Autophagy is a cellular process by which cytosolic constituents are sequestered in a double-membrane, the phagophore, which elongates to form a membrane bound autophagosome. After fusion with lysosomes, the content of the autolysosome is degraded. Under stress conditions, autophagy can serve as a survival mechanism through the non-selective degradation of cytosolic constituents for recycling. In tumors and tumor microenvironment, autophagy is induced by metabolic stress. While the role of autophagy in the biology of cancer cells has been abundantly discussed [Citation1], its function in tumor-infiltrating immune cells has received less attention. In particular, the putative contribution of autophagy to the differentiation of CD4 T cells, an essential event that shapes adaptive immune responses, is incompletely understood. How can autophagy affect CD4 T cell fate? What are the consequences of this biological process for anticancer immune responses?
Early investigations revealed that T cell receptor stimulation induces autophagy, which contributes to T cell survival and antigen-dependent proliferation. Li et al. accordingly detected autophagosomes in activated TH1 and TH2 effector cells that were differentiated in vitro from naive T cells [Citation2]. More recently, Wei et al. found that autophagy controlled the survival and stability of regulatory T cells [Citation3]. However, the contribution of autophagy to the differentiation of effector T cells from naïve T cells remains unclear.
Our study showed that while autophagy failed to modulate TH1, TH2 and TH17 cell differentiation, it markedly dampened IL-9 secretion from TH9 cells [Citation4]. Given that we and others previously ascribed potent anticancer effects to TH9 cells [Citation5], this finding suggested that autophagy could restrict TH9 cell anticancer properties. We explored the mechanisms responsible for these observations and found that PU.1, the master TH9 cell transcription factor, is recruited by p62 to be degraded through selective autophagy (). In line with this, TH9 cells treated with chloroquine, which inhibits lysosomal function and autophagic degradation, have increased IL-9 secretion and anticancer effects. We eventually showed that genetic autophagy inhibition in T cells leads to IL-9-dependent inhibition of tumor growth in mice bearing MC38 colon cancer and B16 melanoma [Citation4].
Figure 1. Selective autophagy prevents TH9 cell differentiation. During TH9 cell differentiation ubiquitinated (Ub) PU.1 is recruited by p62, which recognizes K63 ubiquitination through its UBA domain. PU.1 is then degraded by selective autophagy, which leads to reduced IL-9 expression and anticancer immune responses in the tumor microenvironment. Chloroquine treatment, which inhibits lysosomal functions, prevents PU.1 degradation and drives enhanced TH9 cell-derived IL-9 secretion and anticancer effects.
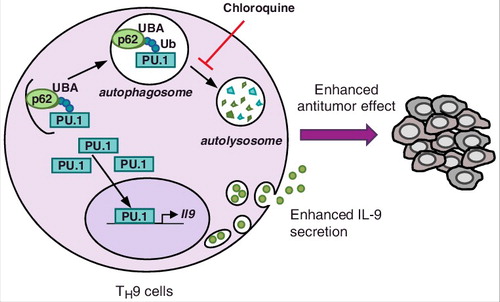
While these results lend further support to the anticancer functions played by TH9 cells, they suggest that the induction of autophagy in tumor microenvironment represses TH9 cell development and/or function. These findings are actually in line with previous studies indicating low frequencies of TH9 cells in mouse and human tumor lesions [Citation6]. The blockade of autophagy in vivo may thus restore anticancer immunity not only by disrupting the stability of regulatory T cells but also by promoting TH9 cell-dependent anticancer immune responses.
Beyond their activity in cancer, TH9 cells can promote tissue inflammation. Indeed, they have first been described to induce colitis in mice [Citation7], and subsequent studies showed that IL-9 expression is significantly higher in patients with inflammatory bowel disease. Conversely, autophagy can downregulate inflammation, as illustrated by the promotion of intestinal inflammation in the absence of autophagy. Our findings thus raise the hypothesis that autophagy possibly controls inflammation by repressing TH9 cell inflammatory properties.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- White E. The role for autophagy in cancer. J Clin Invest. 2015;125(1):42–46. doi:10.1172/JCI73941. PMID:25654549.
- Li C, Capan E, Zhao Y, et al. Autophagy is induced in CD4+ T cells and important for the growth factor-withdrawal cell death. J Immunol. 2006;177(8):5163–5168. doi:10.4049/jimmunol.177.8.5163. PMID:17015701.
- Wei J, Long L, Yang K, et al. Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat Immunol. 2016;17:277–285. doi:10.1038/ni.3365. PMID:26808230.
- Rivera Vargas T, Cai Z, Shen Y, et al. Selective degradation of PU.1 during autophagy represses the differentiation and antitumour activity of TH9 cells. Nat Commun. 2017;8(1):559. doi:10.1038/s41467-017-00468-w. PMID:28916785.
- Végran F, Berger H, Boidot R, et al. The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells. Nat Immunol. 2014;15(8):758–766. doi:10.1038/ni.2925. PMID:24973819.
- Purwar R, Schlapbach C, Xiao S, et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med. 2012;18:1248–1253. doi:10.1038/nm.2856. PMID: 22772464.
- Dardalhon V, Awasthi A, Kwon H, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol. 2008;9(12):1347–1355. doi:10.1038/ni.1677. PMID:18997793.
