ABSTRACT
Arsenic trioxide (ATO) has been reported to exert its anti-cancer activities in human cancers. However, the molecular mechanism of ATO-triggered anti-tumor activity has not been fully elucidated. Recently, multiple studies demonstrated that ATO could regulate miRNAs in human cancers. Therefore, in this study, we investigated whether ATO regulated let-7a in breast cancer cells. We found that ATO upregulated let-7a level in breast cancer cells. We also found that up-regulation of let-7a inhibited cell growth and induced apoptosis and retarded cell migration and invasion. We also observed that up-regulation of let-7a enhanced cell growth inhibition and invasion suppression induced by ATO treatment. Our findings suggest that ATO suppressed cell growth, stimulated apoptosis, and retarded cell invasion partly via upregulation of let-7a in breast cancer cells. Our study provides a new anti-tumor mechanism of ATO treatment in breast cancer.
Introduction
Breast cancer is the most commonly diagnosed cancer in women in the United States.Citation1 In 2017, an estimated more than 252, 000 women will be diagnosed with breast cancer, and 40,610 will die due to breast cancer in the US.Citation1 Mammography screening has been applied for detection of breast cancer at early stage. Several treatments including immunotherapy have improved the benefit of breast cancer patients. However, breast cancer is still the second cancer-related death in women, suggesting that it is pivotal to discover new agents for treating breast cancer.
ATO (arsenic trioxide) has been documented to exhibit tumor suppressive function in human cancers including breast cancer.Citation2–4 ATO-induced cell cycle arrest is possibly due to demethylation and alterations in the expression level of the cell cycle-related genes.Citation5 ATO was reported to down-regulate cancer procoagulant activity in breast cancer cells.Citation6 EZH2 (enhancer of zeste 2 polycomb repressive complex 2) mediated ATO-induced apoptosis via regulating the Wnt signaling pathway in acute myeloid leukemia cells.Citation7 ATO promoted paclitaxel cytotoxicity in paclitaxel-resistent breast cancer cells.Citation8 ATO and cotylenin A cooperatively suppressed cell proliferation and cell invasion activity in breast cancer cells.Citation9 ATO overcome rapamycin-induced feedback activation of Akt and Erk (extracellular regulated protein kinases) signaling to enhance the anti-tumor effects in breast cancer.Citation10 ATO induced cell growth arrest via regulation of FOXO3a (forkhead box O3a) and IKKβ (Ikβ kinase β) expression and localization in breast cancer cells.Citation11 ATO induced cell apoptosis via activation of caspase-3 and suppression of HERG (human ether-a-go-go-related gene) channels in breast cancer cells.Citation12 These reports indicated that ATO exerts its functions via regulation of cell signaling pathways.
It has been well known that microRNAs (miRNAs) are critically involved in regulation of cell growth, cell cycle, migration, and invasion in human cancers including breast cancer.Citation13 As non-coding RNAs, miRNAs negatively regulate its targets expression mainly through post-transcription.Citation14 Clearly, miRNAs play oncogenic or tumor suppressive roles because its targets have different biological functions.Citation15 Among these miRNAs, let-7a was well studied in human cancers. Multiple studies have validated that let-7a exhibits anti-tumor activity in various types of human cancers. It has been identified that circulating level of let-7a decreased in breast cancer patients and correlated with nodal status and estrogen receptor status, suggesting that let-7a could be a novel breast cancer biomarker.Citation16 Let-7a decreased cell proliferation, migration, and invasion via inhibition of CCR7 (C-C chemokine receptor type 7) in breast cancer cells.Citation17 Lin28 induced epithelial-to-mesenchymal transition and stemness via down-regulation of let-7a in breast cancer cells.Citation18 Androgen receptor decreased c-Myc and Kras expression by upregulation of let-7a expression in breast cancer.Citation19 Therefore, upregulation of let-7a could be useful for the treatment of human cancers.
ATO has been reported to govern the expression of several miRNAs.Citation20 For instance, ATO attenuated the invasion potential via demethylation-activated miR-491 in human liver cancer cells.Citation21 ATO down-regulated miR-125b expression and subsequently increased its target gene Bak1 expression in glioma cells.Citation22 ATO induced cell apoptosis via down-regulation of miR-376a in retinoblastoma cells.Citation23 Although these reports indicated that ATO could regulate expression of several miRNAs, the detailed mechanism is uncertain. In the current study, we investigated the anti-tumor function of ATO in breast cancer cells. We further explored the role of let-7a in breast cancer cells and determined whether ATO could regulate the expression of let-7a in breast cancer cells. We also determined whether ATO exerted its anti-cancer activity via up-regulation of let-7a in breast cancer. We found that ATO-mediated tumor suppressive function was in part via upregulation of let-7a. Our study suggested that ATO could be a potential agent to upregulation of let-7a in breast cancer.
