ABSTRACT
Seleno-short-chain chitosan (SSCC) was a synthesized chitosan derivative with the molecular weight of 4826.986 Da. The study is aimed to investigate cytotoxicity of SSCC on human breast cancer MCF-7 and BT-20 cells and explore apoptosis-related mechanism in vitro. The MTT (3- [4,5-Dimethylthiazol-2-yl]-2, 5-diphenylterazolium bromide) assay showed that SSCC exhibited significantly cytotoxic effects on MCF-7 and BT-20 cells in a dose- and time-dependent manner, and the effective inhibitory concentration was 100 μg/ml and 200 μg/ml, respectively. Apoptosis assay of these two kinds of cells was determined by Hoechst 33,342/PI and Annexin V-FITC/PI double staining. The cell cycle assay showed that SSCC triggered S and G2/M phase cell cycle arrest in MCF-7 cells and S phase cell cycle arrest in BT-20 cells in a time-dependent manner. Further studies demonstrated that SSCC led to the generation of reactive oxygen species (ROS) and the disruption of mitochondrial membrane potential (MMP) in these two kinds of cells. N- acetyl-L cysteine (NAC), as a radical scavenger, significantly inhibited the generation of ROS and decreased the apoptosis of MCF-7 and BT-20 cells. Moreover, the expression of mitochondrial apoptosis-related proteins was detected by western blot assay. SSCC up-regulated the expression of Bax, down-regulated the expression of Bcl-2, subsequently increased the release of cytochrome c from mitochondria to cytoplasm, and activated the cleavage of caspase-9 and −3, which finally induced apoptosis in MCF-7 and BT-20 cells in vitro. Consequently, these data indicated that SSCC could induce apoptosis of MCF-7and BT-20 cells in vitro by mitochondrial pathway.
Introduction
Breast cancer is one of the most common malignant tumors in threating women’s health [Citation1]. Annually, about 1.6 million women are diagnosed and 40,290 patients die from breast cancer all over the world [Citation2]. In China, breast cancer has become the primary threat to women’s health and they often occur among the young women [Citation3]. Surgery, chemotherapy, and radiotherapy have become the most common means of tumor therapy. However, a large number of clinical experiments have shown these therapies are very harmful to the health of patients and the effects are not satisfied [Citation4]. For instance, numerous reports demonstrated that bevacizumal-associated adverse effects have been observed in clinical trials, including gastrointestinal perforation, thromboembolic events, hypertension, and wound healing complications [Citation5–Citation7]. Meanwhile, as the development of medicine, more and more examples have illustrated that cytotoxic chemotherapy and endocrine therapy are presented [Citation8]. Therefore, it is urgent to search for a novel and effective antitumor drug with low toxicity for breast cancer treatments.
Apoptosis, known as programmed cell death, which may occur via death receptor-dependent (extrinsic) or mitochondrial (intrinsic) pathway. Mitochondrial apoptosis pathway is a physiological and selective cell death process that acts on a crucial role in balance cell proliferation and apoptosis [Citation9]. Low levels of ROS plays a very important role in cell proliferation and differentiation of normal cells. However, the excess expression of intracellular ROS could lead to cell death through indiscriminately damaging lipids, proteins and DNA [Citation10]. Previous studies have shown that ROS could trigger apoptosis signal and control a serious of physiological and pathological cell death processes [Citation11]. For mitochondrial apoptosis pathway, ROS could directly activate the mitochondrial permeability transition and result in the loss of mitochondrial membrane potential (MMP) and the membrane integrity [Citation12]. Subsequently, cytochrome c is released from mitochondria into cytosol and activates the caspase-cascades system, which eventually contributes to tumor cell apoptosis [Citation13].
