (ATP) maintained cell growth, so apoptosis will be induced once intracellular ATP is depleted. We then examined miR-638 effects on cellular ATP levels and apoptosis in ST cells. The ATP level was dramatically decreased in miR-638 mimic-transfected ST cells, and miR-638 inhibitor elevated the ATP level (, ). We also found that apoptosis was significantly increased in miR-638 mimic-transfected ST cells, and miR-638 inhibitor suppressed apoptosis (, ). These results demonstrated that miR-638 might promote immature Sertoli cell apoptosis by ATP depletion.
Figure 4. miR-638 promotes immature Sertoli cells apoptosis through inactivating PI3K/AKT signaling pathway. ST cells were treated with miR-638 mimics or NC and miR-638 inhibitors or inhibitor NC. (A) Content of ATP was measured by ELISA assay in ST cells transfected with miR-638 mimics or NC. Relative ATP level of NC group was normalized with the miR-638 mimics group. (B) Content of ATP was measured by ELISA assay in ST cells transfected with miR-638 inhibitors or inhibitor NC. Relative ATP level of miR-638 inhibitor was normalized with inhibitor NC group. (C) Cell apoptosis of ST cells transfected with miR-638 mimics or NC was measured 48 h after transfection using Annexin-V/PI staining, followed by flow cytometer analysis. UR: late apoptosis, LR: early apoptosis. (D) Cell apoptosis of ST cells transfected with miR-638 inhibitors or inhibitor NC was measured 48 h after transfection using Annexin-V/PI staining, followed by flow cytometer analysis. (E) p-PI3K (phospho Tyr458) protein levels were detected using Western blot assay. (F) p-AKT (phospho Ser473) protein levels were detected using Western blot assay. Data are presented as the mean ± S.D. (three independent replicates per group). * P < 0.05, ** P < 0.01.
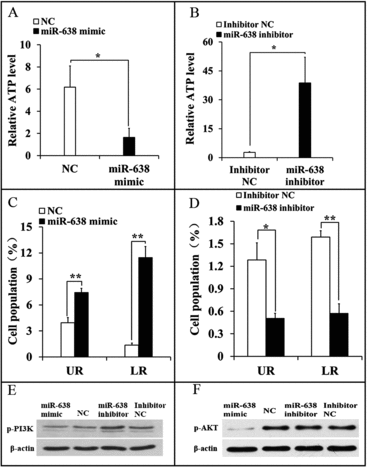
PI3K signaling molecule is induced by growth factor receptor stimulation and its activation is essential for cell growth and cell cycle entry.Citation19–21 PI3K/AKT activation was required for c-MYC stabilization and apoptosis, and protected B-cell lymphoma cell line from anti-IgM-induced apoptosis.Citation22–24 We next measured the effect of miR-638 on activation of PI3K and AKT. Compared to the controls, the expression of p-PI3K and p-AKT was decreased by miR-638 mimics but elevated by miR-638 inhibitors (, ). These results suggested that miR-638 mimics inactivated PI3K and its downstream molecules, thus we speculated that miR-638 promoted apoptosis of ST cells by inhibiting PI3K/AKT pathway.
miR-638 promotes immature Sertoli cells apoptosis through SPAG1
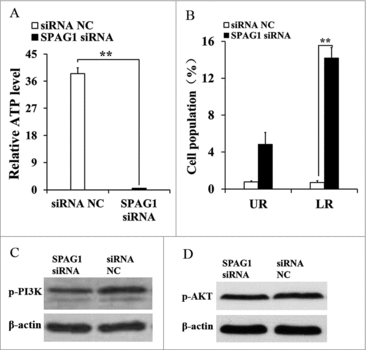
To further determine the molecular mechanism of apoptosis induced by miR-638, we detected the effect of its target gene SPAG1 on apoptosis of ST cells. Consistently, SPAG1 siRNA suppressed the ATP level (), enhanced the apoptosis (), and repressed the activation of PI3K and AKT (, ). The effect of the SPAG1 siRNA on apoptosis of ST cells was consistent with the effect caused by the miR-638 mimics, which indicated that miR-638 promoted immature Sertoli cell apoptosis through its target gene SPAG1.