Results
ATO inhibited cell growth in a dose-dependent manner. To define whether ATO treatment could suppress cell growth, MTT assay was used to measure the cell growth inhibition in MCF-7 and SK-BR-3 cells after different concentrations of ATO treatments. Our MTT results showed that ATO significantly suppressed cell growth in a dose-dependent manner in both breast cancer cells (). Specifically, we found that 8μM ATO treatment caused 40–50% cell growth inhibition in breast cancer cells. Moreover, 10μM ATO treatment led to about 70% cell growth inhibition. Therefore, ATO inhibited breast cancer cell growth.
Figure 1. Effect of ATO on cell growth and apoptosis. (A) MTT assay was used to measure cell proliferation in MCF-7 and SK-BR-3 cells after ATO treatment for 72 hours. *P<0.05, **P<0.01, ***P<0.001, compared with control. (B) Apoptotic cell death was examined using Annexin V-FITC/PI method in breast cancer cells after ATO treatment for 48 hours.
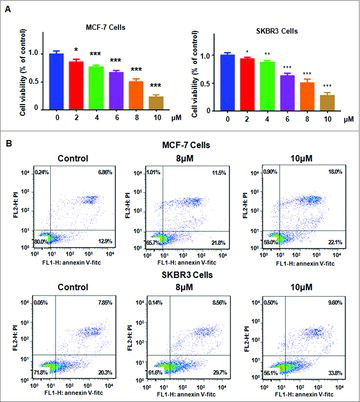
ATO induced cell apoptosis in breast cancer cells. To investigate whether ATO treatment could trigger cell apoptosis, Annexin V-FITC/PI and FACS were conducted in MCF-7 and SK-BR-3 cells after different concentrations of ATO treatment. Our results showed that ATO treatment caused increased the percentage of apoptotic cells in both breast cancer cells (). The percentage of apoptotic cells was increased from 19% in control group to 33% in 8μM ATO-treated group in MCF-7 cells ( top panel). Similar finding was observed in SK-BR-3 cells ( bottom panel). Strikingly, ATO treatment induced cell apoptosis in a dose-dependent manner in breast cancer cells.
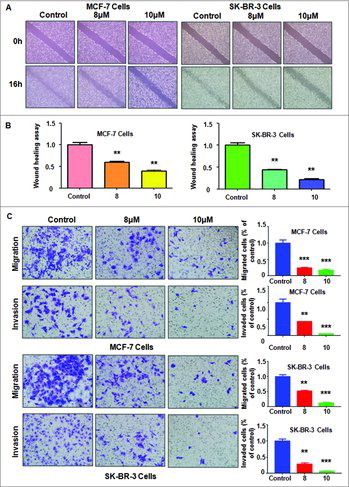
ATO inhibited cell migration and invasion. To dissect whether ATO could govern cell migration in breast cancer cells, a scratch wound-healing assay was used to measure cell migration in MCF-7 and SK-BR-3 cells after different concentrations of ATO treatment. Our wound healing assay results demonstrated that ATO retarded cell migration in a dose-dependent manner in both breast cancer cell lines ( and ). To validate whether ATO inhibited cell invasive activity, Transwell chamber assay were used to detect the cell migration and invasion in breast cancer cells after ATO treatment. We observed that ATO treatment has inhibitory effects on cell migration and invasion in a dose-dependent manner (). Altogether, our finding suggests that ATO retarded cell motility activity.
Figure 2. Effect of ATO on cell migration and invasion. (A) Cell migration was determined using Wound healing assay in breast cancer cells after ATO treatment. (B) Quantitative results are illustrated for panel A.**P<0.01, compared with control. (C) Left panel: Cell invasion was measured using Transwell inserts with Matrigel in MCF-7 (top panel) and SK-BR-3 (bottom panel) breast cancer cells after ATO treatment for 24 hours. Right panel: Quantitative results are shown for left panel.**P<0.01, ***P<0.001, compared with control.
ATO treatment increased let-7a level. It has been known that let-7a plays anti-tumor role in human cancers. Thus, we wondered whether let-7a is involved in ATO-mediated anti-tumor activity in breast cancer cells. To answer this concern, we measured the let-7a level in MCF-7 and SK-BR-3 cells treated with 8μM ATO. We found that ATO treatment significantly increased let-7a level in both breast cancer cells (). Our results clearly showed that ATO could be a potential stimulator of let-7a in breast cancer cells.
Figure 3. Effect of let-7a mimics on cell growth and apoptosis. (A) The expression of let-7a was measured by real-time RT-PCR in MCF-7 and SK-BR-3 cells after 8 μM ATO treatment for 72 hours. ***P<0.001, compared with control. (B) MTT assay was conducted in breast cancer cells after ATO treatment or let-7a mimics or the combination. *P<0.05, compared with control; #P<0.05, compared with ATO alone or let-7a mimics alone. ATO: 8 μM ATO; Mimics: let-7a mimics: Both: 8 μM ATO plus let-7a mimics. (C) Cell apoptosis was detected by Annexin V-FITC/PI method in MCF-7 and SK-BR-3 cells after ATO treatment or let-7a mimics or the combination. ATO: 8 μM ATO; Mimics: let-7a mimics: Both: 8 μM ATO plus let-7a mimics.
