ABSTRACT
ONC201, founding member of the imipridone class of small molecules, is currently being evaluated in advancer cancer clinical trials. We explored single agent and combinatorial efficacy of ONC201 in preclinical models of hematological malignancies. ONC201 demonstrated (GI50 1–8 µM) dose- and time-dependent efficacy in acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myelogenous leukemia (CML), chronic lymphocytic leukemia (CLL), diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma (MCL), Burkitt's lymphoma, anaplastic large cell lymphoma (ALCL), cutaneous T-cell lymphoma (CTCL), Hodgkin's lymphoma (nodular sclerosis) and multiple myeloma (MM) cell lines including cells resistant to standard of care (dexamethasone in MM) and primary samples. ONC201 induced caspase-dependent apoptosis that involved activation of the integrated stress response (ATF4/CHOP) pathway, inhibition of Akt phosphorylation, Foxo3a activation, downregulation of cyclin D1, IAP and Bcl-2 family members. ONC201 synergistically reduced cell viability in combination with cytarabine and 5-azacytidine in AML cells. ONC201 combined with cytarabine in a Burkitt's lymphoma xenograft model induced tumor growth inhibition that was superior to either agent alone. ONC201 synergistically combined with bortezomib in MM, MCL and ALCL cells and with ixazomib or dexamethasone in MM cells. ONC201 combined with bortezomib in a Burkitt's lymphoma xenograft model reduced tumor cell density and improved CHOP induction compared to either agent alone. These results serve as a rationale for ONC201 single-agent trials in relapsed/refractory acute leukemia, non-Hodgkin's lymphoma, MM and combination trial with dexamethasone in MM, provide pharmacodynamic biomarkers and identify further synergistic combinatorial regimens that can be explored in the clinic.
Introduction
ONC201 is a first-in-class anti-cancer agent and founding member of the imipridone class of small molecules that are defined by a unique core chemical structure Citation[1]. The broad spectrum anti-cancer efficacy of ONC201 in solid tumors and hematological malignancies Citation[1,2] is driven by activation of an ATF4/CHOP-mediated integrated stress response (ISR) that ultimately results in dual inhibition of Akt/ERK, Foxo3a activation and DR5/TRAIL-mediated apoptosis Citation[3,4]. ONC201 also targets chemotherapy-resistant cancer stem cells in a Akt/ERK/Foxo3a/DR5/TRAIL-dependent manner in solid tumors and hematological malignancies Citation[4-6].
The ONC201 first-in-human Phase I trial provided evidence of safety, therapeutic pharmacokinetics (PK), prolonged pharmacodynamics (PD) and shrinkage of metastatic lesions Citation[7]. ONC201 has exhibited durable objective response and a benign safety profile in a phase II recurrent glioblastoma clinical trial Citation[8]. ONC201 is also being evaluated in various relapsed/refractory hematological malignancies such as acute leukemias, high-risk myelodysplastic syndromes, non-Hodgkin's lymphoma (NHL) and multiple myeloma (MM) Citation[1]. In the current study, we evaluated the single agent efficacy and mechanism of action of ONC201 in preclinical models of hematological malignancies.
ONC201 exhibits sustained engagement of signaling pathways and prolonged anti-cancer efficacy that outlives its PK. The unique PK-PD disconnect, robust safety profile and oral infrequent dosing suggest that ONC201 is an attractive combinatorial therapeutic Citation[1]. Considering ongoing trials in hematological malignancies are predominantly single agent trials, in the current study, we also explored the combinatorial efficacy of ONC201 with standard-of-care agents in acute leukemia, NHL and MM.
Materials and methods
Cell culture and reagents
ONC201-resistant RKO cells have been generated previously using concentration gradient incubations Citation[3]. MM cell lines including dexamethasone sensitive and resistant cells were obtained from Dr. Nathan G. Dolloff. Karpas 299 cells were obtained from Sigma Aldrich and cultured as per Sigma Aldrich recommendations. All other cell lines were obtained from the American Type Culture Collection (ATCC) and cultured as per ATCC recommendations. Cells were authenticated every month by bioluminescence, growth and morphological observation. ONC201 was provided by Oncoceutics, Inc.
Western blot
Western blotting was performed as described previously Citation[2,3,5]. Briefly, lysates were prepared and evaluated with protein assay (Biorad). LDS sample buffer and reducing agent (Invitrogen) were added for SDS-PAGE. After transfer, primary and secondary antibody incubations were performed, and signal was detected using a chemiluminescent detection kit, followed by autoradiography.