Figure 5. miR-638 promotes immature Sertoli cells apoptosis through SPAG1. ST cells were transfected with SPAG1 siRNA or siRNA NC (A) Content of ATP was measured by ELISA assay. Relative ATP level of siRNA NC group was normalized with the SPAG1 siRNA group. (B) Cell apoptosis was measured 48 h after transfection using Annexin-V/PI staining, followed by flow cytometer analysis. UR: late apoptosis, LR: early apoptosis. (C) p-PI3K (phospho Tyr458) protein levels were detected using Western blot assay. (D) p-AKT (phospho Ser473) protein levels were detected using Western blot assay. Data are presented as the mean ± S. D. (three independent replicates per group). * P < 0.05, ** P < 0.01.
SPAG1 is required for spermatogonia proliferation
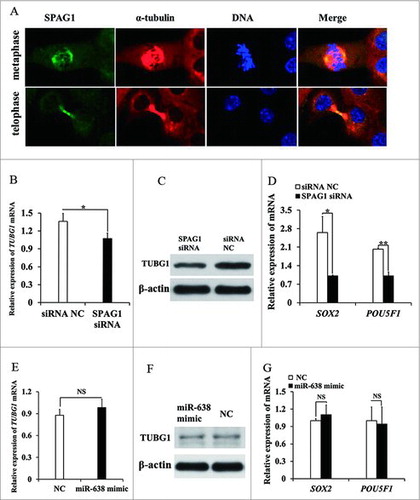
To further explore the possible roles of SPAG1 in spermatogenesis, we utilized immuno-staining to examine the localization of SPAG1 in ST cells. We found that when ST cells entered into mitosis, microtubules were organized to form bipolar spindles, meanwhile SPAG1 signal was assembled into astral structures in metaphase, spindle midzone in telophase but not midbody, as evidenced by co-localization with α-tubulin at these mitotic apparatuses (). In SPAG1 siRNA transfected ST cells, TUBG1 expression was significantly decreased at protein and mRNA expression level (, ). However, TUBG1 mRNA and protein level remained unchanged between miR-638 mimic groups and the control groups (, ). Next, we examined the expression of marker genes including SOX2 and POU5F1 for spermatogonia proliferation. Our results showed that the mRNA levels of SOX2 and POU5F1 were significantly decreased in SPAG1 siRNA-transfected ST cells compared to the controls (). However, the levels of SOX2 and POU5F1 mRNA were similar in both miR-638-transfected ST cells and the controls (). miR-638 had no effect on spermatogonia proliferation, possibly because miR-638 regulated SPAG1 only in a certain extent, but not enough to deplete SPAG1's biological function on spermatogonia proliferation. Overall, these data suggested that SPAG1 was required for spermatogonia proliferation.
Figure 6. SPAG1 is required for spindle morphogenesis. (A) Cellular localization of SPAG1 was detected by immunofluorescent analysis. ST cells at indicated stages were immunostained for SPAG1 (green), microtubule (red; α-tubulin) and DNA (blue). (B) ST cells were treated with nocodazole for 9 h, following transfected with SPAG1 siRNA or siRNA NC. Total cellular lysates were prepared for Q-PCR of TUBG1 gene. (C) Total cellular lysates were extracted from ST cells transfected with SPAG1 siRNA or siRNA NC, and then prepared for Western blot of TUBG1. (D) Total cellular lysates were extracted from ST cells transfected with SPAG1 siRNA or siRNA NC, and then prepared for Q-PCR of SOX2 and POU5F1 gene. (E) ST cells transfected with miR-638 mimics or NC were prepared for Q-PCR of TUBG1 gene. (F) ST cells transfected with miR-638 mimics or NC were prepared for Western blot of TUBG1. (G) Total cellular lysates were extracted from ST cells transfected with miR-638 mimics or NC, and then prepared for Q-PCR of SOX2 and POU5F1 gene. Data are presented as the mean ± S. D. (three independent replicates per group). * P < 0.05, N.S. = non-significant.