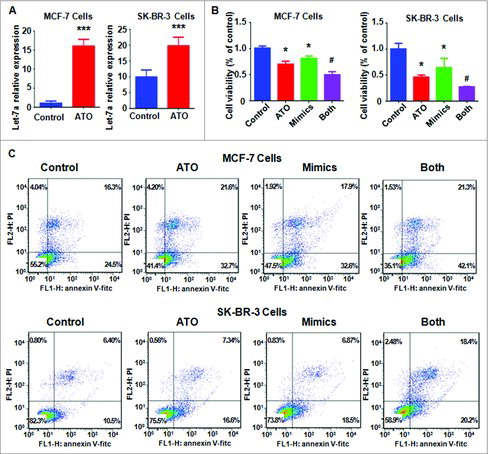
Let-7a mimics inhibited cell growth. To explore the effect of let-7a on cell growth in breast cancer cells, MTT assay was performed to measure cell viability in MCF-7 and SK-BR-3 cells after let-7a mimics transfection for 72 hours. We found that let-7a mimics inhibited cell growth in both breast cancer cells (). Importantly, let-7a mimics enhanced cell growth inhibition induced by ATO treatment (). This finding indicated that up-regulation of let-7a could promote ATO-mediated cell growth inhibition in breast cancer cells.
Let-7a mimics induced apoptosis. We detected the cell apoptosis in MCF-7 and SK-BR-3 cells after let-7a mimics transfection. We found that up-regulation of let-7a led to increased percentage of apoptotic cells in both breast cancer cells (). Furthermore, let-7a mimics plus with ATO treatment resulted in higher percentage of apoptotic cells compared with let-7a mimics alone or ATO alone (). This result suggested that up-regulation of let-7a enhanced ATO-induced apoptosis in breast cancer cells.
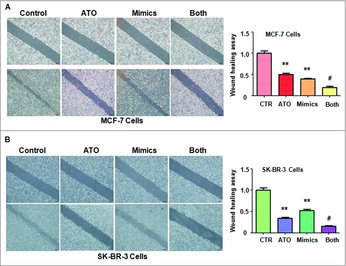
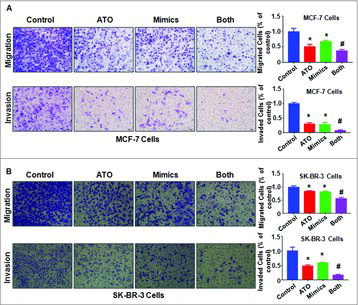
Let-7a mimics retarded cell motility. To investigate the function of let-7a in regulation of cell migration, a scratch wound-healing assay was performed in MCF-7 and SK-BR-3 cells after let-7a mimics transfection. Our results showed that let-7a mimics suppressed cell motility in both breast cancer cell lines ( and ). Moreover, let-7a mimics in combination with ATO treatment led to a lower level of migration compared with let-7a alone or ATO treatment alone ( and ).
Figure 4. Effect of let-7a on cell motility. (A) Left panel: Cell migration was measured using Wound-healing assay in MCF-7 breast cancer cells after ATO treatment or let-7a mimics or the combination. Right panel: Quantitative results are illustrated for left panel. **P<0.01, compared with control; #P<0.05, compared with ATO alone or let-7a mimics alone. ATO: 8 μM ATO; Mimics: let-7a mimics: Both: 8 μM ATO plus let-7a mimics. (B) Left panel: Wound-healing assay was performed to measure the cell migration in SK-BR-3 cells after ATO treatment or let-7a mimics or the combination. Right panel: Quantitative results are illustrated for left panel. **P<0.01, compared with control; #P<0.05, compared with ATO alone or let-7a mimics alone.
Let-7a mimics inhibited cell migration and invasion. We used Matrigel invasion chamber assay to determine whether let-7a retarded cell migration and invasion in MCF-7 and SK-BR-3 cells after let-7a mimics transfection. We found that let-7a mimics suppressed cell migration and invasion in MCF-7 and SK-BR-3 cells ( and ). Notably, let-7a mimics plus with ATO treatment led to less numbers of migrated and invaded cells compared with ATO treatment alone or let-7a mimics alone ( and ). This result suggested that let-7a mimics enhanced ATO-induced inhibition of cell invasion in breast cancer cells.
Figure 5. Effect of let-7a on cell migration and invasion. (A-B) Left panel: The cell migration and invasion was measured using Transwell inserts with or without Matrigel in MCF-7 (A) and SK-BR-3 (B) breast cancer cells after ATO treatment or let-7a mimics or the combination for 24 hours. ATO: 8 μM ATO; Mimics: let-7a mimics: Both: 8 μM ATO plus let-7a mimics. Right panel: Quantitative results are illustrated for left panel. *P<0.05, compared with control; #P<0.05, compared with ATO alone or let-7a mimics alone.