Immunofluorescence staining
Immunofluorescence for patient-derived cells was performed as previously described Citation[9]. Patient derived mantle cell lymphoma (MCL) cells were treated with 5 µM ONC201 for 72 hours. Cells were harvested and spun onto microscope slides with cytospin. Cells were fixed with 4% paraformaldehyde in PBS for 20 minutes. After three washes with PBS, cells were blocked with 5% goat serum for 30 minutes and then incubated with primary antibody Anti-Cyclin D1 antibody [EPR2241] (ab134175) diluted in 5% goat serum overnight. After three washes with PBS, cells were incubated with Goat anti-Rabbit IgG (H+L) Secondary Antibody, Alexa Fluor® 488 conjugate (Thermo Fisher Scientific A-11034) for 2 hours in dark. After three washes with PBS, cells were counterstained with DAPI.
Sub-G1 analysis and cell viability assays
For Sub-G1 analysis, cells were treated, trypsinized, ethanol-fixed, stained with propidium iodide (Sigma) and analyzed by flow cytometry as previously described Citation[2]. Cell viability was determined as described previously using the CellTitre-Glo reagent (Promega) Citation[5].
Burkitt's lymphoma xenograft model
Ramos (Burkitt's lymphoma) cell line was transduced with lentiviral GFP to enable bioluminescent imaging of mice during the study. A pure GFP+ Ramos cell line was obtained via FACS cell sorting and serial passaging. Five week-old female immunodeficient SCID mice [NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ] were injected subcutaneously in both rear flank regions with ∼107 viable human lymphoma cells (harvested from sterile cell culture) suspended in 100 µL matrigel suspension. All subcutaneous tumors were established by Day 8–10 as detected by bioluminescent imaging. The mice were studied in 4 treatment arms for each study as indicated. The mice were humanely euthanized in week 3–4 of treatment when the tumor volume reached the maximum allowable size per the approved institutional IACUC protocol. Tumors were harvested for histology and immunohistochemistry analysis was performed as described previously Citation[2,5].
Statistical analysis
Data are presented as the mean ± standard deviation or standard error of mean from at least three replicates. The Student's two-tailed t-test in Excel (Microsoft) was used for pairwise analysis. Tumor growth in different treatment arms was analyzed by One-way & Two-way ANOVA test. Combination Indices (CI) were determined using CalcuSyn.
Results
ONC201 induced caspase-dependent apoptosis in leukemia is accompanied by inhibition of Akt, IAP and Bcl-2 family members
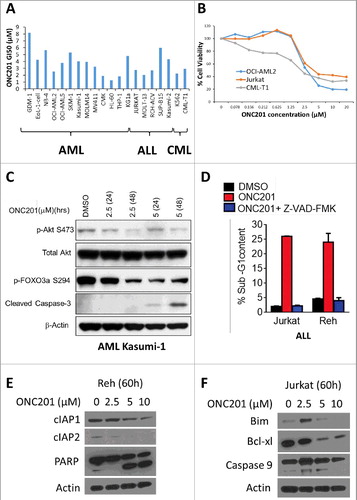
ONC201 was evaluated in 20 human leukemia cell lines: 13 acute myeloid leukemia (AML), 5 acute lymphoblastic leukemia (ALL) and 2 chronic myelogenous leukemia (CML) cell lines. We observed a dose-dependent decrease in cell viability in AML, ALL and CML cells (GI50 1–8 µM) ( and ). ONC201 also decreased cell viability in 2 primary chronic lymphocytic leukemia (CLL) patient samples with 48–72h treatment (Fig S1A). Consistent with the previously reported late-stage signaling effects of ONC201 in solid tumor cells Citation[2], dose- and time-dependent induction of apoptosis (caspase-3 cleavage), inhibition of Akt and Foxo3a phosphorylation was observed in AML cells treated with 2.5 µM or 5 µM of ONC201 for 24–48 hr (). Sub-G1 analysis confirmed that ONC201 induced apoptosis in ALL cells and a pan-caspase inhibitor reduced ONC201-mediated apoptosis (). Consistent with previous findings Citation[2], ONC201-mediated apoptosis in ALL cells involved PARP cleavage and caspase-9 activation ( and ). Anti-apoptotic Bcl-2 family members Bcl-2 and Bcl-xl Citation[10] were downregulated while the pro-apoptotic Bcl-2 family member Bim Citation[10] was upregulated in response to ONC201 treatment in ALL cells. ONC201 also downregulates the inhibitor of apoptosis (IAP) family proteins cIAP1 and cIAP2 in ALL cells, which are known to confer a poor clinical prognosis Citation[11,12]. Thus, ONC201 demonstrated robust single agent efficacy in leukemia that involves caspase-mediated apoptosis.