Chemoprevention means that natural or synthetic chemical agents could not only induce apoptosis or death of tumor cells and display few toxic effects on normal cells but also protect against cancer initiation [Citation14–Citation17]. Due to the biological activity and low toxic nature, selenium played a critical role in regulating the functions of intracellular proteins and inducing tumor cell apoptosis [Citation18]. A great number of reports have confirmed that selenium is able to activate estrogen receptor to some extent [Citation19,Citation20]. More important, selenium also displays obvious cytotoxicity on breast cancer cells [Citation21–Citation23]. For example, Pi et al. [Citation24], illustrated that 5 μg/ml folic acid modified selenium nanoparticles (FA-SeNPs) could suppress the proliferation of MCF-7 cells effectively in vitro. Meanwhile, selenium induced human breast MCF-7cells apoptosis through modulation of phospho-Akt and its downstream substrates [Citation25]. Besides, polysaccharides acting as immune stimulates have shown a wide application prospect in prevention and therapy of tumor [Citation26]. Hu et al. [Citation27], demonstrated that chenopodium quinoa polysaccharide had a significant proliferation inhibition action against MCF-7 cells in a dose- and time-dependent manner. Chitosan, a linear-abundant polysaccharide composed mainly of β-(1–4)-linked 2-deoxy-2-amino-D-gulcopyranose and partially of β-(1–4)-linked 2-deoxy-2-acetamido-D-glucopyranose, is derived from N-deacetylation of chitin [Citation28]. Owing to its unique physical and chemical properties and biological functions, chitosan has been one of the most fascinating biopolymers for antitumor drugs [Citation29]. Researches showed that chitosan could act on tumor cells directly to interfere with cell metabolism, inhibit cell growth, or induce cell apoptosis [Citation30]. As Michela et al. [Citation31] demonstrated that marine diatom cocconeis scutellum and eicosapentaenoic acid (EPA) contributed to proliferation inhibition and apoptosis of BT-20 cells in vitro. Seleno-short-chain chitosan (SSCC) was a synthesized seleno-short-chain chitosan with the molecular weight of 4826.986 Da [Citation32]. In our previous studies, SSCC could induce apoptosis of leukemia K562 cells and lung cancer A549 cells in vitro [Citation28,Citation32]. However, it was still no clear whether SSCC could induce the apoptosis of breast cancer cells in vitro. Thus, in this study, we examined whether SSCC could induce apoptosis of MCF-7 and BT-20 cells and investigated the potential apoptosis mechanism.
Results
Cytotoxicity of SSCC on breast cancer cells assay
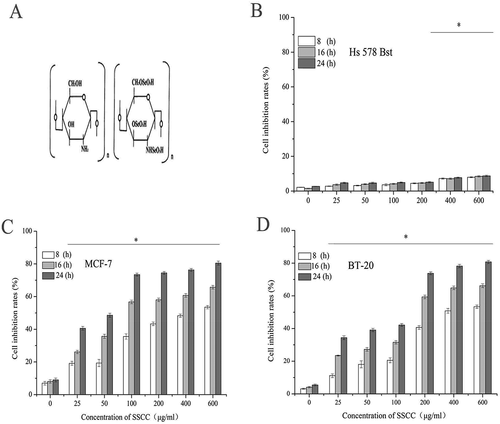
The chemical structure of seleno-short-chain chitosan (SSCC) was showed in ). In order to evaluate toxic effects of SSCC on MCF-7, BT-20 cells and normal breast Hs 578 Bst cells in vitro, cell proliferation inhibition was examined by MTT (3- [4,5-Dimethylthiazol-2-yl]-2, 5-diphenylterazolium bromide) method. The result in , ) showed that SSCC inhibited proliferation of these two kinds of cells in a dose- and time-dependent manner. When MCF-7 and BT-20 cells were treated with 100 μg/ml and 200 μg/ml SSCC for 16 h, the inhibition rate of two kinds of cells had a significant increase and attained to 80% (P < 0.05), respectively. Meanwhile, with the increasing of concentration and treatment time of SSCC, we observed that the toxic effects on this two kind s of cells hardly increased. In contrast, normal breast Hs 578 Bst cells were survival at the highest concentration of 600 μg/ml SSCC (P < 0.05). It is clear that SSCC exhibited few toxic effects on normal breast cells Hs 578 Bst. Therefore, 100 μg/ml and 200 μg/ml SSCC were used in the following experiments in MCF-7 and BT-20 cells, separately.