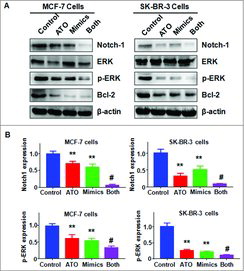
Let-7a mimics inhibited Notch-1 expression. It has been reported that Notch-1 is a potential target gene of let-7a. Therefore, we sought to investigate whether ATO could inhibit Notch-1 expression via up-regulation of let-7a in breast cancer cells. We found that let-7a mimics inhibited the expression of Notch-1 in MCF-7 and SK-BR-3 cells (). Our Western blotting results also showed that ATO down-regulated the expression of Notch-1 in breast cancer cells (). Remarkably, ATO treatment in combination with let-7a mimics led to a greater degree of Notch-1 inhibition compared with ATO treatment alone or let-7a mimics alone (). Consistently, let-7a mimics and ATO treatment inhibited the expression of Notch-1 downstream genes including p-ERK and Bcl-2 in breast cancer cells (). These data suggested that ATO decreased Notch-1 expression partly due to up-regulation of let-7a.
Figure 6. Effect of ATO on the expression of Notch-1 pathway. (A) The expression of Notch-1, ERK, and Bcl-2 was measured by Western blotting analysis in breast cancer cells after ATO treatment or let-7a mimics or the combination. ATO: 8 μM ATO; Mimics: let-7a mimics: Both: 8 μM ATO plus let-7a mimics. (B) Quantitative results are illustrated for panel A. **P<0.01, compared with control; #P<0.05, compared with ATO alone or let-7a mimics alone.
Discussion
Emerging studies have demonstrated that let-7a expression was reduced in breast tumoral tissues in comparison with normal margin tissues.Citation32 Moreover, let-7a expression in human breast cancers is associated with apoptotic expression genes, such as caspase-3, Bcl-2 (B-cell lymphoma 2), and p53.Citation32 One study showed that Her2 (human epidermal growth factor receptor 2) increased PARP1 (poly ADP-ribose polymerase) protein via suppression of the let-7a miRNA in breast cancer cells.Citation33 In addition, let-7a was significantly lower in human Her2 positive breast tumors and inversely correlated with PARP1 protein levels.Citation33 Another study found that let-7a was involved in the anti-angiogenesis effects of the interval exercise training and hormone therapy in breast cancer.Citation34 Wang et al. found that let-7a inhibited cell proliferation and migration via targeting Lin28 in breast cancer cells.Citation35 Sun et al. reported that let-7a was involved in miR-208a-regulated breast cancer stem cells.Citation36 Moreover, lower expression of let-7a induced chemoresistance via enhancing cellular apoptosis in breast cancer cells.Citation37 Furthermore, let-7a regulated mammosphere formation capacity through Ras/NF-κB (nuclear factor kappa B) and Ras/MAPK (mitogen-activated protein kinase) /ERK pathway in breast cancer stem cells.Citation38 Further study identified that let-7a plays an important role as a tumor suppressor via targeting HMGA1 (high mobility group A1) in breast cancer.Citation39 Strikingly, let-7a increased cell sensitivity to doxorubicin via induction of mitochondrial ROS (reactive oxygen species) and up-regulation of oxidative stress responsive genes in breast cancer cells.Citation40 In line with these reports, we found that let-7a inhibited cell growth, induced apoptosis and retarded cell migration and invasion in breast cancer cells. Taken together, let-7a could be a potential target for the treatment for human breast cancer.
Notch signaling pathway has been characterized to play an oncogenic role in human breast cancer. ATO has been reported to inhibit Notch pathway in several human cancers.Citation41–43 For example, ATO depleted the cancer stem-like cell population in gliomas via inhibition of Notch pathway.Citation41,43 Similarly, ATO inhibited repopulation of neurosphere derived from glioblastoma by down-regulation of Notch pathway.Citation44 Hu et al found that ATO inhibited the proliferation of myeloma cell line through suppression of Notch signaling pathway.Citation42 Yang et al. reported that ATO exerted anti-lung cancer activity via inhibition of Notch-1.Citation45 In line with these findings, we observed that ATO down-regulated Notch-1 expression in breast cancer cells. These reports identified that ATO could inhibit the expression of Notch-1 in human cancers. Several studies have revealed that Notch-1 inhibited let-7a expression.Citation46 Overexpression of Notch-1 led to decreased expression of let-7a in pancreatic cancer cells.Citation46 Metformin treatment caused upregulation of let-7a in pancreatic cancer cells.Citation47 Curcumin analogue inhibited pancreatic tumor growth by upregulation of several miRNAs including let-7a.Citation48 In this study, we found that ATO upregulated let-7a expression in breast cancer cells. Altogether, ATO exerts its anti-cancer activity in part via inhibition of Notch-1/let-7a in breast cancer. It is well known that ATO is mainly used for treatment of PML (promyelocytic leukemia) patients.Citation49 Therefore, deeper investigation is required to explore whether ATO exerts its physiological function via up-regulation of let-7a using mouse models in breast cancer.