Figure 1. ONC201 induced caspase-dependent apoptosis in leukemia is accompanied by inhibition of Akt, IAP and Bcl-2 family members. (A) GI50 of ONC201 in viability assay with indicated AML, ALL and CML cell lines at 72 h post-treatment with concentrations from 78 nM up to 20 µM. (B) Representative dose response curves for (A). (C) Western blot for indicated proteins in DMSO/ONC201-treated AML Kasumi-1 cells at indicated concentrations and time points. (D) % Apoptosis (Sub-G1 cells) in DMSO/ONC201-treated (5 µM, 48h) with or without caspase inhibitor Z-VAD-FMK in ALL Jurkat and Reh cells. (E) Western blot analysis of ONC201-treated ALL Reh and (F) Jurkat cells at indicated concentrations and time point.
ONC201-induced apoptosis in MM involves activation of ISR and IAP downregulation
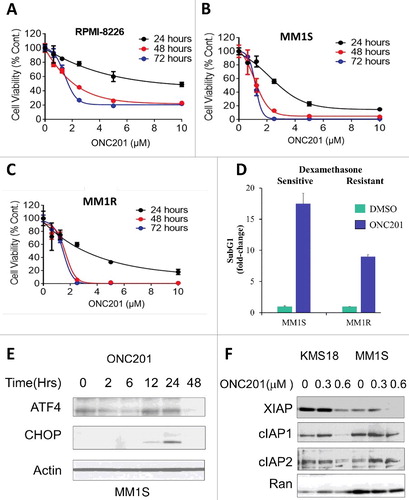
Next, the anti-cancer efficacy of ONC201 was evaluated in MM. Low micromolar concentrations of ONC201 reduced MM cell viability in a time- and dose-dependent manner (). Dexamethasone has been used in the treatment of MM for decades and is frequently combined with other standard-of-care therapies used to treat this disease Citation[13]. ONC201 reduced cell viability and induced apoptosis in dexamethasone-resistant MM.1R cells with efficacy comparable to dexamethasone-sensitive MM.1S cells (). Consistent with previous findings Citation[3,4], ONC201 caused activation of the ISR pathway, as observed with induction of ATF/CHOP in MM cells (). Downregulation of all IAP family proteins simultaneously is important for induction of apoptosis in MM cells Citation[14] and XIAP is reported to be highly up-regulated in MM cells Citation[15]. Similar to acute leukemia, ONC201 treatment of MM cells resulted in a dose-dependent decrease in the anti-apoptotic proteins XIAP, cIAP1 and cIAP2. Thus, ONC201 is efficacious as a single agent in MM.
Figure 2. ONC201 induced apoptosis in MM involved induction of ISR and IAP inhibition. Dose response curve for cell viability assay of ONC201-treated (A) RPMI-8226, (B) MM.1S and (C) dexamethasone resistant MM.1R cells at indicated concentrations and time points. (D) % Apoptosis (Sub-G1 cells, fold change) in DMSO/ONC201-treated (5 µM, 48h) MM.1S and MM.1R cells. (E) Western blot for indicated proteins in ONC201-treated (0.625 µM) MM.1S cells at indicated time points. (F) Western blot for indicated proteins in ONC201-treated (48 h) KMS18 and MM.1S cells at indicated concentrations.
ONC201 inhibited cell viability in lymphoma independent of subtype, triggered an ISR and downregulated cyclin D1
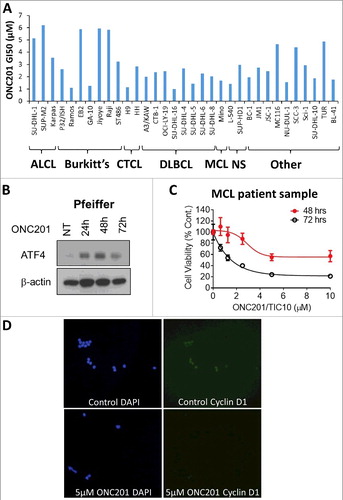
ONC201 was evaluated in 33 human lymphoma cell lines: 3 anaplastic large cell lymphoma (ALCL), 7 Burkitt's lymphoma, 2 cutaneous T-cell lymphoma (CTCL), 8 diffuse large B-cell lymphoma (DLBCL), 1 MCL, 2 nodular sclerosis (NS) and 10 other lymphoma cell lines. ONC201 demonstrated low micromolar range efficacy (GI50 1–6 µM) across all lymphoma subtypes ( and S1B). Low micromolar concentrations of ONC201 reduced cell viability of MCL and DLBCL cell lines and primary cells in a time- and dose-dependent manner (, S1A and S1C). Consistent with previous findings Citation[3,4], ONC201 caused activation of the ISR pathway, as observed with induction of ATF4 in Pfeiffer DLBCL cells (). Overexpression of cyclin D1 is a diagnostic feature of MCL Citation[16]. Consistent with previous findings and ISR activation Citation[3], ONC201 reduced cyclin D1 expression in patient-derived MCL cells (). Thus, ONC201 is broadly active as a single agent across various lymphoma subtypes.