Figure 1. The cytotoxicity of SSCC on breast cancer MCF-7 and BT-20 cells and normal breast Hs 578Bst cells. (a) The chemical structure of seleno-short-chain chitosan (SSCC). (b), (c) and (d) Columns stand for inhibition rates of SSCC on normal breast cells, MCF-7 cells and BT-20 cells, after treatment with SSCC (25 – 600 μg/ml) for 8 – 24 h, separately. The inhibition rate of cells was determined by MTT method. *p < 0.05 compared with control group was considered as statistically significant difference
Morphological changes of SSCC on breast cancer cells assay
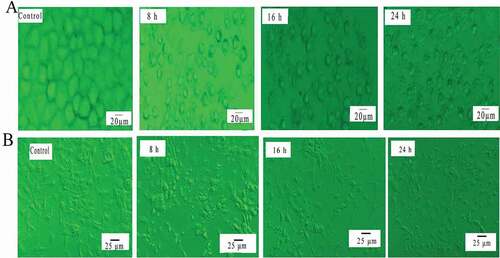
In order to observe toxic effects of SSCC on this two types of cells, cell morphology was observed under an inverted microscope. The result () showed that cell surface morphology of untreated group was complete and connections between the cells were dense. However, as the development of cultured time, we observed that cells gradually flattened and even collapsed from original three-dimensional. Apoptosis characteristics including cell shrinkage, cell volume reduction, apoptosis bodies and morphological collapse was also observed. Therefore, it is no doubt that SSCC had a markedly cytotoxicity on breast cancer cells.
Figure 2. Morphological changes of cells. (a) Morphological changes of MCF-7 cells were detected by inverted microscope (magnification, x20). Cells were exposed to 100 μg/ml SSCC for 8 – 24 h. (b) Morphological changes of BT-20 cells were observed by inverted microscope (magnification, x20), after incubation with 200 μg/ml SSCC for 8 – 24 h
Apoptosis assay of breast cancer cells
Cell apoptosis was measured by Hoechst 33,342/PI staining. Hoechst 33,342 is a kind of blue fluorescent dye, and it could bind with DNA within the nucleus [Citation33]. Thus, the living cells showed light blue. PI is a nucleic acid dye that only passes through the cell membrane of apoptotic cell and dead cell and displays light red [Citation34,Citation35]. The result in showed that untreated MCF-7 and BT-20 cells expressed weak blue. After treated with SSCC for 8 h, nuclei fragments were discovered in MCF-7 and BT-20 cells and exhibited lighted blue. As the development of incubating time, the volume of cells became smaller and emitted bright blue and weak red. When the incubation time reached to 24 h, both cells displayed bright blue and bright red, which indicated a large number of dead cells existed in this period.
Figure 3. SSCC induced apoptosis of breast cancer cells. (a) After incubation with appointed concentration of SSCC MCF-7 and BT-20 cells for 8 – 24 h, cells apoptosis was analyzed using Hoechst 33,342/PI double staining and observed under inverted fluorescence microscope (magnification, x 20). (b) Apoptosis rates of MCF-7 and BT-20 cells were detected by Annexin V-FITC/PI double staining. NAC, a kind of free radical scavenger, was used in this work. a and a’ Control