Discussion
Spermatogenesis is the process in which spermatozoa are produced from spermatogonial stem cells by way of mitosis and meiosis.Citation25 Sertoli cells are the somatic cells of the testes that are necessary for spermatogenesis. Sertoli cells facilitate the successful progression of germ cells to spermatozoa by providing critical factors and by controlling the environment within the seminiferous tubules.Citation16 Immature Sertoli cells have proliferation capacity while mature Sertoli cells don't have, so the number of mature Sertoli cells depends on immature Sertoli cells, thus immature Sertoli cells are important for spermatogenesis.Citation26
Several studies have reported that miRNAs are involved in spermatogenesis. For example, miR-210 expression was up-regulated in the testes of sterile men with non-obstructive azoospermia (NOA), and miR-210 regulated spermatogenesis through targeting insulin-like growth factor 2 (IGF2) in male infertility.Citation27 The targeted disruption of miR-17-92 in adult mice testes resulted in severe testicular atrophy, empty seminiferous tubules, and depressed sperm production.Citation28 miR-34b/c and miR-449 were specifically expressed in post-mitotic spermatocyte and round spermatid in mice testes, whose deficiency damaged meiosis and spermatozoa maturation and resulted in oligoasthenoteratozoospermia and infertility.Citation29
Previous studies focused on miR-638's role in cell proliferation, and tumorigenesis,Citation30,31 however, the function of miR-638 on spermatogenesis is largely unknown. In this study, we identified that miR-638 directly targeted SPAG1 gene, a gene playing important roles in spermatogenesis.Citation13 miR-638 inhibited ST cell growth and promoted cell apoptosis, indicating that miR-638 regulates pig spermatogenesis possibly via SPAG1 gene.
PI3K/AKT activation was required for c-MYC stabilization which is essential for cell cycle progression.Citation24 Our results showed that miR-638 inhibited ST cell proliferation and significantly decreased the expression of p-PI3K, p-AKT, c-MYC, CCND1 and CDK4 (, ; , ). As illustrated by our findings, miR-638 inactivated the PI3K/ AKT pathway and its downstream c-MYC- stimulated cell cycle pathway, thereby inhibited ST cell growth. Interestingly, PI3K/ AKT pathway and its downstream cell cycle regulators including c-MYC were dramatically suppressed when SPAG1 was depleted. These results demonstrated that miR-638 inactivated PI3K/AKT signaling pathway through direct repression of its target gene SPAG1.
Apoptosis is a process of programmed cell death and occurs via the death receptor (extrinsic) pathway or the mitochondrial (intrinsic) pathway.Citation32,33 The mitochondria- facilitated intrinsic pathway is started by the initial release of small amounts of CytC from mitochondria, followed by the loss of mitochondrial membrane potential (DYm), ATP depletion and caspase activation.Citation22,34,35 In the cytoplasm, CytC combines with Apaf-1 which recruits caspase 9 to form the apoptosome, and activates caspase 3 subsequently.Citation36,37 In our present study, ATP level was significantly suppressed when miR-638 mimics were transfected into ST cells. This effect was significantly blocked by miR-638 inhibitors. Similarly, SPAG1 siRNA also abrogated the ATP production. These results indicated that miR-638 interfered with mitochondrial changes that leaded to mitochondrial dysfunction and apoptosis.
Previous studied have showed that PI3K/AKT pathway as a critical mediator of the protection from apoptosis.Citation38 PI3K/AKT pathway is required for apoptosis and activation of PI3K/AKT protected B-cell lymphoma cell line from anti-IgM-induced apoptosis.Citation22 AKT is an important regulator of cellular survival which suppresses the activities and/or expression of various pro-apoptotic proteins.Citation39,40 Inhibition of the PI3K/AKT pathway resulted in the cleavage of caspase 3 in chicken embryo fibroblast cells at an early phase of Newcastle disease virus infection.Citation41 In this study, we found that miR-638 prevented activation of PI3K and AKT (, ). Therefore, we speculated that miR-638 promoted ST cells apoptosis through suppressing activation of PI3K/AKT pathway, and by stimulating mitochondrial dysregulation and subsequent caspase activation.