Figure 3. ONC201 inhibited lymphoma cell viability independent of subtype, triggered an ISR and downregulated cyclin D1. (A) GI50 of ONC201 in viability assay with indicated ALCL, Burkitt's lymphoma, CTCL, DLBCL, MCL, NS and other lymphoma cell lines at 72 h post-treatment with concentrations from 78 nM up to 20 µM. (B) Western blot for indicated proteins in ONC201-treated (1.25 µM) Pfeiffer DLBCL cells at indicated time points. (C) % Cell viability curves at indicated concentrations and time points for ONC201 in primary MCL cells. (D) Cyclin D1 immunofluorescence for patient-derived MCL cells treated with DMSO or 5 µM ONC201 for 72 h. DAPI is used a nuclear stain.
ONC201 synergizes with chemotherapy, hypomethylating agents and proteasome inhibitors in vitro and in vivo
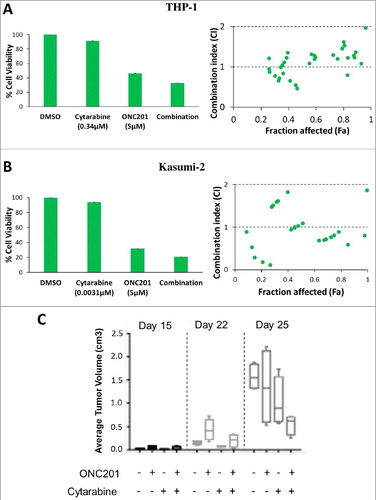
We tested the efficacy of ONC201 in combination with approved therapies in hematological malignancies in vitro and in vivo. We evaluated the combinatorial efficacy of ONC201 with cytarabine. ONC201 demonstrated synergistic reduction in cell viability in combination with cytarabine confirmed by combination indices in AML cell lines CMK (Fig S2A and Table S1), THP-1 ( and Table S3), HL60 (Fig S2B and Table S4) and ALL Kasumi-2 cells ( and Table S2). ONC201 combined with cytarabine in a Burkitt's lymphoma xenograft model exhibited superior tumor growth inhibition compared to either agent alone ( and S3). Thus, ONC201 combines synergistically with cytarabine in vitro and in vivo.
Figure 4. ONC201 combines synergistically with cytarabine in vitro and in vivo. Representative % cell viability bar chart at indicated concentrations and combination index (CI) versus fraction affected (Fa) plots for ONC201 and cytarabine, either as single agent or in combination (72 h) in (A) THP-1 and (B) Kasumi-2 cells. CI < 1 indicates synergism, CI = 1 indicates additive effect and CI > 1 indicates antagonism. Raw data shown in Table S2 and S3. (C) Average tumor volume (cm3) on indicated days post treatment for ONC201 (100 mg/kg, weekly, oral gavage) and cytarabine (75 mg/kg/day on day 1/2/3, every 3 weeks, intraperitoneal injection) either as single agent or in combination in a Ramos Burkitt's lymphoma subcutaneous xenograft model in SCID mice (4 mice per group).