Materials and methods
Cell culture and reagents: MCF-7 and SK-BR-3 cell lines were bought from the ATCC Company (American Type Culture Collection, Manassas, VA, USA). Cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum at 37°C in a 5% CO2 humidified atmosphere. ATO (>99.5%, cat. No. 17971) and MTT 3-(4,5-dimethythiazol- 2-yl)-2,5-diphenyl tetrazolium bromide. are obtained from Sigma (St. Louis, Mo). ATO was dissolved in 1mM NaOH to make 1mM stock solution and was added directly to the medium at different concentrations. Let-7a mimics were bought from GenePharma Company (Shanghai, China). Anti-Notch-1 (ICN), anti-ERK, anti-p-ERK, anti-Bcl-2, and the secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
MTT assay: MCF-7and SK-BR-3 cells (5 × 103) were seeded in a 96-well culture plate for overnight and then treated with different concentrations of ATO or let-7a mimics or the combination for 72 hours. Cell proliferation was determined by MTT assay as described previously.Citation50
Apoptosis analyses: MCF-7and SK-BR-3 cells were cultured in 6-well plate overnight and then treated with different concentration of ATO or let-7a mimics or the combination for 48 hours, respectively. Then, Annexin V-FITC/PI staining and FACS were performed to measure the apoptosis as described before.Citation51
Wound healing assay: MCF-7 and SK-BR-3 cells were cultured in 6-well plate for 48 hours. After the cells are almost confluent, the straight scratch wound was established using a pipette tip. Then, cells were treated with 8 μM ATO or let-7a mimics or combination for 16 hours. Photographic images of the scratched area were taken from each well at 0 h and 16 hours, respectively.
Invasion assay: The invasion assay was measured in MCF-7 and SK-BR-3 cells treated with 8μM ATO or let-7a mimics or the combination by Transwell inserts with Matrigel. After incubation for 24h, the invading cells were fixed and stained with Giemsa solution. The stained invasive cells were photographed under a microscope.
miRNA real-time reverse transcriptase-PCR: To determien whether ATO treatment could regulate the expression of let-7a in MCF-7 and SK-BR-3 cells, let-7a level was measured in breast cancer cells after ATO treatment by real-time reverse transcriptase-PCR (RT-PCR) assay as described previously.Citation50
Let-7a mimics tranfection: MCF-7and SK-BR-3 cells were seeded in six-well plates and transfected with let-7a mimics or the nonspecific control as described previously.Citation52
Western blotting assay: To measure the expression of Notch-1 in MCF-7and SK-BR-3 cells treated with 8μM ATO or let-7a mimics or the combination for 72 hours, Western blotting analysis was performed. The cells were lysed in the cold lysis buffer and the proteins were separated on SDS-PAGE and immunoblotted with indicated antibodies as described previously.Citation53
Statistical Analysis: All statistical analyses were performed using GraphPad Prism 4.0 (Graph pad Software, La Jolla, CA) by ANOVA (analysis of variance) with Dunnett's test. Values are expressed as the mean± standard error of the mean. P<0.05 was considered as statistically significant.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This work is supported by grant from National Natural Science Foundation of China (NSFC 81502126). This work is also supported in part by Natural Science Foundation of Anhui (1508085SMH232) and the program for graduate research of Bengbu Medical College (Byycx1315 and Byycx1616). This work was supported by grant from the priority academic program development of Jiangsu higher education institutions.
Reference
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi:10.3322/caac.21387.
- Wu DD, Lau ATY, Yu FY, Cai NL, Dai LJ, Ok Kim M, Jin DY, Xu YM. Extracellular signal-regulated kinase 8-mediated NF-kappaB activation increases sensitivity of human lung cancer cells to arsenic trioxide. Oncotarget. 2017;8:49144–49155.
- Noguera NI, Pelosi E, Angelini DF, Piredda ML, Guerrera G, Piras E, Battistini L, Massai L, Berardi A, Catalano G, et al. High-dose ascorbate and arsenic trioxide selectively kill acute myeloid leukemia and acute promyelocytic leukemia blasts in vitro. Oncotarget. 2017;8:32550–32565.
- Tai S, Xu L, Xu M, Zhang L, Zhang Y, Zhang K, Liang C. Combination of Arsenic trioxide and Everolimus (Rad001) synergistically induces both autophagy and apoptosis in prostate cancer cells. Oncotarget. 2017;8:11206–11218.
- Moghaddaskho F, Eyvani H, Ghadami M, Tavakkoly-Bazzaz J, Alimoghaddam K, Ghavamzadeh A, Ghaffari SH. Demethylation and alterations in the expression level of the cell cycle-related genes as possible mechanisms in arsenic trioxide-induced cell cycle arrest in human breast cancer cells. Tumour Biol. 2017;39:1010428317692255. doi:10.1177/1010428317692255. PMID:28218039
- Hoffman EA, Gizelska K, Mirowski M, Mielicki W. Arsenic trioxide downregulates cancer procoagulant activity in MCF-7 and WM-115 cell lines in vitro. Contemp Oncol (Pozn). 2015;19:108–112.
