ABSTRACT
Doxorubicin (DOX) is often first-line treatment of undifferentiated/unclassified soft tissue sarcoma (USTS). However, the DOX response rate for USTS patients is low. Individualized precision-medicine technology that could identify DOX responders as well as non-responders would be of high value to cancer patients. In the present study, we established 5 patient-derived orthotopic xenograft (PDOX) nude mouse models from 5 USTS patients and evaluated the efficacy of DOX in each PDOX model. USTS's were grown orthotopically in the right thigh of nude mice to establish the PDOX models. Two weeks after implantation, the mouse models were randomized into two groups of 8 mice each: untreated control; and DOX (3 mg/kg, i.p., once a week for 2 weeks). DOX showed significant growth inhibition in only 2 USTS PDOX models out of 5 (p = 0.0054, p = 0.0055, respectively) on day 14 after initiation. DOX was ineffective in the other 3 PDOX models. However, even in the DOX-sensitive cases, DOX could not regress the PDOX tumors responding to treatment. The present study has important implications since this is the first in vivo study to compare the DOX sensitivity for USTS on multiple patient tumors. We showed that only two of five USTS were responsive to DOX, despite DOX being first line chemotherapy for USTS. The 3 resistant cases should not be treated with DOX clinically, in order to spare the patients' unnecessary toxicity. This PDOX model is useful for precise individualized drug sensitivity testing, especially for rare heterogeneous recalcitrant sarcomas such as USTS.
Introduction
Soft tissue sarcoma (STS) is a heterogeneous group of neoplasms comprising more than 50 subtypes. Undifferentiated/unclassified soft-tissue sarcoma (USTS) is described in the fourth edition of the World Health Organization (WHO) Classification of Tumors of Soft Tissue and Bone in 2013 [Citation1]. USTS is the most common type of STS observed in adults [Citation2–4].
Surgical resection remains the only curative option for USTS which often metastasizes and then is not a candidate for surgery [Citation5–7]. Anthracycline-based chemotherapy, including doxorubicin (DOX), is first-line treatment of USTS, but the DOX response rate for USTS patients is low [Citation8,Citation9]. The use of DOX for USTS is controversial since the disease is heterogeneous. Therefore, novel more effective individualized and precision treatment is necessary for USTS.
Toward this goal, our laboratory pioneered the patient-derived orthotopic xenograft (PDOX) nude mouse model with the technique of surgical orthotopic implantation (SOI), including breast [Citation10], ovarian [Citation11], lung [Citation12], cervical [Citation13,Citation14], colon [Citation15–17], stomach [Citation18], pancreatic [Citation19–23], melanoma [Citation24–28], and sarcoma [Citation29–38]. The PDOX model, developed by our laboratory over the past 30 years, has many advantages over subcutaneous-transplant models which are growing ectopically under the skin [Citation39].
In the present study, we established PDOX nude-mouse models with USTS from 5 patients and evaluated DOX efficacy for each patient.
Results and discussion
USTS PDOX nude mouse models were established from 5 patients. Patient characteristics from 5 patients are shown in . The median age was 60 years (range 56–65). Three male and 2 female patients were included. Tumor presentation was 3 primary and 2 recurrent cases.
Table 1. Patients characteristics.
DOX showed significant growth inhibition in only 2 PDOX models (p = 0.0054, p = 0.0055, respectively) and resistance in 3 on day 14 after treatment initiation ( and ).
Figure 1. DOX sensitivity of 5 different USTS PDOX models. Bar graphs show relative tumor volume at post-treatment relative to pre-treatment tumor volume. Error bars: ± SD.
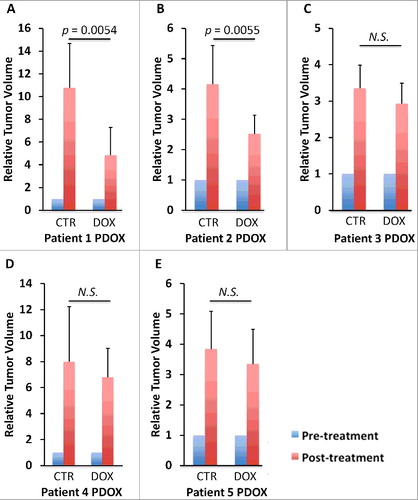
Figure 2. Treated and untreated PDOX tumors. Tumors from USTS PDOX models of patients 1 and 4 are shown before and after treatment with DOX. Scale bar: 5 mm.