group; b and b’ Treatment with 5 mM NAC for 1 h; c and c’ Treatment with SSCC for 8 h; d and d’ Treatment with SSCC for 16 h; e and e’ Treatment with SSCC for 24 h; f and f’ Treatment with 5 mM NAC for 1 h following treatment with SSCC for 24 h. (c) and (d) columns represent the proportion of two kinds of apoptotic cells after incubation with appointed concentration of SSCC for 8 – 24 h in the presence or absence of NAC. *p < 0.05 compared with control group was considered as statistically significant difference
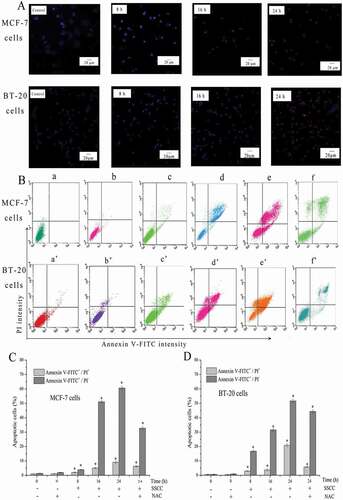
The detection of cell apoptosis rate was achieved by Annexin V-FITC/PI staining and measured by flow cytometry. The untreated cells showed low or negative staining with both Annexin V and PI, indicating the presence of a large number of viable cells. The total Annexin V-positive stands for the early and late apoptotic cells. Thus, as displayed in ), with the increase of incubated time, the proportion of normal cells gradually decreased, meanwhile, the number of early apoptosis, late apoptosis and necrotic cells increased. After MCF-7 cells exposed to 100 μg/ml SSCC for 8 – 24 h, the apoptosis ratio increased from 3.04% to 69.73%. For BT-20 cells, the apoptosis ratio increased from 6.54% to 74.78% after exposed to 200 μg/ml SSCC for 8 – 24 h. These results showed SSCC caused similar apoptotic effects on different breast cancer cells. Moreover, NAC acted as a free radical scavenger was used in this study. The results showed that pretreatment with 5 mM NAC for 1 h following treated with appointed concentration of SSCC for 24 h could effectively block the apoptotic cell death on MCF-7and BT-20 cells. The proportion of apoptotic cell declined from 69.73%. to 43.07% and from 74.78% to 49.38% in both cells, respectively (P < 0.05). Thus, it is clear that SSCC could induce breast cancer cell apoptosis in a time-dependent manner.
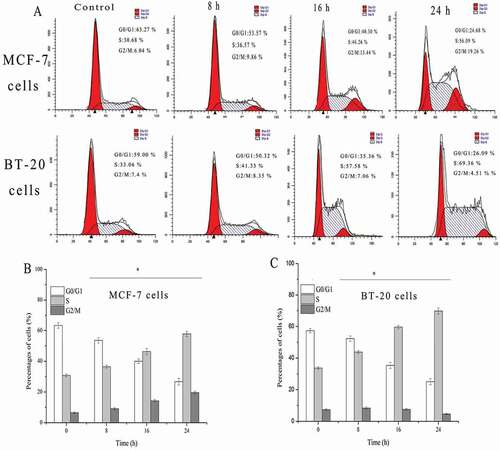
Cell cycle assay in breast cancer cells
To investigate whether cell growth inhibition owing to cell cycle arrested at a specific phase, cell cycle regulation was analyzed by flow cytometry. The result was showed in ). When MCF-7 cells were treated with 100 μg/ml SSCC for 8 – 24 h, the percentages of G0/G1 phase cell significantly decreased from 63.27% to 24.68% (P < 0.05). In contrast, the proportion of cell in S phase increased from 30.68% to 56.09%, and the ratio of cells in G2/M phase increased from 6.04% to 19.26% (P < 0.05). Thus, cell cycle might be arrested in the S and G2/M phase. Simultaneously, when BT-20 cells were treated with 200 μg/ml SSCC for the same time, the proportion of cells in S phase was significantly increased from 41.33% to 69.36% (P < 0.05). These results indicated that SSCC-induced apoptosis suppression on MCF-7 and BT-20 cells were possibly mediated by slowing down cellular progress in S and G2/M phase, and S phase, respectively.