Sertoli cells, the somatic cells of testis, are the main structural component of the seminiferous tubules. Sertoli cells play vital roles in modulating spermatogenesis, because they can provide structural supports to the developing germ cells, stimulate germ cell movement and secrete growth factor to nourish germ cells.Citation1,26 Each Sertoli cell supports a limited number of germ cells, and Sertoli cell number is the main contributory factor responsible for sperm production and the adulthood testis size.Citation42,43 During the pre-pubertal period of development such as at 60 days of age, germ cells multiply and differentiate in a synchronous fashion in testis; but at 180 days of age, spermatozoa are produced asynchronously and the testis contains all kinds of germ cells.Citation44 Therefore, spermatocytes appeared in 60-d testes, and 180-d pig testes have various types of sperm cells.Citation12,45 Our previous study showed miR-638 was up-regulated in 180-d (mature) testes compared to 60-d (immature) testes of Large White boars.Citation12 Based on miR-638 promoting ST cells apoptosis and the important roles of ST cells on spermatogonia, we speculated that miR-638 might contribute to elimination of damaged spermatogonia. Our results showed that SPAG1 depletion suppressed levels of SOX2 and POU5F1 mRNA while miR-638 had no such effects on levels of SOX2 and POU5F1 mRNA, probably because miR-638 was modulated by several target genes and resulted in a cumulative effect.
In summary, miR-638 inhibits immature Sertoli cell growth by indirectly inactivating PI3K/AKT pathway via SPAG1 gene. We then speculate that miR-638 functions in pig spermatogenesis by determining the fate of immature Sertoli cells.
Materials and methods
Cell culture and transfection: The swine testicular (ST) cell line (ATCC, https://www.atcc.org/Products/All/CRL-1746) was isolated from swine fetal testes of 80 to 90 day pigs and was identified as a collection of immature porcine Sertoli cells in our previous report. ST cells were cultured in high-glucose Dulbecco's modified Eagle's medium (HyClone) with 10% (v/v) fetal bovine serum (Gibco, https://www.thermofisher.com/order/catalog/product/26140079?ICID=search-product) at 5% CO2 and 37°C. The cells were transfected with the miR-638 mimics, negative control (NC) (GenePharma), miR-638 inhibitors and inhibitor NC (GenePharma), SPAG1 siRNA or siRNA NC (Ribo) (Table S1) using Lipofectamine 2000 transfection reagent or Lipogectamine RNAiMAX (Invitrogen, https://www.thermofisher.com/order/catalog/product/11668019). Opti-MEM I Reduced Serum Medium (Gibco, https://www.thermofisher.com/cn/zh/home/life-science/cell-culture/mammalian-cell-culture/classical-media/opti-mem.html?icid=fr-optimem-main) was used to dilute Lipofectamine 2000 transfection reagent (Life Technologies) and nucleic acids.
Dual-luciferase reporter assays: A fragment from the 3′-UTR of SPAG1 mRNA harboring putative miR-638 binding sites was amplified by reverse transcription- polymerase chain reaction (RT-PCR) with the primer pair SPAG1-3′UTR-PF and SPAG1-3′UTR-PR (Table S2) using total RNA extracted from untreated ST cells with the HP Total RNA Kit (Omega Bio-tek, http://www.omegabiotek.com.cn/template/productShow.aspx?i=100000091137943&m=129002) and sub-cloned into pmirGLO dual-luciferase miRNA target expression vector (Promega, https://cn.promega.com/products/reporter-assays-and-transfection/reporter-vectors-and-cell-lines/pmirglo-dual-luciferase-mirna-target-expression-vector/). ST cells were seeded in a 24-well plate at a density of 1.5 × 105/ml with Dulbecco minimum essential medium (DMEM) supplied with 10% fetal bovine serum (FBS) medium (Gibco, https://www.thermofisher.com/order/catalog/product/11965092?ICID=search-product). Twenty four hours later, ST cells were co-transfected with the 3′UTR luciferase reporter vectors and the miR-638 mimics or miR-638 inhibitors. After 24 h transfection, cells were collected for luciferase activity assays with the Dual-Glo Luciferase Assay System (Promega, https://cn.promega.com/products/reporter-assays-and-transfection/reporter-assays/dual_glo-luciferase-assay-system/).
Quantitative (Q)-PCR: Total RNA was extracted from ST cells with the HP Total RNA Kit (Omega Bio-tek). Cellular RNA was extracted 48 h after transfection. qPCR was performed on the Bio-Rad CFX96 system (Bio-Rad) using the iTaq Universal SYBR Green Supermix (Bio-Rad). Primers used in the qPCR (Table S2) were designed using primer5.0 software. All PCRs were performed in triplicate. Gene expression levels were normalized to the expression of β-actin using Gene Expression Macro software (Bio-Rad) by using 2−Δ Δ Ct value.