The body weight on day 14, compared with day 0, did not significantly differ between control and treatment group in each PDOX model (). There were no animal deaths in any group.
Figure 3. Effect of each treatment on mouse body weight. Bar graphs shows mouse body weight in each mouse at pre- and post-treatment.
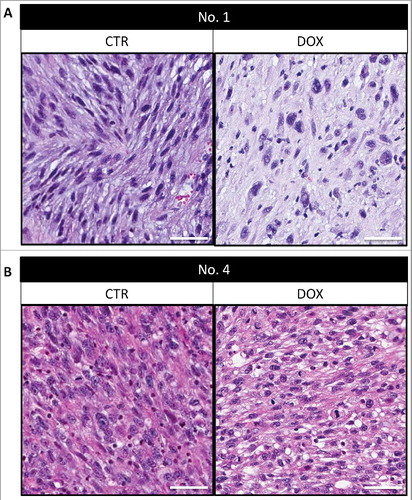
Histologically, untreated control tumors comprised mostly viable cells. In DOX-sensitive tumor No. 1 described in , some necrosis and fibrosis were observed after DOX treatment (A). In contrast, in DOX-resistant tumor No. 4, there were no notable changes after DOX treatment compared to the control (B).
Figure 4. Tumor histology. Tumors from PDOX models 1 and 4 are shown both untreated and after treatment. Scale bars: 50 μm.
In the present study, DOX could inhibit tumor growth in only 2 of 5 PDOX models. The other 3 PDOX models were resistant. In addition, even in the sensitive cases, there was no tumor regression. In such cases, a second drug to effectively combine with DOX could possibly be identified. Future experiments will focus on this goal. The present results demonstrate the urgent need for individualized, precision treatment of USTS. The DOX-resistant PDOX cases should not receive DOX therapy in the clinic. The probable result would be toxicity without efficacy. In the current study, recurrent tumors were both DOX-sensitive and- resistant. Therefore, the PDOX model can be used for precise individualized drug-sensitivity testing, especially for rare recalcitrant heterogeneous sarcomas [Citation29–38].
DOX resistance has been reportedly due to a number of different cellular changes including: overexpression of efflux transporters, reduction of α topoisomerase II (TOP2A) [Citation40,Citation41], p53 mutation [Citation42], activation of NF-κB [Citation43], elevated FOXO3 [Citation44], activation of the PI3K/Akt pathway [Citation45], activation of the MEK/ERK pathway [Citation46], as well as other possible mechanisms, including DNA repair.
Previously-developed concepts and strategies of highly-selective tumor targeting can take advantage of molecular targeting of tumors, including tissue-selective therapy which focuses on unique differences between normal and tumor tissues [Citation47–52].
Conclusion
The present study has demonstrated significant differences of DOX sensitivity of 5 different USTS PDOX models. These results demonstrate the powerful potential of the PDOX model for precision oncology and suggest that patients with failed DOX therapy could possibly benefit from drugs shown to be active against PDOX models of their tumor.
Materials and methods
Mice
Athymic nu/nu nude mice (AntiCancer Inc., San Diego, CA), 4–6 weeks old, were used in this study. Animals were housed in a barrier facility on a high efficacy particulate arrestance (HEPA)-filtered rack under standard conditions of 12-hour light/dark cycles. The animals were fed an autoclaved laboratory rodent diet. All mouse surgical procedures and imaging were performed with the animals anesthetized by subcutaneous injection of a ketamine mixture (0.02 ml solution of 20 mg/kg ketamine, 15.2 mg/kg xylazine, and 0.48 mg/kg acepromazine maleate). The response of animals during surgery was monitored to ensure adequate depth of anesthesia. The animals were observed on a daily basis and humanely sacrificed by CO2 inhalation if they met the following humane endpoint criteria: severe tumor burden (more than 20 mm in diameter), prostration, significant body weight loss, difficulty breathing, rotational motion and body temperature drop. All animal studies were conducted in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Animals under Assurance Number A3873-1.
