BAX is a well-known pro-apoptotic protein that forms a pore to release cytochrome c from mitochondria in stressed cells undergoing apoptosis. Now Brayer et al find a new function for BAX in unstressed cells, to regulate cell cycle progression by functioning within the nucleus.Citation1
Stress responses either rescue vital functions or terminate cells when the damage is too severe. Apoptosis is perhaps the best characterized terminal stress response, a form of cell suicide involving cell and nuclear fragmentation that is executed by caspase proteases. In most metazoan organisms, apoptosis regulation also involves mitochondria. Pro-apoptotic signaling releases cytochrome c from the mitochondrial intermembrane space to the cytosol, where it participates in a complex that initiates caspase activity. BAX is one of several homologous proteins that undergo homo-oligomerization to form the pores in the outer mitochondrial membrane through which cytochrome c is released.Citation2
While pro-apoptotic signals recruit BAX to mitochondria in stressed cells, in unstressed cells, BAX is held in an inactive conformation that inhibits insertion into the mitochondrial membrane. But while BAX is inactive for cell death, it turns out it may be busy doing other things. Mailleux and colleagues noticed that a portion of BAX protein is present in the nucleus in unstressed cells. Whereas the knockdown or knockout of BAX reduced cell proliferation, the expression of a nuclear-targeted BAX, engineered with a nuclear localization sequence at the N-terminus (NLS-BAX), but not a nuclear-excluded BAX with a nuclear export sequence (NES-BAX), enhanced proliferation, suggesting that BAX could function within the nucleus to control the cell cycle in unstressed conditions.
The authors further found that when BAX was in the nucleus, it was bound to chromatin and positioned at key sites to regulate CDKN1A, the gene encoding for the cyclin-dependent kinase inhibitor protein p21. Chromatin immunoprecipiation studies placed BAX at the CDKN1A transcriptional start site and at one of two responsive elements for the p53 tumor suppressor protein, a known inducer of p21 expression. Lowered expression or knockout of BAX increased p21 expression, while NLS-BAX, but not NES-BAX, reduced p21 levels, demonstrating control over expression of this important cell cycle regulatory protein by BAX in unstressed conditions, in a manner distinct from mitochondrial functions.
Does nuclear BAX have physiologic functions? The authors went on to show that BAX was nuclear localized during branching morphogenesis in human fetal lung tissue, and that nuclear BAX controlled the expression of additional genes in primary human lung fibroblasts, including alpha smooth muscle actin and collagen 1, which are typically upregulated during myofibroblastic differentiation. Tissues from patients suffering from idiopathic pulmonary fibrosis (IPF) also showed increased nuclear BAX staining, as did cells from lung adenocarcinoma specimens, suggesting that nuclear functions of BAX could contribute significantly to normal and pathophysiology in a manner involving nuclear function that is distinct from apoptosis regulation.
These findings build on previous work from other groups who also implicated BAX in non-death functions. BAX has been observed in the nucleus in previous studies,Citation3,4 and was implicated in controlling cell cycle progression,Citation5 but the function of nuclear BAX had not been examined directly. Other non-death functions of BAX are also proposed. At mitochondria, for example, BAX is reported to contribute to maintaining respiration in unstressed conditions.Citation6 These studies and the current findings suggest that BAX contributes significantly to unstressed physiology, to maintain respiratory capacity or proliferative potential, at least in part by acting from within the nucleus in close proximity to chromatin.
The findings by Mailleux and colleagues raise numerous interesting questions to be further pursued. For example, how is BAX function controlled in the nucleus? What is the molecular basis of the interaction with BAX and chromatin? Does BAX utilize binding partners? Furthermore, anti-apoptotic Bcl-2 family proteins have been implicated in controlling proliferation,Citation5 and binding between Bcl-2 and BAX has been reported in the nucleus.Citation4 Although the authors in the current study did not identify Bcl-2, or Bcl-xL, in nuclear fractions, it may still be important to consider potential crosstalk with BAX nuclear functions.
Potential connections with the p53 pathway may also be interesting to explore. p53 signaling dynamics are known to be complex and involve oscillatory patterns.Citation7 The transcriptional changes that result from p53 induction either lead to growth arrest, to allow for the restoration of vital cell functions when damage can be repaired, or induce apoptosis when the damage is too severe. As BAX is a p53-induced gene, and Mailleux and colleagues find BAX residing at a p53-responsive element near CDKN1A, BAX could regulate negative feedback on p53-mediated transactivation of p21. This suggests that BAX could contribute to coordinating cell fate decisions, but whether nuclear BAX can compete with p53 will require further studies. These exciting findings by Mailleux and colleagues suggest that BAX could participate in the regulation of cell fate decisions in response to stress, and that in unstressed conditions, BAX supports proliferation by acting within the nucleus.Citation1
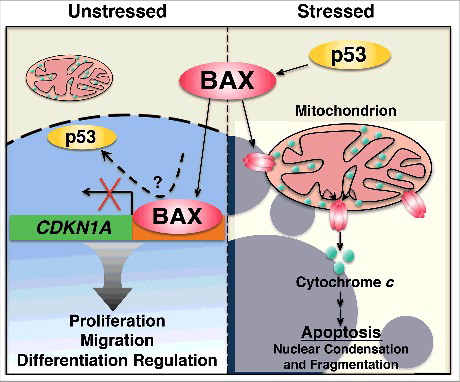
Figure 1. BAX functions in the nucleus. In unstressed conditions BAX functions in the nucleus to regulate gene expression programs linked to proliferation, migration and differentiation. BAX interacts with the CDKN1A promoter where it could compete with p53. When cells are stressed, p53 activates BAX, which forms pores in the mitochondrial outer membrane to release cytochrome c and initiate apoptosis.
References
- Brayer S JA, Jaillet M, Gregianin E, Mahmoudi S, Somme JM, Fabre A, Mordant P, Cazes A, Crestani B, Mailleux A. The pro-apoptotic BAX protein influences cell growth and differentiation from the nucleus in healthy interphasic cells. Cell Cycle. 2017;16(21):2108–2118. doi:10.1080/15384101.2017.1371882. PMID:28933587
- Jurgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci U S A. 1998;95:4997–5002. doi:10.1073/pnas.95.9.4997. PMID:9560217
- Salah-eldin A, Inoue S, Tsuda M, Matsuura A. Abnormal intracellular localization of Bax with a normal membrane anchor domain in human lung cancer cell lines. Japanese J Cancer Res. 2000;91:1269–77. doi:10.1111/j.1349-7006.2000.tb00914.x.
- Hoetelmans RW. Nuclear partners of Bcl-2: Bax and PML. DNA Cell Biol. 2004;23:351–4. doi:10.1089/104454904323145236. PMID:15231068
- Janumyan Y, Cui Q, Yan L, Sansam CG, Valentin M, Yang E. G0 function of BCL2 and BCL-xL requires BAX, BAK, and p27 phosphorylation by Mirk, revealing a novel role of BAX and BAK in quiescence regulation. J Biol Chem. 2008;283:34108–20. doi:10.1074/jbc.M806294200. PMID:18818203
- Boohaker RJ, Zhang G, Carlson AL, Nemec KN, Khaled AR. BAX supports the mitochondrial network, promoting bioenergetics in nonapoptotic cells. American journal of physiology Cell Physiol. 2011;300:C1466–78. doi:10.1152/ajpcell.00325.2010. PMID:21289292
- Purvis JE, Karhohs KW, Mock C, Batchelor E, Loewer A, Lahav G. p53 dynamics control cell fate. Science. 2012;336:1440–4. doi:10.1126/science.1218351. PMID:22700930