Western blotting: Cells were scraped and lysed in 200 μl ice-cold RIPA lysis buffer (Beyotime). After 5 min on ice, lysates were clarified (14 000 × g, 4°C, 5 min). Five μg proteins were boiled in 5 × SDS buffer for 5 min, separated by SDS-PAGE and transferred to PVDF membranes (Millipore). Then the membranes were blocked with skim milk and then incubated overnight at 4°C with primary antibodies specific for SPAG1 (Santa Cruz, ABclonal), p-PI3K (Cell Signalling Technology), p-AKT (Affinity), c-MYC (ABclonal), CDK4 (ABclonal), CCNE1 (Santa Cruz), CCND1 (Abcam), TUBG1 (Santa Cruz). Beta- actin (Wuhan Guge, http://www.servicebio.cn/ncykt/201703071004.html) was served as loading control. An Image Quant LAS4000 mini (GE Healthcare Life Sciences) was used to detect protein expression.
Cell proliferation analysis: ST cells were seeded on a 16-well (E-) Plate with 5000 cells per well and allowed to grow for 12–24 h. Cell proliferation were monitored using an xCELLigence RTCA DP instrument (Roche Applied Science) and MTT assay.
Cell apoptosis analysis: Cell apoptosis was analyzed using the Annexin V-FITC Apoptosis Detection Kit (KeyGEN BioTECH) according to manufacturer's instructions. Briefly, the ST cells were harvested through trypsinization (without EDTA) and washed twice with phosphate-buffered saline (HyClone). After centrifugation, the sediment was re-suspended in 500 μl binding buffer and incubated with FITC-conjugated Annexin V and PI. The samples then were analyzed by FACSCalibur Flow Cytometry (Becton Dickinson).
Cell cycle analysis: Cell cycle was analyzed with the Cell Cycle Detection Kit (KeyGEN BioTECH) according to the manufacturer's protocol. Briefly, after 48 h transfection, the ST cells were fixed by 70% (v/v) ethanol and incubated overnight at −20°C. Before analysis, cells were incubated with 50 mg/ml propidium iodide (PI) for 30 min at 4°C. The cell cycle was analyzed using FACSCalibur Flow Cytometry (Becton Dickinson) and the ModFit software (Verity Software House). Twenty thousand events were analyzed per sample.
ATP assessment: ST cells were cultured in a six-well plate with 2 ml medium. ST cells were treated with miR-638 mimics or NC and miR-638 inhibitors or inhibitor NC and SPAG1 siRNA or siRNA NC. After 48 h, ATP concentration was evaluated by using the enzyme-linked immunosorbent assay (ELISA) kit (Enzo Life Sciences). Relative ATP level of one group was normalized with the other group.
Immunofluorescence and confocal microscopy: ST cells were plated on chamber slides at 60–70% confluency, washed with PBS, and fixed with 4% paraformaldehyde- PBS for 20 min, then permeabilized in PBS containing 0.5% Triton X-100 for 20 min. ST cells were then incubated with proper primary antibodies and the corresponding secondary antibodies subsequently. For double-staining, after secondary antibody incubation, the ST cells were processed with the second primary antibodies following the incubation with the corresponding secondary antibodies. DNA was counterstained with DAPI (1 μg/ml) for 10 min at room temperature. Finally, ST cells were mounted on glass slides with DABCO and examined with confocal laser scanning microscope (Zeiss LSM 510 META) equipped with a Plan-Apochromat 63 × /1.4 oil DIC objective. Confocal images were further processed with Zeiss LSM Image Browser and Adobe Photoshop (Adobe Systems Inc.).
For immunolabelling, anti-SPAG1 antibody (Santa Cruz) at 1:50 dilution; anti-α-tubulin (Affinity) at 1:1000 dilution were used as the primary antibodies. FITC- or Cy3-labelled antibodies were used as the secondary antibodies at 1:200 dilution.
Bioinformatics method and statistical analysis: The miR-638 targets were predicted by RNAhybrid (https://bibiserv.cebitec.uni-bielefeld.de/rnahybrid?id=rnahybrid_view_submission). Statistical analyses based on two-tailed Student's t-tests were used for P-value calculations. Significance was determined at a 95% confidence interval. All data are expressed as the mean ± standard deviation (S.D.).