Patient-derived tumors
The tumors diagnosed with USTS were resected in the Department of Surgery, University of California, Los Angeles (UCLA). Undifferentiated soft tissue sarcomas include those that show no specific identifiable line of differentiation by currently-available methods including immunohistochemistry, special stains, and cytogenetic or molecular-pathology techniques. Written informed consent was provided by each patient. The Institutional Review Board (IRB) of UCLA approved this experiment [Citation25,Citation26]. Of the 2 relapsed patients, one failed adjuvant chemotherapy and the other was untreated. Patient no. 2, who relapsed, previously failed pazopanib, then received gemcitabine and docetaxel, where the patient's disease was stabilized, then underwent tumor resection which was used to construct the PDOX model. Patient no. 4 received DOX, ifosfamide and MESNA, followed by radiation. However, the tumor continued to grow and the patient underwent tumor resection from which her PDOX was constructed. The patient subsequently succumbed to her disease.
Establishment of PDOX models of USTS by surgical orthotopic implantation (SOI)
Fresh samples of the USTS of the patients were obtained and transported immediately to the laboratory at AntiCancer, Inc., on wet ice. The samples were cut into 5-mm fragments and implanted subcutaneously in nude mice. After the subcutaneously-implanted tumors grew to more than 10 mm in diameter, the tumors were then harvested and cut into small fragments (3 mm3). After nude mice were anesthetized with the ketamine solution described above, a 5-mm skin incision was made on the right high thigh, then the biceps femoris was split to make space. A single tumor fragment was implanted orthotopically into the space to establish the PDOX model. The wound was closed with a 6-0 nylon suture (Ethilon, Ethicon, Inc., NJ, USA).
Treatment study design in the USTS PDOX model
PDOX mouse models were randomized into two groups of 8 mice each: untreated control (n = 8); and DOX (3 mg/kg, i.p., once a week for 2 weeks, n = 8). Tumor length and width were measured both pre- and post-treatment. Tumor volume was calculated with the following formula: Tumor volume (mm3) = length (mm) × width (mm) × width (mm) × 1/2. Data are presented as mean ± SD. The tumor volume ratio is defined as the tumor volume at post-treatment point relative to pre-treatment tumor volume.
Histological examination
Fresh tumor samples were fixed in 10% formalin and embedded in paraffin before sectioning and staining. Tissue sections (5 μm) were deparaffinized in xylene and rehydrated in an ethanol series. Hematoxylin and eosin (H&E) staining was performed according to standard protocols. Histological examination was performed with a BHS System Microscope (Olympus Corporation, Tokyo, Japan). Images were acquired with INFINITY ANALYZE software (Lumenera Corporation, Ottawa, Canada) [Citation25,Citation26].
Statistical analysis
JMP version 11.0 was used for all statistical analyses. Significant differences for continuous variables were determined using the Mann-Whitney U test. Bar graphs expressed average values and error bar showed SD. A probability value of P ≤ 0.05 was considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study was supported in part by National Cancer Institute grant CA213649.
Additional information
Funding
References
- Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46:95–104. doi:10.1097/PAT.0000000000000050. PMID:24378391
- Poremba C. Soft tissue sarcomas: the role of histology and molecular pathology for differential diagnosis. Verh Dtsch Ges Pathol. 2006;90:59–72. PMID:17867581
- Al-Agha OM, Igbokwe AA. Malignant fibrous histiocytoma: between the past and the present. Arch Pathol Lab Med. 2008;132:1030–1035. PMID:18517265
- Matushansky I, Charytonowicz E, Mills J, et al. MFH classification: differentiating undifferentiated pleomorphic sarcoma in the 21st Century. Expert Rev Anticancer Ther. 2009;9:1135–1144. doi:10.1586/era.09.76. PMID:19671033
- Spira AI, Ettinger DS. The use of chemotherapy in soft-tissue sarcomas. Oncologist. 2002;7:348–359. doi:10.1634/theoncologist.7-4-348. PMID:12185297
- Grimer R, Judson I, Peake D, et al. Guidelines for the management of soft tissue sarcomas. Sarcoma. 2010;2010:506182. PMID:20634933
- Italiano A, Mathoulin-Pelissier S, Cesne AL, et al. Trends in survival for patients with metastatic soft-tissue sarcoma. Cancer. 2011;117:1049–1054. doi:10.1002/cncr.25538. PMID:20945333
- Casali PG, Blay JY, ESMO/CONTICANET/EUROBONET Consensus Panel of experts. Soft tissue sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):198–203. doi:10.1093/annonc/mdq209.