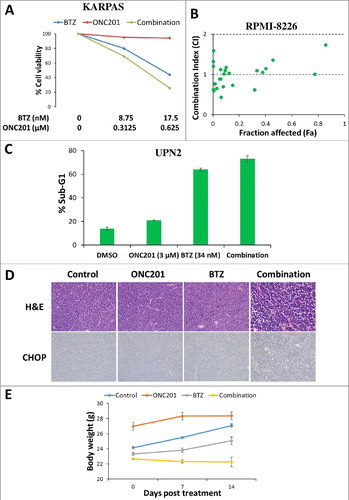
Next, we tested the combinatorial efficacy of ONC201 with hypomethylating agent 5-azacytidine in AML Citation[17]. ONC201 demonstrated synergistic reduction in cell viability in combination with 5-azacytidine that was confirmed by combination indices in AML cell lines HL-60 ( and Table S5), MOLM14 ( and Table S6) and MV411 ( and Table S7). We also tested the efficacy of dexamethasone Citation[13], as well as the proteasome inhibitors bortezomib Citation[18] and ixazomib Citation[19], in combination with ONC201. ONC201 synergistically reduced cell viability in combination with dexamethasone or ixazomib in RPMI-8226 MM cells (Figure S4, Table S8 and S9). ONC201 in combination with bortezomib synergistically reduced cell viability (, and Table S10) in KARPAS 299 ALCL cells and RPMI-8226 MM cells and improved apoptosis induction () in UPN2 MCL cells. ONC201 combined with bortezomib was evaluated in a Burkitt's lymphoma xenograft model. Histological evaluation of tumor xenografts revealed a reduced tumor cell density at 3–4 weeks and enhanced CHOP induction at 48 hours with combination treatment () compared to either agent alone. Tumors in the control group had predominantly viable tumor cells at 48 hours post-treatment. The monoagent groups showed minimal cell death with bortezomib alone and modest cell death with ONC201 as monoagent in clustered regions. In contrast, the combination group showed extensive global tumor necrosis with nuclear debris. The combination treatment did not result in body weight loss (). Thus, ONC201 is a promising combinatorial therapy that synergizes with approved leukemia, lymphoma and MM therapies.
Figure 5. ONC201 combines synergistically with 5-azacytidine in AML in vitro. Representative % cell viability curves at indicated concentrations and combination index (CI) versus fraction affected (Fa) plots for ONC201 and 5-azacytidine (5-aza), either as single agent or in combination (72 h) in (A) HL-60, (B) MOLM14 and (C) MV411 cells. CI < 1 indicates synergism, CI = 1 indicates additive effect and CI > 1 indicates antagonism. Raw data shown in Table S5, S6 and S7.
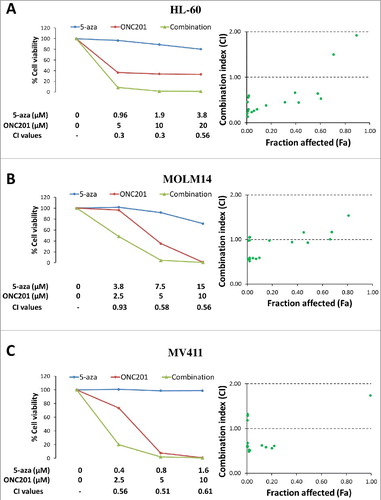
Figure 6. ONC201 combines synergistically with bortezomib in vitro and in vivo. (A) Representative % cell viability curves at indicated concentrations in KARPAS 299 ALCL cells and (B) combination index (CI) versus fraction affected (Fa) plot in RPMI-8226 MM cells, for ONC201 and bortezomib (BTZ) either as single agent or in combination (72 h). (C) % Apoptosis (Sub-G1 cells) in UPN2 MCL cells treated with ONC201 and BTZ at indicated concentrations either as single agent or in combination (72 h). (D) Representative H&E (40X) at end point 3–4 week's post-treatment initiation and CHOP (20X) staining 48 h post treatment initiation for ONC201 (100 mg/kg, weekly, intraperitoneal injection) and BTZ (0.3 mg/kg, twice weekly, intraperitoneal injection) either as single agent or in combination in a Ramos Burkitt's lymphoma subcutaneous xenograft model in SCID mice (7 mice per group). (E) Body weight (g) for mice in (D) at indicated days post treatment.
Discussion
ONC201 is a broad-spectrum anti-cancer agent that induces p53-independent apoptosis in solid tumors and hematological malignancies Citation[1]. ONC201 targets cancer cells via activation of the ATF4/CHOP-mediated ISR and inhibition of Akt/ERK that ultimately results in DR5/TRAIL-mediated apoptosis Citation[2-4]. ONC201 has demonstrated robust safety and clinical benefit in advanced solid tumors including objective response, metastatic tumor shrinkage and stable disease in recurrent glioblastoma, prostate and endometrial cancer patients Citation[7,8].
Preclinical studies have previously demonstrated ONC201 efficacy in acute leukemia, MM and lymphoma. In accordance with ONC201-mediated activation of the ISR that B cells are highly sensitive to, lymphoma and myeloma were identified as the most ONC201-sensitive tumor types in a >1000 cell line screen Citation[1]. ONC201 in vitro efficacy was confirmed in pediatric NHL cell lines that involved TRAIL- and caspase-dependent cell death Citation[20]. ONC201 has demonstrated in vivo efficacy with prolongation of survival in an Eµ-myc transgenic B-cell lymphoma model Citation[2]. ONC201 also demonstrated low micromolar efficacy in AML and MCL including apoptosis in patient-derived cells that are resistant to standard of care therapies and in vivo anti-leukemic stem cell activity Citation[4].