- Zhang H, Gu H, Li L, Ren Y, Zhang L. EZH2 mediates ATO-induced apoptosis in acute myeloid leukemia cell lines through the Wnt signaling pathway. Tumour Biol. 2016;37:5919–5923.
- Bakhshaiesh TO, Armat M, Shanehbandi D, Sharifi S, Baradaran B, Hejazi MS, Samadi N. Arsenic trioxide promotes paclitaxel cytotoxicity in resistant breast cancer cells. Asian Pac J Cancer Prev. 2015;16:5191–5197. doi:10.7314/APJCP.2015.16.13.5191.
- Kasukabe T, Okabe-Kado J, Kato N, Honma Y, Kumakura S. Cotylenin A and arsenic trioxide cooperatively suppress cell proliferation and cell invasion activity in human breast cancer cells. Int J Oncol. 2015;46:841–848. doi:10.3892/ijo.2014.2760.
- Guilbert C, Annis MG, Dong Z, Siegel PM, Miller WH, Jr., Mann KK. Arsenic trioxide overcomes rapamycin-induced feedback activation of AKT and ERK signaling to enhance the anti-tumor effects in breast cancer. PLoS One. 2013;8:e85995. doi:10.1371/journal.pone.0085995. PMID:24392034
- Liu W, Gong Y, Li H, Jiang G, Zhan S, Liu H, Wu Y. Arsenic trioxide-induced growth arrest of breast cancer MCF-7 cells involving FOXO3a and IkappaB kinase beta expression and localization. Cancer Biother Radiopharm. 2012;27:504–512. doi:10.1089/cbr.2012.1162.
- Wang Y, Zhang Y, Yang L, Cai B, Li J, Zhou Y, Yin L, Yang BF, Lu YJ. Arsenic trioxide induces the apoptosis of human breast cancer MCF-7 cells through activation of caspase-3 and inhibition of HERG channels. Exp Ther Med. 2011;2:481–486. doi:10.3892/etm.2011.224.
- Kasinski AL, Slack FJ. Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer. 2011;11:849–864.
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi:10.1038/nrc1997.
- van Kouwenhove M, Kedde M, Agami R. MicroRNA regulation by RNA-binding proteins and its implications for cancer. Nat Rev Cancer. 2011;11:644–656. doi:10.1038/nrc3107.
- Heneghan HM, Miller N, Lowery AJ, Sweeney KJ, Newell J, Kerin MJ. Circulating microRNAs as novel minimally invasive biomarkers for breast cancer. Ann Surg. 2010;251:499–505. doi:10.1097/SLA.0b013e3181cc939f.
- Kim SJ, Shin JY, Lee KD, Bae YK, Sung KW, Nam SJ, Chun KH. MicroRNA let-7a suppresses breast cancer cell migration and invasion through downregulation of C-C chemokine receptor type 7. Breast Cancer Res. 2012;14:R14. doi:10.1186/bcr3098. PMID:22251626
- Liu Y, Li H, Feng J, Cui X, Huang W, Li Y, Su F, Liu Q, Zhu J, Lv X, et al. Lin28 induces epithelial-to-mesenchymal transition and stemness via downregulation of let-7a in breast cancer cells. PLoS One. 2013;8:e83083. doi:10.1371/journal.pone.0083083. PMID:24349438
- Lyu S, Yu Q, Ying G, Wang S, Wang Y, Zhang J, Niu Y. Androgen receptor decreases CMYC and KRAS expression by upregulating let-7a expression in ER-, PR-, AR+ breast cancer. Int J Oncol. 2014;44:229–237. doi:10.3892/ijo.2013.2151.
- Liang H, Li X, Wang L, Yu S, Xu Z, Gu Y, Pan Z, Li T, Hu M, Cui H, et al. MicroRNAs contribute to promyelocyte apoptosis in As2O3-treated APL cells. Cell Physiol Biochem. 2013;32:1818–1829. doi:10.1159/000356615.
- Wang X, Jiang F, Mu J, Ye X, Si L, Ning S, Li Z, Li Y. Arsenic trioxide attenuates the invasion potential of human liver cancer cells through the demethylation-activated microRNA-491. Toxicol Lett. 2014;227:75–83. doi:10.1016/j.toxlet.2014.03.016.
- Chen S, Zhu L, Huang J, Cai Y, Lu X, Yang Q, Wu Q, Chen C, Wang Z. Arsenic trioxide targets miR-125b in glioma cells. Curr Pharm Des. 2014;20:5354–5361. doi:10.2174/1381612820666140128204132.