Figure 4. The effects of SSCC on cells cycle distribution. (a) MCF-7 cells (100 μg/ml) and BT-20 (200μg/ml) cells were treated with SSCC for 8 – 24 h and the number of cells in every phase was analyzed by flow cytometry. (b) and (c) Columns represent the percentages of corresponding cell cycle phase after treatment with SSCC for 8 – 24 h in MCF-7 cells and BT-20 cells, separately. *p < 0.05 compared with control group was considered as statistically significant difference
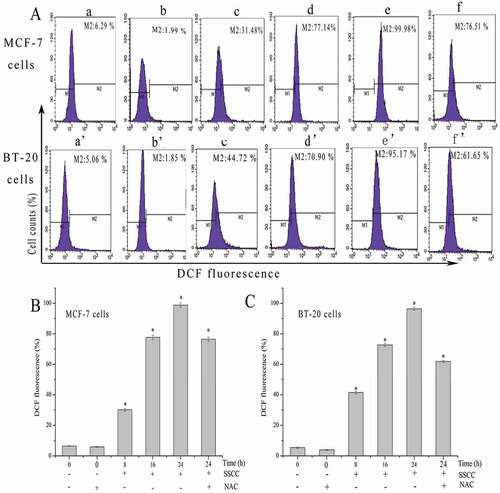
Effects of SSCC on ROS generation
To detect the effects of SSCC on intracellular ROS, the generation of ROS was measured by DCFH-DA staining. DCFH-DA is a kind of non-fluorescent dye which could be hydrolyzed into DCFH by esterase after entering cells. Finally, DCFH becomes DCF because of the generation of ROS [Citation36]. As shown in ), the peaks of control group were in the left, and with the development of treatment time, the peaks of two kinds of cells gradually changed from left to right and the levels of ROS increased from 6.29% to 99.98%, and 5.06% to 95.17% in a time-dependent manner (P < 0.05), respectively. Moreover, NAC acted as a free radical scavenger was used in this work. The results showed that after pretreatment with 5 mM NAC for 1 h following treated with appointed concentration of SSCC for 24 h, intracellular ROS decreased from 99.98% to 76.51% and 95.14% to 61.65% in MCF-7 cells and BT-20 cells, respectively (P < 0.05). These results illustrated that SSCC induced MCF-7 and BT-20 cells apoptosis through triggering the generation of ROS.
Figure 5. ROS generation in MCF-7 and BT-20 cells was tested by DCFH-DA staining and analyzed using flow cytometry. NAC, a kind of free radical scavenger, inhibited SSCC-induced generation of ROS. (a) The fluorescence changes after incubation with SSCC for 8 – 24 h and the presence or absence of NAC in MCF-7 (100 μg/ml) and BT-20 cells (200 μg/ml). a and a’ Control group; b and b’ Treatment with 5 mM NAC for 1 h; c and c’ Treatment with SSCC for 8 h; d and d’ Treatment with SSCC for 16 h; e and e’ Treatment with SSCC for 24 h; f and f’ Treatment with 5 mM NAC for 1 h followed by treatment with SSCC for 24 h, separately. (b) and (c) Columns representing the levels of intracellular ROS after treatment with SSCC for 8 – 24 h and the presence or absence of NAC in MCF-7 cells and BT-20 cells, respectively. *p < 0.05 compared with control group was considered as statistically significant difference
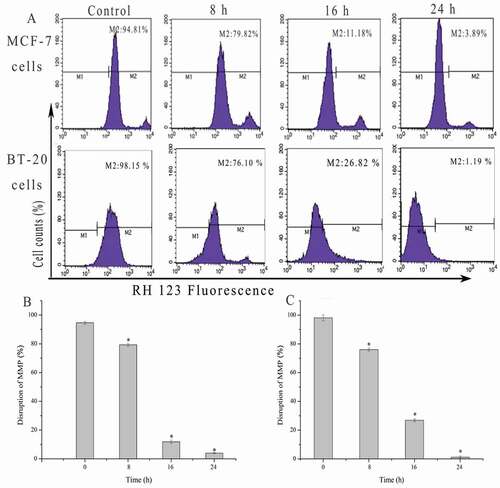
Effects of SSCC mitochondrial membrane potential (MMP)
Researches showed that the disruption of MMP is closely related to mitochondrial apoptosis pathway. The result ()) displayed that fluorescence intensity of Rhodamine123 in MCF-7 and BT-20 cells markedly decreased from 98.56% to 14.16% and 98.15% to 1.19% in a time-dependent manner after incubation with appointed concentration of SSCC for 8 – 24 h (P < 0.05), respectively. The result indicated that SSCC could significantly induce the apoptosis of MCF-7 and BT-20 cells by mitochondrial pathway.