- Leahy M, Garcia Del Muro X, Reichardt P, et al. Chemotherapy treatment patterns and clinical outcomes in patients with metastatic soft tissue sarcoma. The SArcoma treatment and Burden of Illness in North America and Europe (SABINE) study. Ann Oncol. 2012;23:2763–2770. doi:10.1093/annonc/mds070. PMID:22492696
- Fu X, Le P, Hoffman RM. A metastatic-orthotopic transplant nude-mouse model of human patient breast cancer. Anticancer Res. 1993;13:901–904. PMID:8352558
- Fu X, Hoffman RM. Human ovarian carcinoma metastatic models constructed in nude mice by orthotopic transplantation of histologically-intact patient specimens. Anticancer Res. 1993;13:283–286. PMID:8517640
- Wang X, Fu X, Hoffman RM. A new patient-like metastatic model of human lung cancer constructed orthotopically with intact tissue via thoracotomy in immunodeficient mice. Int J Cancer. 1992;51:992–995. doi:10.1002/ijc.2910510626. PMID:1639545
- Hiroshima Y, Zhang Y, Zhang N, et al. Establishment of a patient-derived orthotopic xenograph (PDOX) model of HER-2-positive cervical cancer expressing the clinical metastatic pattern. PLoS One. 2015;10:e0117417. doi:10.1371/journal.pone.0117417. PMID:25689852
- Murakami T, Kiyuna T, Kawaguchi K, et al. The irony of highly-effective bacterial therapy of a patient-derived orthotopic xenograft (PDOX) model of Ewing's sarcoma, which was blocked by Ewing himself 80 years ago. Cell Cycle. 2017;16:1046–1052. doi:10.1080/15384101.2017.1304340. PMID:28296559
- Hiroshima Y, Maawy A, Metildi CA, et al. Successful fluorescence-guided surgery on human colon cancer patient-derived orthotopic xenograft mouse models using a fluorophore-conjugated anti-CEA antibody and a portable imaging system. J Laparoendosc Adv Surg Tech A. 2014;24:241–247. doi:10.1089/lap.2013.0418. PMID:24494971
- Fu X, Besterman JM, Monosov A, et al. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc Natl Acad Sci USA. 1991;88:9345–9349. doi:10.1073/pnas.88.20.9345. PMID:1924398
- Metildi CA, Kaushal S, Luiken GA, et al. Fluorescently-labeled chimeric anti-CEA antibody improves detection and resection of human colon cancer in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J Surg Oncol. 2014;109:451–458. doi:10.1002/jso.23507. PMID:24249594
- Furukawa T, Kubota T, Watanabe M, et al. Orthotopic transplantation of histologically intact clinical specimens of stomach cancer to nude mice: correlation of metastatic sites in mouse and individual patient donors. Int J Cancer. 1993;53:608–612. doi:10.1002/ijc.2910530414. PMID:8436434
- Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci USA. 1992;89:5645–5649. doi:10.1073/pnas.89.12.5645. PMID:1608975
- Kawaguchi K, Igarashi K, Murakami T, et al. MEK inhibitors cobimetinib and trametinib, regressed a gemcitabine-resistant pancreatic cancer patient-derived orthotopic xenograft (PDOX). Oncotarget. 2017;8:47490–47496. doi:10.18632/oncotarget.17667. PMID:28537897
- Hiroshima Y, Zhang Y, Murakami T, et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R in combination with anti-angiogenesis therapy on a pancreatic cancer patient-derived orthotopic xenograph (PDOX) and cell line mouse models. Oncotarget. 2014;5:12346–12357. doi:10.18632/oncotarget.2641. PMID:25402324
- Hiroshima Y, Maawy A, Zhang Y, et al. Metastatic recurrence in a pancreatic cancer patient derived orthotopic xenograft (PDOX) nude mouse model is inhibited by neoadjuvant chemotherapy in combination with fluorescence-guided surgery with an anti-CA 19–9-conjugated fluorophore. PLoS One. 2014;9:e114310. doi:10.1371/journal.pone.0114310. PMID:25463150