Our study demonstrates low micromolar in vitro activity of ONC201 in a broad panel of AML, ALL, CML, DLBCL, MCL, Burkitt's lymphoma, ALCL, CTCL, NS, MM cell lines and CLL, DLBCL, MCL primary samples. ONC201 induced apoptosis in MM cells resistant to standard of care dexamethasone. Pharmacokinetic results from the phase I study have shown that ONC201 can achieve the micromolar levels required for anti-cancer efficacy in our studies Citation[7]. Mechanistically, ONC201 induced caspase-dependent apoptosis that involved activation of the ISR (ATF4/CHOP) pathway, inhibition of Akt phosphorylation, Foxo3a activation, downregulation of cyclin D1, IAP and Bcl-2 family members. Thus, ONC201 demonstrates robust broad spectrum single agent efficacy in hematological malignancies.
The relatively short half-life, prolonged anti-cancer efficacy, unique mechanism of action, robust safety profile and oral infrequent dosing provide an opportunity to combine ONC201 with approved anti-cancer therapies in the clinic Citation[1]. ONC201 has been previously shown to combine synergistically with several FDA approved cancer therapies such as sorafenib Citation[21], gemcitabine Citation[22], taxanes and bevacizumab Citation[2]. ONC201 also synergizes with Bcl-2 inhibitors in leukemia, lymphoma Citation[4] and glioblastoma cells Citation[23]. Our results demonstrate that ONC201 was synergistic with approved leukemia therapies such as chemotherapy cytarabine Citation[24] and hypomethylating agent 5-azacytidine Citation[17]. Cytarabine and 5-azacytidine have been shown to enhance cytotoxicity in combination with ISR activation Citation[25,26], Akt inhibition Citation[27,28] and TRAIL Citation[29-31] providing a mechanistic rationale for synergy with ONC201. Within leukemia, AML represents a major unmet need with a 5-year survival rate of 26% Citation[32]. With no recent approvals, standard treatment does not exist for relapsed AML patients, and the prognosis is particularly worse for elderly patients that cannot tolerate chemotherapy Citation[33]. ONC201 could help improve clinical benefit in combination with cytarabine and 5-azacytidine without adding to the burden of toxicity. Additionally, we show that ONC201 synergizes with approved lymphoma and MM therapies such as dexamethasone Citation[1] and proteasome inhibitors bortezomib and ixazomib Citation[18,19]. Interestingly, ONC201 synergy with proteasome inhibitors appears to be a class effect that could be potentially explained by ISR activation via different mechanisms Citation[3,4]. ONC201 could potentially help achieve durable responses and improve survival in combination with approved lymphoma and MM therapies especially in relapse/refractory patients Citation[34,35]. Thus, ONC201 synergistically combines with approved leukemia, lymphoma and MM therapies in vitro and in vivo.
In summary, these results serve as a rationale for ongoing ONC201 single agent trials in relapsed/refractory acute leukemia (NCT02392572), NHL (NCT02420795), MM (NCT02609230) and combination trial with dexamethasone in relapsed/refractory MM (NCT02863991). Our findings suggest that ONC201 may be an important therapeutic option for patients with hematological malignancies who have developed resistance to approved therapies. Additionally, our results point to specific standard-of-care therapies that may be combined with ONC201 to exert synergistic anti-tumor activity without adding to the burden of toxicity.
Disclosure of Potential conflict of interest
V.V.P. and J.E.A. are employees and stockholders of Oncoceutics, Inc. W.O. and W.S.E-D. are co-founders and stockholders of Oncoceutics, Inc. W.S.E-D. is fully compliant with institutional disclosure requirements and conflict of interest rules.
Supplementary.rar
Download (3.7 MB)Acknowledgements
The work was presented in part at the 58th American Society of Hematology Annual Meeting (December 2016). W.S.E-D. is an American Cancer Society Research Professor.
Additional information
Funding
References
- Allen JE, Kline CLB, Prabhu VV, et al. Discovery and clinical introduction of first-in-class imipridone ONC201. Oncotarget. 2016.https://doi.org/10.18632/oncotarget.11814.