Figure 6. Mitochondrial membrane potential (MMP) disruption in MCF-7 and BT-20 cells was measured by Rhodamine 123 staining and analyzed using flow cytometry. (a) MCF-7 (100 μg/ml) and BT-20 cells (200 μg/ml) were treated with SSCC for 8 – 24 h. (b) and (c) Columns stand for the percentages of MMP disruption after treatment with specified concentration of SSCC for 8 – 24 h in MCF-7 and BT-20 cells, respectively. *p < 0.05 compared with control group was considered as statistically significant difference
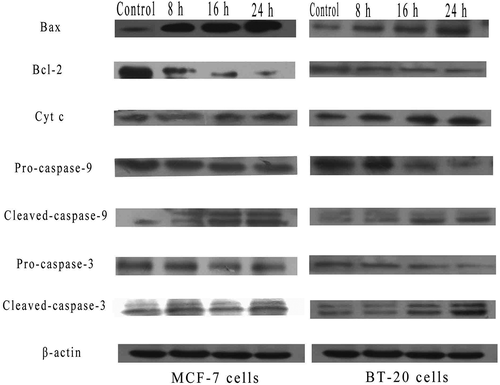
Effects of SSCC on apoptosis-related regulators involved in mitochondrial pathway
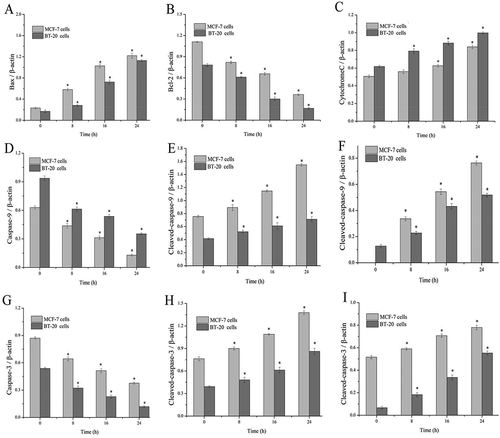
To explore the mechanism of SSCC-induced cells apoptosis, the levels of mitochondrial apoptosis-related proteins were measured by western blotting. As showed in , when MCF-7 and BT-20 cells were treated with appointed concentration of SSCC, the level of Bax increased while the level of Bcl-2 decreased in a time-dependent manner (P < 0.05). Next, the protein level of cytochrome c time-dependently increased after treatment with SSCC. The result showed that SSCC could promote the release of cytochrome c from mitochondrial into cytoplasm. Simultaneously, cleaved-caspase-9 and −3 were activated, which indicated inevitably apoptosis occurred in cells. Therefore, these results suggested that SSCC induced apoptosis of MCF-7 and BT-20 cells by MMP.
Figure 7. SSCC-induced mitochondrial apoptosis relevant-protein on MCF-7 cells and BT- 20 cells were measured by western blotting. Photos from A to I represent the ratio of Bax, Bcl-2, cytochrome c, pro-caspase-9, cleaved-caspase-9, pro-caspase-3, and cleaved-caspase-3 to β-actin after incubation with appointed concentration of SSCC for 8 – 24 h, respectively. *P< 0.05 compared with the control group was considered as significantly different
Discussions
In this study, a synthesized seleno-short-chain chitosan (SSCC) was explored owing to its prominent antitumor activity on breast cancer cells and low toxic effect on normal cell in vitro. MTT assay showed that SSCC could inhibit MCF-7 and BT-20 cells viability in a dose- and time-dependent manner. Especially, 100 μg/ml and 200 μg/ml SSCC caused markedly cytotoxicity and inhibition rate attained to 80% after incubation for 16 h in MCF-7 cells and BT-20 cells, separately. However, SSCC exhibited few toxicity on normal human breast Hs 578 Bst cells, which provided a theoretical basic for its application in clinical breast cancer treatment. The results were in agreement with our previous study that SSCC suppressed the growth of suspended human leukemia K562 and lung A549 cells and exhibited low toxic effect on normal mouse embryonic fibroblasts NIH3T3 and normal lung cells MRC-5. However, the most effective inhibition concentration of SSCC on K562 and A549 cells were 100 μg/ml and 200 μg/ml, separately, which may be associated with the type of tumor cells [Citation28,Citation32]. Thus, our results showed that SSCC could effectively inhibited breast cancer cells growth in vitro.