Conflicts of interest
The authors declare no conflict of interest.
1380130_Supplemental_Material.docx
Download MS Word (24.6 KB)Additional information
Funding
References
- Hai Y, Hou J, Liu Y, Liu Y, Yang H, Li Z, He Z. The roles and regulation of Sertoli cells in fate determinations of spermatogonial stem cells and spermatogenesis. Semin Cell Dev Biol. 2014;29:66-75. https://doi.org/10.1016/j.semcdb.2014.04.007. PMID: 24718316
- Feng HL. Molecular biology of male infertility. Arch Androl. 2003;49:19-27. PMID: 12647775
- Lian J, Zhang X, Tian H, Liang N, Wang Y, Liang C, Li X, Sun F. Altered microRNA expression in patients with non-obstructive azoospermia. Reprod Biol Endocrinol. 2009;7:13; https://doi.org/10.1186/1477-7827-7-13. PMID: 19210773
- Huang H, Tian H, Duan Z, Cao Y, Zhang XS, Sun F. microRNA-383 impairs phosphorylation of H2AX by targeting PNUTS and inducing cell cycle arrest in testicular embryonal carcinoma cells. Cell Signal. 2014;26:903-11. https://doi.org/10.1016/j.cellsig.2014.01.016. PMID: 24462707
- Lian J, Tian H, Liu L, Zhang XS, Li WQ, Deng YM, Yao GD, Yin MM, Sun F. Downregulation of microRNA-383 is associated with male infertility and promotes testicular embryonal carcinoma cell proliferation by targeting IRF1. Cell Death Dis. 2010;1:e94; https://doi.org/10.1038/cddis.2010.70. PMID: 21368870
- Li C, Chen S, Li H, Chen L, Zhao Y, Jiang Y, Liu Z, Liu Y, Gao S, Wang F, et al. MicroRNA-16 modulates melatonin-induced cell growth in the mouse-derived spermatogonia cell line GC-1 spg cells by targeting Ccnd1. Biol Reprod. 2016;95:57; https://doi.org/10.1095/biolreprod.115.138313. PMID: 27465135
- Liu Y, Liu WB, Liu KJ, Ao L, Cao J, Zhong JL, Liu JY. Overexpression of miR-26b-5p regulates the cell cycle by targeting CCND2 in GC-2 cells under exposure to extremely low frequency electromagnetic fields. Cell Cycle. 2016;15:357-67. https://doi.org/10.1080/15384101.2015.1120924. PMID:26637059
- Yao C, Sun M, Yuan Q, Niu M, Chen Z, Hou J, Wang H, Wen L, Liu Y, Li Z, et al. MiRNA-133b promotes the proliferation of human Sertoli cells through targeting GLI3. Oncotarget. 2016;7:2201-19. PMID: 26755652; https://doi.org/10.18632/oncotarget.6876
- Sand M, Skrygan M, Sand D, Georgas D, Hahn SA, Gambichler T, Altmeyer P, Bechara FG. Expression of microRNAs in basal cell carcinoma. Br J Dermatol. 2012;167:847-55. https://doi.org/10.1111/j.1365-2133.2012.11022.x. PMID: 22540308
- Wang JL, Hu Y, Kong X, Wang ZH, Chen HY, Xu J, Fang JY. Candidate microRNA biomarkers in human gastric cancer: a systematic review and validation study. PLoS One. 2013;8:e73683; https://doi.org/10.1371/journal.pone.0073683. PMID: 24040025
- Zhang J, Fei B, Wang Q, Song M, Yin Y, Zhang B, Ni S, Guo W, Bian Z, Quan C, et al. MicroRNA-638 inhibits cell proliferation, invasion and regulates cell cycle by targeting tetraspanin 1 in human colorectal carcinoma. Oncotarget. 2014;5:12083-96. https://doi.org/10.18632/oncotarget.2499. PMID: 25301729
- Luo L, Ye L, Liu G, Shao G, Zheng R, Ren Z, Zuo B, Xu D, Lei M, Jiang S, et al. Microarray-based approach identifies differentially expressed microRNAs in porcine sexually immature and mature testes. PLoS One. 2010;5:e11744; https://doi.org/10.1371/journal.pone.0011744. PMID: 20805883
- Liu N, Qiao Y, Cai C, Lin W, Zhang J, Miao S, Zong S, Koide SS, Wang L. A sperm component, HSD-3.8 (SPAG1), interacts with G-protein beta 1 subunit and activates extracellular signal-regulated kinases (ERK). Front Biosci. 2006;11:1679-89. PMID: 16368546