- Allen JE, Krigsfeld G, Mayes PA, et al. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci Transl Med. 2013;5(171):171ra17.https://doi.org/10.1126/scitranslmed.3004828. PMID:23390247
- Kline CL, Van den Heuvel APJ, Allen JE, et al. ONC201 kills solid tumor cells by triggering an integrated stress response dependent on ATF4 activation by specific eIF2alpha kinases. Sci Signal. 2016;9(415):ra18.https://doi.org/10.1126/scisignal.aac4374. PMID:26884600
- Ishizawa J, Kojima K, Chachad D, et al. ATF4 induction through an atypical integrated stress response to ONC201 triggers p53-independent apoptosis in hematological malignancies. Sci Signal. 2016;9(415):ra17.https://doi.org/10.1126/scisignal.aac4380. PMID:26884599
- Prabhu VV, Allen JE, Dicker DT, et al. Small-Molecule ONC201/TIC10 Targets Chemotherapy-Resistant Colorectal Cancer Stem-like Cells in an Akt/Foxo3a/TRAIL-Dependent Manner. Cancer Res. 2015;75(7):1423–1432.https://doi.org/10.1158/0008-5472.CAN-13-3451. PMID:25712124
- Prabhu VV, et al. ONC201 Depletes Cancer Stem Cells in Refractory Cancer Patient Samples. Blood. 2014;124(21):5219.
- Stein MN, Bertino JR, Kaufman HL, et al. First-in-human Clinical Trial of Oral ONC201 in Patients with Refractory Solid Tumors. Clin Cancer Res. 2017.https://doi.org/10.1158/1078-0432.CCR-16-2658.
- Arrillaga-Romany I, Chi AS, Allen JE, et al. A phase 2 study of the first imipridone ONC201, a selective DRD2 antagonist for oncology, administered every three weeks in recurrent glioblastoma. Oncotarget. 2017.https://doi.org/10.18632/oncotarget.17837.
- Matthew EM, Zhou L, Yang Z, et al. A multiplexed marker-based algorithm for diagnosis of carcinoma of unknown primary using circulating tumor cells. Oncotarget. 2016;7(4):3662–3676.https://doi.org/10.18632/oncotarget.6657. PMID:26695546
- Reed JC. Bcl-2-family proteins and hematologic malignancies: history and future prospects. Blood. 2008;111(7):3322–3330.https://doi.org/10.1182/blood-2007-09-078162. PMID:18362212
- Pluta A, Wierzbowska A, Cebula-Obrzut B, et al. Prognostic value of inhibitor of apoptosis protein family expression in patients with acute myeloid leukemia. Leuk Lymphoma. 2015;56(9):2529–2535.https://doi.org/10.3109/10428194.2014.1003052. PMID:25549803
- Fulda S. Inhibitor of apoptosis proteins in hematological malignancies. Leukemia. 2009;23(3):467–476.https://doi.org/10.1038/leu.2008.329. PMID:19039324
- Alexanian R, Dimopoulos MA, Delasalle K, et al. Primary dexamethasone treatment of multiple myeloma. Blood. 1992;80(4):887–890. PMID:1498331
- Ramakrishnan V, Painuly U, Kimlinger T, et al. Inhibitor of apoptosis proteins as therapeutic targets in multiple myeloma. Leukemia. 2014;28(7):1519–1528.https://doi.org/10.1038/leu.2014.2. PMID:24402161
- Desplanques G, Giuliani N, Delsignore R, et al. Impact of XIAP protein levels on the survival of myeloma cells. Haematologica. 2009;94(1):87–93.https://doi.org/10.3324/haematol.13483. PMID:19001278
- Ott MM, Bartkova J, Bartek J, et al. Cyclin D1 expression in mantle cell lymphoma is accompanied by downregulation of cyclin D3 and is not related to the proliferative activity. Blood. 1997;90(8):3154–3159. PMID:9376597
- Steensma DP. Can hypomethylating agents provide a platform for curative therapy in myelodysplastic syndromes? Best Pract Res Clin Haematol. 2012;25(4):443–451.https://doi.org/10.1016/j.beha.2012.10.007. PMID:23200541
- Teicher BA, Tomaszewski JE. Proteasome inhibitors. Biochem Pharmacol. 2015;96(1):1–9.https://doi.org/10.1016/j.bcp.2015.04.008. PMID:25935605
- Muz B, Ghazarian RN, Ou M, et al. Spotlight on ixazomib: potential in the treatment of multiple myeloma. Drug Des Devel Ther. 2016;10:217–226. PMID:26811670
