Meiosis is a specialized cell division that reduces the chromosome number of germ cells by exactly half, thereby ensuring that gametes have precisely half the complement of chromosomes as somatic cells. This reduction in ploidy is achieved through the coupling of a single round of DNA replication with two tandem nuclear divisions, meiosis I and meiosis II. During meiosis I, homologs (i.e., chromosomes of different parental origin) are separated, whereas in meiosis II, sister chromatids are separated. Since the separation of sister chromatids also occurs in mitosis, it is the separation of homologs that makes meiosis unique. Ironically, the correct separation of homologs starts with their pairing.
In early prophase I, a proteinaceous macromolecule known as the synaptonemal complex (SC) begins to form. The SC facilitates the pairing of homologs by adhering them along their entire lengths, leading to the formation of recombination intermediates between homologs. As cells exit prophase I, the SC abruptly disassembles and the recombination intermediates are converted into crossovers. Although the SC is essential for efficient crossing over, if left intact until anaphase I, it would oppose the microtubule forces responsible for separating homologs, potentially resulting in severe chromosomal nondisjunction. Despite this, and in contrast to SC formation, relatively little is known about SC disassembly [Citation1].
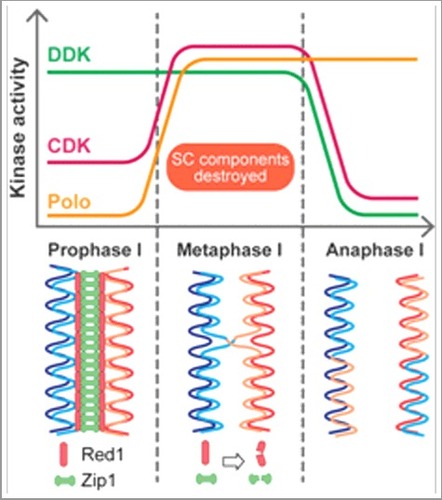
Exit from prophase I in budding yeast is achieved through upregulation of Ndt80, the master transcription factor of meiosis. From the hundreds of genes that Ndt80 upregulates, the only gene product essential for SC destruction is Polo-like kinase (Polo) [Citation2]. We recently reported that, in addition to Polo, Dbf4-dependent Cdc7 kinase (DDK) and cyclin-dependent kinase (CDK) play important roles in regulating SC disassembly [Citation3]. The efficiency of SC disassembly, as determined by immunofluorescence microscopy, was found to show a strong positive correlation with DDK-Polo interaction strength. Examination of whole cell extracts revealed that SC disassembly correlated with a drastic decline in the levels of major SC components, indicating that the disassembly mechanism mediated by DDK and Polo involves protein destruction. Moreover, upon depletion of Dbf4 or Cdc7, which comprise the regulatory and catalytic subunits of DDK, respectively, Polo-driven SC destruction became inefficient. Similarly, inactivation of CDK greatly hindered SC destruction. However, in both cases, SC destruction still occurred, albeit inefficiently. These findings highlighted a coordinated effort by three fundamental cell cycle kinases in promoting SC destruction.
Whereas DDK activity is high in prophase I, Polo levels are relatively low until exit from prophase I. In parallel, CDK activity is also enhanced through the upregulation of cyclins. Thus, the three kinases have distinct activity profiles during meiosis. However, DDK activity is swiftly ablated at the completion of metaphase I through anaphase-promoting complex/cyclosome-mediated destruction of Dbf4, effectively creating a narrow window of time in the cell cycle when the activities of all three kinases coincide (). It is within this timeframe that the three kinases collaborate to destroy the SC. At the molecular level, Polo and CDK collaboratively phosphorylate Dbf4 at the prophase I-metaphase I transition; this phosphorylation is important for efficient SC destruction [Citation4]. Thus, Dbf4 serves as the hub of a phosphorylation-based signalling network involving DDK, Polo, and CDK. This phosphorylation then triggers a downstream cascade to promote Polo-mediated SC destruction. This could potentially involve direct phosphorylation of Polo by CDK, which has been shown to promote Polo activity in mitosis [Citation4]. We favour the idea that at least one of these kinases directly phosphorylates SC components. Consistent with this hypothesis, it has been demonstrated that partially purified DDK can phosphorylate SC components in vitro [Citation5]. Moreover, DDK, Polo and CDK are members of a cell cycle-regulated protein destruction system that divides replication initiation into two distinct phases of origin licensing and firing [Citation6]. Such a phospho-degron system is likely to be involved in the destruction of SC components.
Figure 1. The activities of fundamental cell cycle kinases overlap during the prophase I-metaphase I transition, leading to efficient destruction of SC proteins Red1 and Zip1 before anaphase I. See the text for more details.
Historically, DDK has a well established role in DNA replication, but more recently, it was shown to play critical roles in promoting meiotic DSB formation in prophase I of meiosis [Citation7]. In contrast, Polo was known to function primarily during anaphase to promote the destruction of sister chromatid cohesion. We have now provided compelling evidence that Polo-driven SC destruction at the prophase I-metaphase I transition is coordinated by DDK via a direct interaction with, and phosphorylation of, Dbf4. In addition to providing insight into the regulation of SC destruction, these findings allow us to attach a clear functional significance to the DDK-Polo interaction, which has remained enigmatic since its discovery. It is possible that this DDK-Polo axis, along with CDK, is also important for events that occur within the same time frame, such as crossover maturation and establishment of sister kinetochore mono-orientation [Citation2,Citation7]. It remains a high priority to determine whether this physical and functional interaction is evolutionarily conserved. The precise interplay between these highly conserved cell cycle kinases should be the subject of future research, especially when considering the link between their dysregulation and human disorders.
Disclosure of potential conflicts of interest
Authors declare no conflict of interest.
References
- Tsubouchi H, Argunhan B, Tsubouchi T. Shaping meiotic chromosomes with SUMO: a feedback loop controls the assembly of the synaptonemal complex in budding yeast. Microbial Cell. 2016;3:126–128 doi:10.15698/mic2016.03.486. PMID:28357343
- Sourirajan A, Lichten M. Polo-like kinase Cdc5 drives exit from pachytene during budding yeast meiosis. Genes Dev. 2008;22:2627–2632 doi:10.1101/gad.1711408. PMID:18832066
- Argunhan B, Leung WK, Afshar N, et al. Fundamental cell cycle kinases collaborate to ensure timely destruction of the synaptonemal complex during meiosis. Embo J. 2017;36:2488–2509. doi:10.15252/embj.201695895. PMID: 28694245
- Mortensen EM, Haas W, Gygi M, et al. Cdc28-dependent regulation of the Cdc5/Polo kinase. Curr Biol. 2005;15:2033–2037. doi:10.1016/j.cub.2005.10.046. PMID:16303563
- Chen X, Suhandynata RT, Sandhu R, et al. Phosphorylation of the synaptonemal complex protein Zip1 regulates the crossover/noncrossver decision during yeast meiosis. PLOS Biol. 2015;13:e1002329. doi:10.1371/journal.pbio.1002329. PMID:26682552
- Reuswigg KU, Zimmerman F, Galanti L, et al. Robust replication control is generated by temporal gaps between licensing and firing phases and depends on degradation of firing factor Sld2. Cell Reports. 2016;17:556–569. doi:10.1016/j.celrep.2016.09.013. PMID:27705801
- Matos J, Lipp JJ, Bogdanova A, et al. Dbf4-dependent Cdc7 kinase links DNA replication to the segregation of homologous chromosomes in meiosis I. Cell. 2008;135:662–678. doi:10.1016/j.cell.2008.10.026. PMID:19013276