- Hiroshima Y, Maawy AA, Katz MH, et al. Selective efficacy of zoledronic acid on metastasis in a patient-derived orthotopic xenograph (PDOX) nude-mouse model of human pancreatic cancer. J Surg Oncol. 2015;111:311–315. doi:10.1002/jso.23816. PMID:25394368
- Yamamoto M, Zhao M, Hiroshima Y, et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R on a melanoma patient-derived orthotopic xenograft (PDOX) nude-mouse model. PLoS One. 2016;11:e0160882. doi:10.1371/journal.pone.0160882. PMID:27500926
- Kawaguchi K, Murakami T, Chmielowski B, et al. Vemurafenib-resistant BRAF-V600E mutated melanoma is regressed by MEK targeting drug trametinib, but not cobimetinib in a patient-derived orthotopic xenograft (PDOX) mouse model. Oncotarget. 2016;7:71737–71743. PMID:27690220
- Kawaguchi K, Igarashi K, Murakami T, et al. Tumor-targeting Salmonella typhimurium A1-R combined with Temozolomide regresses malignant melanoma with a BRAF-V600 mutation in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget. 2016;7:85929–85936. PMID:27835903
- Kawaguchi K, Igarashi K, Murakami T, et al. Salmonella typhimurium A1-R targeting of a chemotherapy resistant BRAF-V600E melanoma in a patient-derived orthotopic xenograft (PDOX) model is enhanced in combination with either vemurafenib or temozlomide. Cell Cycle. 2017;16:1288–1294. doi:10.1080/15384101.2017.1314420. PMID:28622068
- Kawaguchi K, Igarashi K, Murakami T, et al. Tumor-targeting Salmonella typhimurium A1-R sensitizes melanoma with a BRAF-V600E mutation to vemurafenib in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J Cell Biochem. 2017;118:2314–2319. doi:10.1002/jcb.25886. PMID:28106277
- Murakami T, Singh AS, Kiyuna T, et al. Effective molecular targeting of CDK4/6 and IGF-1R in a rare FUS-ERG fusion CDKN2A-deletion doxorubicin-resistant Ewing's sarcoma patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget. 2016;7:47556–47564. doi:10.18632/oncotarget.9879. PMID:27286459
- Murakami T, DeLong J, Eilber FC, et al. Tumor-targeting Salmonella typhimurium A1-R in combination with doxorubicin eradicate soft tissue sarcoma in a patient-derived orthotopic xenograft PDOX model. Oncotarget. 2016;7:12783–12790. doi:10.18632/oncotarget.7226. PMID:26859573
- Murakami T, Li S, Han Q, et al. Recombinant methioninase effectively targets a Ewing's sarcoma in a patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget. 2017;8:35630–35638. doi:10.18632/oncotarget.15823. PMID:28404944
- Igarashi K, Kawaguchi K, Murakami T, et al. High efficacy of pazopanib on an undifferentiated spindle-cell sarcoma resistant to first-line therapy is identified with a patient-derived orthotopic xenograft (PDOX) nude mouse model. J Cell Biochem. 2017;118:2739–2743. doi:10.1002/jcb.25923. PMID:28176365
- Igarashi K, Kawaguchi K, Kiyuna T, et al. Temozolomide combined with irinotecan caused regression in an adult pleomorphic rhabdomyosarcoma patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget. 2017;8:75874–75880. doi:10.18632/oncotarget.16548. PMID:29100276
- Igarashi K, Kawaguchi K, Kiyuna T, et al. Patient-derived orthotopic xenograft (PDOX) mouse model of adult rhabdomyosarcoma invades and recurs after resection in contrast to the subcutaneous ectopic model. Cell Cycle. 2017;16:91–94. doi:10.1080/15384101.2016.1252885. PMID:27830986
- Igarashi K, Kawaguchi K, Murakami T, et al. Intra-arterial administration of tumor-targeting Salmonella typhimurium A1-R regresses a cisplatin-resistant relapsed osteosarcoma in a patient-derived orthotopic xenograft (PDOX) mouse model. Cell Cycle. 2017;16:1164–1170. doi:10.1080/15384101.2017.1317417. PMID:28494180