- Zhang Y, Wu JH, Han F, Huang JM, Shi SY, Gu RD, Chen XL, He B. Arsenic trioxide induced apoptosis in retinoblastoma cells by abnormal expression of microRNA-376a. Neoplasma. 2013;60:247–253. doi:10.4149/neo_2013_033.
- Shidfar F, Ghaffari SH, Tavoosidana G, Hosseini E, Alimoghaddam K, Ghavamzadeh A. Arsenic trioxide Alters the MicroRNA Expression Profile of U87 glioblastoma. Anticancer Agents Med Chem. 2015;16:247–258. doi:10.2174/1871520615666150629100752.
- Wang J, Li Y, Jiang C. MiR-133b contributes to arsenic-induced apoptosis in U251 glioma cells by targeting the hERG channel. J Mol Neurosci. 2015;55:985–994. doi:10.1007/s12031-014-0455-8.
- Ji H, Li Y, Jiang F, Wang X, Zhang J, Shen J, Yang X. Inhibition of transforming growth factor beta/SMAD signal by MiR-155 is involved in arsenic trioxide-induced anti-angiogenesis in prostate cancer. Cancer Sci. 2014;105:1541–1549. doi:10.1111/cas.12548.
- Jiang F, Wang X, Liu Q, Shen J, Li Z, Li Y, Zhang J. Inhibition of TGF-beta/SMAD3/NF-kappaB signaling by microRNA-491 is involved in arsenic trioxide-induced anti-angiogenesis in hepatocellular carcinoma cells. Toxicol Lett. 2014;231:55–61. doi:10.1016/j.toxlet.2014.08.024.
- Chang YW, Chen MW, Chiu CF, Hong CC, Cheng CC, Hsiao M, Chen CA, Wei LH, Su JL. Arsenic trioxide inhibits CXCR4-mediated metastasis by interfering miR-520h/PP2A/NF-kappaB signaling in cervical cancer. Ann Surg Oncol. 2014;21 Suppl 4:S687–S695.
- Si L, Jiang F, Li Y, Ye X, Mu J, Wang X, Ning S, Hu C, Li Z. Induction of the mesenchymal to epithelial transition by demethylation- activated microRNA-200c is involved in the anti-migration/invasion effects of arsenic trioxide on human breast cancer cells. Mol Carcinog. 2015;54:859–869. doi:10.1002/mc.22157.
- Wang Y, Wang L, Yin C, An B, Hao Y, Wei T, Li L, Song G. Arsenic trioxide inhibits breast cancer cell growth via microRNA-328/hERG pathway in MCF-7 cells. Mol Med Rep. 2015;12:1233–1238.
- Zhang S, Ma C, Pang H, Zeng F, Cheng L, Fang B, Ma J, Shi Y, Hong H, Chen J, et al. Arsenic trioxide suppresses cell growth and migration via inhibition of miR-27a in breast cancer cells. Biochem Biophys Res Commun. 2016;469:55–61. doi:10.1016/j.bbrc.2015.11.071.
- Mansoori B, Mohammadi A, Shirjang S, Baghbani E, Baradaran B. Micro RNA 34a and let-7a expression in human breast cancers is associated with apoptotic expression genes. Asian Pac J Cancer Prev. 2016;17:1887–1890.
- Wielgos ME, Rajbhandari R, Cooper TS, Wei S, Nozell S, Yang ES. Let-7 Status Is Crucial for PARP1 Expression in HER2-Overexpressing Breast Tumors. Mol Cancer Res. 2017;15:340–347. doi:10.1158/1541-7786.MCR-16-0287-T.
- Isanejad A, Alizadeh AM, Amani Shalamzari S, Khodayari H, Khodayari S, Khori V, Khojastehnjad N. MicroRNA-206, let-7a and microRNA-21 pathways involved in the anti-angiogenesis effects of the interval exercise training and hormone therapy in breast cancer. Life Sci. 2016;151:30–40. doi:10.1016/j.lfs.2016.02.090.
- Wang L, Wang YX, Zhang DZ, Fang XJ, Sun PS, Xue HC. Let-7a mimic attenuates CCL18 induced breast cancer cell metastasis through Lin 28 pathway. Biomed Pharmacother. 2016;78:301–307. doi:10.1016/j.biopha.2016.01.028.
- Sun X, Jiang S, Liu J, Wang H, Zhang Y, Tang SC, Wang J, Du N, Xu C, Wang C, et al. MiR-208a stimulates the cocktail of SOX2 and beta-catenin to inhibit the let-7 induction of self-renewal repression of breast cancer stem cells and formed miR208a/let-7 feedback loop via LIN28 and DICER1. Oncotarget. 2015;6:32944–32954. doi:10.18632/oncotarget.5079.