In order to detect whether SSCC-induced MCF-7 and BT-20 cells occurred apoptosis and evaluate the apoptosis rate of them, Hoechst 33,342/PI and Annexin V-FITC/PI staining was thus performed. The morphological observation of Hoechst 33,342/PI showed that SSCC could induce breast cancer cells apoptosis in vitro. Moreover, the result of Annexin V-FITC/PI showed that the number of early and late apoptotic cells significantly increased with the development of treatment time. After incubation with SSCC for 24 h, the percentages of apoptotic cells were increased significantly in MCF-7 (100 μg/ml) and BT-20 cells (200 μg/ml). Moreover, in this study, NAC as a free radical scavenger blocked the toxic effects of SSCC on MCF-7 and BT-20 cells in vitro. Similarly, according to the report of Mahdaviet al [Citation37], 50 μM spiroquinazolinone benzamide led to the apoptosis of MCF-7 cells and the apoptosis rate reached to 28% after treatment for 72 h. Meanwhile, When BT-20 cells were treated with N-Hydroxyphthalimide at 10 μM, the total percentage of apoptotic cells increased about 8.8-fold compared with control [Citation38]. In contrast, SSCC-induced breast cancer cells including MCF-7 and BT-20 cells have a higher apoptosis rate than other chemical compounds. Simultaneously, our findings indicated that SSCC could induce these two types of cells apoptosis through the generation of ROS.
Numerous reports have shown that antitumor agents could block and induce apoptosis at specific times [Citation39]. In this study, SSCC induced MCF-7 cells apoptosis and arrested cell cycle in S and G2/M phase, similarly, it induced BT-20 cells apoptosis and arrested cell cycle in S phase in a time-dependent manner. According to the study of Wang et al. [Citation40] showed that synthesized sarsasapogenin could arrest MCF-7 cells cycle in G2/M phase. Similarly, Chang et al. [Citation41] revealed that after exposed to Capsaicin, MCF-7 and BT-20 cells were arrested in S phase. The result indicated that SSCC- induced apoptosis of MCF-7and BT-20 cells was possibly mediated by slowing down cellular progress in S and G2/M phase and S phase, separately.
ROS is a medium factor of apoptosis signal channel. A large number of researches have shown that high level ROS could lead to DNA destruction, MMP dysfunction, and cell apoptosis [Citation37,Citation42]. In our work, after treatment with appointed concentration of SSCC in MCF-7 and BT-20 cells, the generation of ROS significantly increased and MMP distinctly decreased in a time-dependent manner. Additionally, pretreatment with the free radical scavenger NAC distinctly attenuated the generation of ROS and weakened apoptosis of these two kinds of cells. Similarly, According to the research of Lu et al. [Citation43], after treatment with 10 – 50 μg/ml Crocin, mitochondrial activity of MCF-7 cell was decreased significantly compared with control group. Meanwhile, Jin et al. [Citation44] also demonstrated that daidzein led to the generation of ROS and finally triggered the mitochondrial apoptosis pathway in MCF-7 cells. Thus, our experimental results are in agreement with these researches, ROS acted as an apoptosis signal factor led to the opening of mitochondrial membrane channels and finally induced the apoptosis of cells.
Bcl-2 family is the primary regulator for mitochondrial apoptosis pathway [Citation45]. Bcl-2 family is commonly divided into pro-apoptotic and anti-apoptotic functional protein group [Citation46]. The pro-apoptotic Bcl-2 family protein like Bax, Bak and Bid [Citation47] directly promotes the release of cytochrome c into cytoplasm or inhibits the expression of anti-apoptotic Bcl-2 family protein including Bcl-2, Bcl-Xl and Bcl-w, which plays an important role in regulating the mitochondrial apoptosis pathway [Citation48,Citation49]. In detail, Bcl-2 could change the inner mitochondrial permeability transition pore and repress dissipation of mitochondrial transmembrane potential, which thus could block the release cytochrome c, while Bax promoted the reduction of mitochondrial transmembrane potential and induced cytochrome c release [Citation50,Citation51]. The cytochrome c in cytoplasm facilitates the recruitment and activation of caspase-9, which subsequently activates caspase-3, a crucial activated death protease [Citation52]. In this study, SSCC up-regulating Bax and down-regulating Bcl-2, subsequently incited the release of cytochrome c from mitochondria to cytoplasm, activated the increase of cleaved-caspase-9 and cleaved-caspase-3 and finally induced breast cancer cells apoptosis in vitro. The results were in agreement with other chitosan compounds which induced breast tumor cells apoptosis through the mitochondrial apoptosis pathway. Research founding that Tanshinone IIA (one of pure compounds from Salviae miltiorrhizae) could induce mitochondrial apoptosis by increasing the expression of Bax and activating caspase-3 in BT-20 cells [Citation53]. Similarly, the research of Yan et al. [Citation54] indicated that G. beauts hemolymph-induced MCF-7 cells apoptosis was attributed to the up-regulating of Bad and caspase-3 and down-regulating of Bcl-2. These experiments illustrated that SSCC induced the apoptosis of breast cancer cells through mitochondrial apoptosis pathway.