- Knowles MR, Ostrowski LE, Loges NT, Hurd T, Leigh MW, Huang L, Wolf WE, Carson JL, Hazucha MJ, Yin W, et al. Mutations in SPAG1 cause primary ciliary dyskinesia associated with defective outer and inner dynein arms. Am J Hum Genet. 2013;93:711-20. https://doi.org/10.1016/j.ajhg.2013.07.025. PMID: 24055112
- Ma C, Song H, Guan K, Zhou J, Xia X, Li F. Characterization of swine testicular cell line as immature porcine Sertoli cell line. In Vitro Cell Dev Biol Anim. 2016;52:427-33; https://doi.org/10.1007/s11626-015-9994-8.PMID: 26744029
- Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol. 1998;9:411-6. https://doi.org/10.1006/scdb.1998.0203.PMID: 9813187
- Huang C, Wu D, Khan FA, Jiao X, Guan K, Huo L. The GTPase SPAG-1 orchestrates meiotic program by dictating meiotic resumption and cytoskeleton architecture in mouse oocytes. Mol Biol Cell. 2016;27:1776-85. https://doi.org/10.1091/mbc.E16-02-0132. PMID: 27053660
- Zajac-Kaye M. Myc oncogene: a key component in cell cycle regulation and its implication for lung cancer. Lung Cancer. 2001;34 Suppl 2:S43-6. PMID: 11720740
- Alvarez B, Martínez-A C, Burgering BM, Carrera AC. Forkhead transcription factors contribute to execution of the mitotic programme in mammals. Nature. 2001;413:744-7. https://doi.org/10.1038/35099574. PMID: 11607034
- García Z, Kumar A, Marqués M, Cortés I, Carrera AC. Phosphoinositide 3-kinase controls early and late events in mammalian cell division. EMBO J. 2006;25:655-61. https://doi.org/10.1038/sj.emboj.7600967. PMID: 16437156
- Jones SM, Kazlauskas A. Growth-factor-dependent mitogenesis requires two distinct phases of signalling. Nat Cell Biol. 2001;3:165-72. https://doi.org/10.1038/35055073. PMID: 11175749
- Carey GB, Semenova E, Qi X, Keegan AD. IL-4 protects the B-cell lymphoma cell line CH31 from anti-IgM-induced growth arrest and apoptosis: contribution of the PI-3 kinase/AKT pathway. Cell Res. 2007;17:942-55; https://doi.org/10.1038/sj.cr.2007.90. PMID: 17968425
- Kumar A, Marqués M, Carrera AC. Phosphoinositide 3-kinase activation in late G1 is required for c-Myc stabilization and S phase entry. Mol Cell Biol. 2006;26:9116-25. https://doi.org/10.1128/MCB.00783-06. PMID: 17015466
- Zhu J, Blenis J, Yuan J. Activation of PI3K/Akt and MAPK pathways regulates Myc-mediated transcription by phosphorylating and promoting the degradation of Mad1. Proc Natl Acad Sci U S A. 2008;105:6584-9. https://doi.org/10.1073/pnas.0802785105. PMID: 18451027
- Güneş S, Kulaç T. The role of epigenetics in spermatogenesis. Turk J Urol. 2013;39:181-7. https://doi.org/10.5152/tud.2013.037. PMID: 26328105
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769-84. PMID: 12773099
- Tang D, Huang Y, Liu W, Zhang X. Up-regulation of microRNA-210 is associated with spermatogenesis by targeting IGF2 in male infertility. Med Sci Monit. 2016;22:2905-10. PMID: 27535712
- Xie R, Lin X, Du T, Xu K, Shen H, Wei F, Hao W, Lin T, Lin X, Qin Y, et al. Targeted disruption of miR-17-92 impairs mouse spermatogenesis by activating mTOR signaling pathway. Medicine (Baltimore). 2016;95:e2713; PMID: 26886608; https://doi.org/10.1097/MD.0000000000002713
- Comazzetto S, Di Giacomo M, Rasmussen KD, Much C, Azzi C, Perlas E, Morgan M, O'Carroll D. Oligoasthenoteratozoospermia and infertility in mice deficient for miR-34b/c and miR-449 loci. PLoS Genet. 2014;10:e1004597; https://doi.org/10.1371/journal.pgen.1004597. PMID: 25329700