- Wu J, Li S, Jia W, Deng H, Chen K, Zhu L, Yu F, Su F. Reduced Let-7a Is Associated with Chemoresistance in Primary Breast Cancer. PLoS One. 2015;10:e0133643. doi:10.1371/journal.pone.0133643. PMID:26218285
- Xu C, Sun X, Qin S, Wang H, Zheng Z, Xu S, Luo G, Liu P, Liu J, Du N, et al. Let-7a regulates mammosphere formation capacity through Ras/NF-kappaB and Ras/MAPK/ERK pathway in breast cancer stem cells. Cell Cycle. 2015;14:1686–1697. doi:10.1080/15384101.2015.1030547.
- Liu K, Zhang C, Li T, Ding Y, Tu T, Zhou F, Qi W, Chen H, Sun X. Let-7a inhibits growth and migration of breast cancer cells by targeting HMGA1. Int J Oncol. 2015;46:2526–2534. doi:10.3892/ijo.2015.2949.
- Serguienko A, Grad I, Wennerstrom AB, Meza-Zepeda LA, Thiede B, Stratford EW, Myklebost O, Munthe E. Metabolic reprogramming of metastatic breast cancer and melanoma by let-7a microRNA. Oncotarget. 2015;6:2451–2465. doi:10.18632/oncotarget.3235.
- Zhen Y, Zhao S, Li Q, Li Y, Kawamoto K. Arsenic trioxide-mediated Notch pathway inhibition depletes the cancer stem-like cell population in gliomas. Cancer Lett. 2010;292:64–72. doi:10.1016/j.canlet.2009.11.005.
- Hu J, Huang X, Hong X, Lu Q, Zhu X. Arsenic trioxide inhibits the proliferation of myeloma cell line through notch signaling pathway. Cancer Cell Int. 2013;13:25. doi:10.1186/1475-2867-13-25. PMID:23497375
- Ding D, Lim KS, Eberhart CG. Arsenic trioxide inhibits Hedgehog, Notch and stem cell properties in glioblastoma neurospheres. Acta Neuropathol Commun. 2014;2:31. doi:10.1186/2051-5960-2-31. PMID:24685274
- Wu J, Ji Z, Liu H, Liu Y, Han D, Shi C, Wang C, Yang G, Chen X, Shen C, et al. Arsenic trioxide depletes cancer stem-like cells and inhibits repopulation of neurosphere derived from glioblastoma by downregulation of Notch pathway. Toxicol Lett. 2013;220:61–69. doi:10.1016/j.toxlet.2013.03.019.
- Yang MH, Zang YS, Huang H, Chen K, Li B, Sun GY, Zhao XW. Arsenic trioxide exerts anti-lung cancer activity by inhibiting angiogenesis. Curr Cancer Drug Targets. 2014;14:557–566. doi:10.2174/1568009614666140725090000.
- Bao B, Wang Z, Ali S, Kong D, Li Y, Ahmad A, Banerjee S, Azmi AS, Miele L, Sarkar FH. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett. 2011;307:26–36. doi:10.1016/j.canlet.2011.03.012.
- Bao B, Wang Z, Ali S, Ahmad A, Azmi AS, Sarkar SH, Banerjee S, Kong D, Li Y, Thakur S, et al. Metformin inhibits cell proliferation, migration and invasion by attenuating CSC function mediated by deregulating miRNAs in pancreatic cancer cells. Cancer Prev Res (Phila). 2012;5:355–364. doi:10.1158/1940-6207.CAPR-11-0299.
- Bao B, Ali S, Banerjee S, Wang Z, Logna F, Azmi AS, Kong D, Ahmad A, Li Y, Padhye S, et al. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res. 2012;72:335–345. doi:10.1158/0008-5472.CAN-11-2182.
- McCulloch D, Brown C, Iland H. Retinoic acid and arsenic trioxide in the treatment of acute promyelocytic leukemia: current perspectives. Onco Targets Ther. 2017;10:1585–1601.
- Ma J, Fang B, Zeng F, Ma C, Pang H, Cheng L, Shi Y, Wang H, Yin B, Xia J, et al. Down-regulation of miR-223 reverses epithelial-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Oncotarget. 2015;6:1740–1749. doi:10.18632/oncotarget.2714.
- Xia J, Duan Q, Ahmad A, Bao B, Banerjee S, Shi Y, Ma J, Geng J, Chen Z, Rahman KM, et al. Genistein inhibits cell growth and induces apoptosis through up-regulation of miR-34a in pancreatic cancer cells. Curr Drug Targets. 2012;13:1750–1756. doi:10.2174/138945012804545597.
- Ma J, Cheng L, Liu H, Zhang J, Shi Y, Zeng F, Miele L, Sarkar FH, Xia J, Wang Z. Genistein down-regulates miR-223 expression in pancreatic cancer cells. Curr Drug Targets. 2013;14:1150–1156. doi:10.2174/13894501113149990187.
- Yang Q, Wang Y, Lu X, Zhao Z, Zhu L, Chen S, Wu Q, Chen C, Wang Z. MiR-125b regulates epithelial-mesenchymal transition via targeting Sema4C in paclitaxel-resistant breast cancer cells. Oncotarget. 2015;6:3268–3279.