- Talekar MK, Allen JE, Dickerand DT, et al. ONC201 induces cell death in pediatric non-Hodgkin's lymphoma cells. Cell Cycle. 2015;14(15):2422–2428.https://doi.org/10.1080/15384101.2015.1054086. PMID:26030065
- Allen JE, Prabhu VV, Talekar M, et al. Genetic and Pharmacological Screens Converge in Identifying FLIP, BCL2, and IAP Proteins as Key Regulators of Sensitivity to the TRAIL-Inducing Anticancer Agent ONC201/TIC10. Cancer Res. 2015;75(8):1668–1674.https://doi.org/10.1158/0008-5472.CAN-14-2356. PMID:25681273
- Zhang Q, Wang H, Ran L, et al. The preclinical evaluation of TIC10/ONC201 as an anti-pancreatic cancer agent. Biochem Biophys Res Commun. 2016;476(4):260–266.https://doi.org/10.1016/j.bbrc.2016.05.106. PMID:27233611
- Karpel-Massler G, Bâ M, Shu C, et al. TIC10/ONC201 synergizes with Bcl-2/Bcl-xL inhibition in glioblastoma by suppression of Mcl-1 and its binding partners in vitro and in vivo. Oncotarget. 2015;6(34):36456–36471. PMID:26474387
- Kremer WB. Drugs five years later: cytarabine. Ann Intern Med. 1975;82(5):684–688.https://doi.org/10.7326/0003-4819-82-5-684. PMID:1056159
- Weigert O, Pastore A, Rieken M, et al. Sequence-dependent synergy of the proteasome inhibitor bortezomib and cytarabine in mantle cell lymphoma. Leukemia. 2007;21(3):524–528.https://doi.org/10.1038/sj.leu.2404511. PMID:17268531
- Kiziltepe T, Hideshima T, Catley L, et al. 5-Azacytidine, a DNA methyltransferase inhibitor, induces ATR-mediated DNA double-strand break responses, apoptosis, and synergistic cytotoxicity with doxorubicin and bortezomib against multiple myeloma cells. Mol Cancer Ther. 2007;6(6):1718–1727.https://doi.org/10.1158/1535-7163.MCT-07-0010. PMID:17575103
- Meja K, Stengel C, Sellar R, et al. PIM and AKT kinase inhibitors show synergistic cytotoxicity in acute myeloid leukaemia that is associated with convergence on mTOR and MCL1 pathways. Br J Haematol. 2014;167(1):69–79.https://doi.org/10.1111/bjh.13013. PMID:24975213
- Mao M, Tian F, Mariadason JM, et al. Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents. Clin Cancer Res. 2013;19(3):657–667.https://doi.org/10.1158/1078-0432.CCR-11-1446. PMID:23251002
- Taylor DJ, Parsons CE, Han H, et al. Parallel screening of FDA-approved antineoplastic drugs for identifying sensitizers of TRAIL-induced apoptosis in cancer cells. BMC Cancer. 2011;11:470.https://doi.org/10.1186/1471-2407-11-470. PMID:22044796
- Kaminskyy VO, Surova OV, Vaculova A, et al. Combined inhibition of DNA methyltransferase and histone deacetylase restores caspase-8 expression and sensitizes SCLC cells to TRAIL. Carcinogenesis, 2011;32(10):1450–1458.https://doi.org/10.1093/carcin/bgr135. PMID:21771726
- Eramo A, Pallini R, Lotti F, et al. Inhibition of DNA methylation sensitizes glioblastoma for tumor necrosis factor-related apoptosis-inducing ligand-mediated destruction. Cancer Res. 2005;65(24):11469–11477.https://doi.org/10.1158/0008-5472.CAN-05-1724. PMID:16357155
- Orozco JJ, Appelbaum FR. Unfavorable, complex, and monosomal karyotypes: the most challenging forms of acute myeloid leukemia. Oncology (Williston Park). 2012. 26(8):706–712. PMID:22957403
- Takahashi K, Kantarjian H, Garcia-Manero G, et al. Clofarabine Plus Low-Dose Cytarabine Is as Effective as and Less Toxic Than Intensive Chemotherapy in Elderly AML Patients. Clin Lymphoma Myeloma Leuk. 2016;16(3):163–168 e1–2.https://doi.org/10.1016/j.clml.2015.11.016.
- Goy A, Bernstein SH, Kahl BS, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Ann Oncol. 2009;20(3):520–525.https://doi.org/10.1093/annonc/mdn656. PMID:19074748
- Raza S, Safyan RA, Rosenboum E et al. Optimizing current and emerging therapies in multiple myeloma: a guide for the hematologist. Ther Adv Hematol. 2017;8(2):55–70.https://doi.org/10.1177/2040620716680548. PMID:28203342