- Hiroshima Y, Zhang Y, Zhang N, et al. Patient-derived orthotopic xenograft (PDOX) nude mouse model of soft-tissue sarcoma more closely mimics the patient behavior in contrast to the subcutaneous ectopic model. Anticancer Res. 2015;35:697–701. PMID:25667448
- Kawaguchi K, Igarashi K, Murakami T, et al. Combination of gemcitabine and docetaxel regresses both gastric leiomyosarcoma proliferation and invasion in an imageable patient-derived orthotopic xenograft (iPDOX) model. Cell Cycle. 2017;16:1063–1069. doi:10.1080/15384101.2017.1314406. PMID:28426279
- Kiyuna T, Murakami T, Tome Y, et al. High efficacy of tumor-targeting Salmonella typhimurium A1-R on a doxorubicin- and dactolisib-resistant follicular dendritic-cell sarcoma in a patient-derived orthotopic xenograft PDOX nude mouse model. Oncotarget. 2016;7:33046–33054. doi:10.18632/oncotarget.8848. PMID:27105519
- Hoffman RM. Patient-derived orthotopic xenografts: better mimic of metastasis than subcutaneous xenografts. Nat Rev Cancer. 2015;15:451–452. doi:10.1038/nrc3972. PMID:26422835
- Tewey KM, Rowe TC, Yang L, et al. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984;226:466–468. doi:10.1126/science.6093249. PMID:6093249
- Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer. 2009;9:338–350. doi:10.1038/nrc2607. PMID:19377506
- Zhan M, Yu D, Lang A, et al. Wild type p53 sensitizes soft tissue sarcoma cells to doxorubicin by down-regulating multidrug resistance-1 expression. Cancer. 2001;92:1556–1566. doi:10.1002/1097-0142(20010915)92:6%3c1556::AID-CNCR1482%3e3.0.CO;2-S. PMID:11745235
- Liffers ST, Tilkorn DJ, Stricker I, et al. Salinomycin increases chemosensitivity to the effects of doxorubicin in soft tissue sarcomas. BMC Cancer. 2013;13:490. doi:10.1186/1471-2407-13-490. PMID:24144362
- Hui RC, Francis RE, Guest SK, et al. Doxorubicin activates FOXO3a to induce the expression of multidrug resistance gene ABCB1 (MDR1) in K562 leukemic cells. Mol Cancer Ther. 2008;7:670–678. doi:10.1158/1535-7163.MCT-07-0397. PMID:18347152
- Babichev, Y., et al. PI3K/AKT/mTOR inhibition in combination with doxorubicin is an effective therapy for leiomyosarcoma. J Transl Med. 2016;14:67. doi:10.1186/s12967-016-0814-z. PMID:26952093
- Chandhanayingyong C, Kabaroff L, Datti A, et al. MAPK/ERK signaling in osteosarcomas, Ewing sarcomas and chondrosarcomas: therapeutic implications and future directions. Sarcoma. 2012;2012:404810. doi:10.1155/2012/404810. PMID:22577336
- Blagosklonny MV. Matching targets for selective cancer therapy. Drug Discov Today. 2003;8:1104–7. doi:10.1016/S1359-6446(03)02806-X. PMID:14678733
- Blagosklonny MV. Teratogens as anti-cancer drugs. Cell Cycle. 2005;4:1518–21. doi:10.4161/cc.4.11.2208. PMID:16258270
- Blagosklonny MV. Treatment with inhibitors of caspases, that are substrates of drug transporters, selectively permits chemotherapy-induced apoptosis in multidrug-resistant cells but protects normal cells. Leukemia. 2001;15:936–41. doi:10.1038/sj.leu.2402127. PMID:11417480
- Blagosklonny MV. Target for cancer therapy: proliferating cells or stem cells. Leukemia. 2006;20:385–91. doi:10.1038/sj.leu.2404075. PMID:16357832
- Apontes P, Leontieva OV, Demidenko ZN, et al. Exploring long-term protection of normal human fibroblasts and epithelial cells from chemotherapy in cell culture. Oncotarget. 2011;2:222–33. doi:10.18632/oncotarget.248. PMID:21447859
- Blagosklonny MV. Tissue-selective therapy of cancer. Br J Cancer. 2003;89:1147–51. doi:10.1038/sj.bjc.6601256. PMID:14520435

