“Classification” is a major point of contention in the field of cell death. Ferroptosis is a regulated form of necrosis that was identified in 2012 and is specified by the accumulation of lipid peroxide within the cell. Several studies have demonstrated that the pharmacological inhibition of ferroptosis is beneficial for ischemia reperfusion injury models in multiple tissues, thus suggesting the relevance of ferroptosis to some human diseases. A growing number of studies have recently uncovered the mechanism by which detrimental levels of lipid peroxide accumulate; however, the molecular mechanisms that link lipid peroxide accumulation to the execution of cell death remain largely unknown [Citation1].
Reactive oxygen species (ROS), such as lipid peroxide, are vital signal mediators. ROS induce a diverse array of signaling cascades, including the thioredoxin (Trx) apoptosis signal-regulating kinase 1 (ASK1) axis [Citation2]. We recently revealed that ASK1-p38 MAPK cascade is activated through ferroptosis induced by cold stress () [Citation3]. Importantly, the ASK1 axis lies downstream of lipid peroxide and has no effect on lipid peroxide accumulation. Precise analyses revealed that the ASK1-p38 axis is only partially involved in canonical ferroptosis induced by erastin or RSL3 in a cell-type-specific manner; however, the same axis plays critical roles in cold-stress-induced ferroptosis [Citation3].
Figure 1. Schematic model of cold stress-induced ferroptosis. The ASK1-p38 MAPK axis is activated in response to cold stress and is involved in inducing ferroptosis.
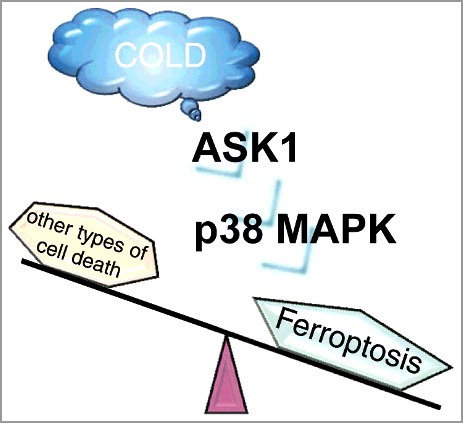
Aberrant fluctuations of environmental cues are harmful for cellular homeostasis. An environmental temperature change is a vital cue and is known to affect the cellular functions in a diverse manner, i.e., enzyme kinetics and membrane fluidity. Our recent dataset revealed that prolonged severe cold stress induces the accumulation of lipid peroxide, which activates the ASK1-p38 axis and promotes ferroptosis [Citation3]. Although lipid peroxide accumulation mediates membrane destruction, we demonstrate that signaling pathways downstream of lipid peroxide may be involved in the execution of cell death, i.e., by disrupting lipid membrane homeostasis. This is a rare example wherein an environmental stress induces ferroptosis, but it is worth mentioning that heat stress also induces ferroptosis-like cell death in plants [Citation4]. Although numerous studies have demonstrated that thermal stress induces ROS production, the precise mechanism through which lipid peroxide accumulates remains unknown.
ROS signaling mediates not only ferroptosis but also apoptosis and parthanatos; intensity, spatiotemporal dynamics, and other features of ROS presumably determine cell fate. One plausible explanation is that multiple signaling pathways are activated in parallel but that only one signal predominates to execute a specific type of cell death in a situation-dependent manner. Our previous study demonstrated that high concentrations of H2O2 induce receptor-interacting protein 1 (RIP1)-independent necrosis via the ASK1-p38 MAPK axis [Citation5]. For cold stress-induced cell death, our preliminary data suggest that the parthanatos pathway is also evoked because PARP1 inhibition also attenuates cold-induced cell death (Hattori, K., Ishikawa, H. and Ichijo, H., unpublished observation). This evidence raises the possibility that ferroptosis and parthanatos intersect and crosstalk in the course of death-inducing signaling mechanisms. In fact, each form of cell death possesses some common features. Nicotinamide adenine dinucleotide (NAD+), an essential factor in cell metabolism, controls various cell death pathways [Citation6]. Of note, reduced NAD+ levels are induced both in ferroptosis [Citation7] and parthanatos [Citation6], suggesting that a common NAD+-dependent mechanism is involved in the execution of necrotic cell death. Given that numerous NAD+-dependent enzymes exist, NAD+ depletion must lead to the disruption of overall cellular homeostasis, including lipid homeostasis on the plasma membranes.
The classification of cell death opens the possibility that manipulating a specific type of cell death may contribute to the development of unique therapeutic interventions because each type of cell death may be involved in a specific type of disease. Nevertheless, we should not overlook the fact that all types of cell death are potentially intertwined. In addition, cell death induced by the fluctuation of environmental cues might be specifically modulated via diverse mechanisms because cells typically sense environmental variation using multiple signaling pathways.
ASK1, as the name suggests, has long been considered an apoptosis-regulating factor [Citation2]. We here suggest that ASK1 is involved in a wider range of ROS-dependent cell death processes, including ferroptosis, as a hub of multiple signaling cascades [Citation3,Citation5].
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Agmon E, Stockwell BR. Lipid homeostasis and regulated cell death. Curr Opin Chem Biol. 2017;39:83–89. doi:10.1016/j.cbpa.2017.06.002. PMID:28645028
- Sakauchi C, Wakatsuki H, Ichijo H, et al. Pleiotropic properties of ASK1. Biochim Biophys Acta BBA – Gen Subj. 2017;1861:3030–3038. doi:10.1016/j.bbagen.2016.09.028. PMID:27693599
- Hattori K, Ishikawa H, Sakauchi C, et al. Cold stress‐induced ferroptosis involves the ASK1‐p38 pathway. EMBO Rep. 2017;18:2067–2078. doi:10.15252/embr.201744228. PMID:28887319
- Distéfano AM, Martin MV, Córdoba JP, et al. Heat stress induces ferroptosis-like cell death in plants. J Cell Biol. 2017;216:463–476. doi:10.1083/jcb.201605110. PMID:28100685
- Watanabe T, Sekine S, Naguro I, et al. Apoptosis signal-regulating kinase 1 (ASK1)-p38 pathway-dependent cytoplasmic translocation of the orphan nuclear receptor NR4A2 is required for oxidative stress-induced necrosis. J Biol Chem. 2015;290:10791–10803. doi:10.1074/jbc.M114.623280. PMID:25752609
- Preyat N, Leo O. Complex role of nicotinamide adenine dinucleotide in the regulation of programmed cell death pathways. Biochem Pharmacol. 2016;101:13–26. doi:10.1016/j.bcp.2015.08.110. PMID:26343585
- Shimada K, Hayano M, Pagano NC, et al. Cell-line selectivity improves the predictive power of pharmacogenomic analyses and helps identify NADPH as biomarker for ferroptosis sensitivity. Cell Chem Biol. 2016;23:225–235. doi:10.1016/j.chembiol.2015.11.016. PMID:26853626