- He M, Lin Y, Tang Y, Liu Y, Zhou W, Li C, Sun G, Guo M. miR-638 suppresses DNA damage repair by targeting SMC1A expression in terminally differentiated cells. Aging (Albany NY). 2016;8:1442-56. https://doi.org/10.18632/aging.100998. PMID: 27405111
- Li P, Liu Y, Yi B, Wang G, You X, Zhao X, Summer R, Qin Y, Sun J. MicroRNA-638 is highly expressed in human vascular smooth muscle cells and inhibits PDGF-BB-induced cell proliferation and migration through targeting orphan nuclear receptor NOR1. Cardiovasc Res. 2013;99:185-93. https://doi.org/10.1093/cvr/cvt082. PMID: 23554459
- Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798-811. https://doi.org/10.1038/sj.onc.1209608. PMID: 16892092
- Nagata S. Fas ligand-induced apoptosis. Annu Rev Genet. 1999;33:29-55. https://doi.org/10.1146/annurev.genet.33.1.29. PMID: 10690403
- Bossy-Wetzel E, Newmeyer DD, Green DR. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 1998;17:37-49. https://doi.org/10.1093/emboj/17.1.37. PMID: 9427739
- Valks DM, Cook SA, Pham FH, Morrison PR, Clerk A, Sugden PH. Phenylephrine promotes phosphorylation of Bad in cardiac myocytes through the extracellular signal-regulated kinases 1/2 and protein kinase A. J Mol Cell Cardiol. 2002;34:749-63. PMID: 12099715
- Adams JM, Cory S. Apoptosomes: engines for caspase activation. Curr Opin Cell Biol. 2002;14:715-20. PMID: 12473344
- Heck DE, Kagan VE, Shvedova AA, Laskin JD. An epigrammatic (abridged) recounting of the myriad tales of astonishing deeds and dire consequences pertaining to nitric oxide and reactive oxygen species in mitochondria with an ancillary missive concerning the origins of apoptosis. Toxicology. 2005;208:259-71. https://doi.org/10.1016/j.tox.2004.11.027. PMID: 15691590
- Chu P, Han G, Ahsan A, Sun Z, Liu S, Zhang Z, Sun B, Song Y, Lin Y, Peng J, et al. Phosphocreatine protects endothelial cells from Methylglyoxal induced oxidative stress and apoptosis via the regulation of PI3K/Akt/eNOS and NF-κB pathway. Vascul Pharmacol. 2017;91:26-35. https://doi.org/10.1016/j.vph.2016.08.012. PMID: 27590258
- Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983-98. https://doi.org/10.1038/sj.onc.1207115. PMID:14663477
- Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59-71. PMID: 15784165
- Kang Y, Yuan R, Zhao X, Xiang B, Gao S, Gao P, Dai X, Feng M, Li Y, Xie P, et al. Transient activation of the PI3K/Akt pathway promotes Newcastle disease virus replication and enhances anti-apoptotic signaling responses. Oncotarget. 2017;8:23551-23563. https://doi.org/10.18632/oncotarget.15796. PMID: 28423596
- Johnson L, Zane RS, Petty CS, Neaves WB. Quantification of the human Sertoli cell population: its distribution, relation to germ cell numbers, and age-related decline. Biol Reprod. 1984;31:785-95. PMID: 6509142
- Orth JM, Gunsalus GL, Lamperti AA. Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology. 1988;122:787-94. https://doi.org/10.1210/endo-122-3-787. PMID: 3125042
- Song H, Zhu L, Li Y, Ma C, Guan K, Xia X, Li F. Exploiting RNA-sequencing data from the porcine testes to identify the key genes involved in spermatogenesis in Large White pigs. Gene. 2015;573:303-9; https://doi.org/10.1016/j.gene.2015.07.057.PMID: 26192463
- Egbunike GN. Development of puberty in Large White boars in a humid tropical environment. Acta Anat (Basel). 1979;104:400-5. PMID: 525235