Conclusions
In conclusion, we confirmed that SSCC could induce apoptosis of breast cancer MCF-7and BT-20 cells and exhibit few toxic effects on normal breast Hs 578Bst cells in vitro. SSCC could induce MCF-7 and BT-20 cells apoptosis and lead to cell cycle arrest in G2/M, S phase and S phase, separately. In addition, our results revealed the apoptosis of MCF-7 and BT-20 cells was related to the over-generation of ROS and the collapsing of MMP. N- acetyl-L cysteine (NAC), as a radical scavenger, significantly inhibited the generation of ROS and decreased the apoptosis of MCF-7 and BT-20 cells. Furthermore, SSCC up-regulated the expression of Bax, down-regulated the expression of Bcl-2, subsequently increased the release of cytochrome c from mitochondria to cytoplasm, and activated the cleavage of caspase-9 and −3, thereby inducing apoptosis in MCF-7 and BT-20 cells in vitro. Taken together, the present study demonstrated that SSCC induced cells apoptosis via mitochondrial pathway. These findings provide a theoretical basis for further clinic application of SSCC as a potential antitumor drug.
Materials and methods
Drugs and reagents
MCF-7 and BT-20 cells lines were obtained from American Type Culture Collection (ATCC.VA). Minimum essential medium (MEM) was purchased from HyClone (Fisher Scientific International). Fetal bovine serum (FBS) was purchased from Every Green (Hangzhou, China). 3- [4, 5-Dimethylthiazol-2-yl]-2, 5-diphenylterazolium bromide (MTT), Dimethyl sulfoxide (DMSO), propidium iodide (PI), and Hoechst 33,342 were obtained from Sigma Chemical (St. Louis, MO, USA). Annexin V- FITC/PI, N-acetyl-Lcysteine (NAC), Protein Iysis Buffer and Bradford Protein Assay Kit were purchased from Keygen Biotech (Nanjing, China). Reactive Oxygen Species Assay Kit, Chemiluminescence (ECL) reagent and Rhodamine 123 were obtained from Beyotime (Suzhou, China). All antibodies are purchased from Tianjin Sungene Biotech Co., Ltd. (Tianjin, China). All other chemical reagents were of analytical grade.
Cell culture
MCF-7 cells, BT-20 cells and normal breast Hs 578 Bst (Tianjin Medical University, Tianjin, China) were cultured in MEM with FBS (10%), penicillin (100 μg/ml) and streptomycin (50 μg/ml) at 37 ℃ in a humidified incubator with 5% CO2 atmosphere.
Preparation of SSCC
Briefly, Seleno-short-China chitosan (SSCC) was synthesized as described previously. The chitosan solution (1%, in 0.5% acetic acid solution) was degraded with 3% H2O2 at room temperature under stirring (200 rpm/4g) for 12 h. Next, SeO2 (1:10 w/v) was added to the solution, and the reaction was completed in 3 h. The reaction mixture was dialyzed (using 5 kDa cutoff dialysis membrane, Sigma Chemical) overnight and precipitated by ethanol to get SSCC (Patent Number CN 1600791A). The chemical structure of SSCC was showed in ).
Cell viability assay
MTT experiment was used to analyze inhibition rates of cells. Breast cancer MCF-7, BT-20 cells and normal breast Hs 578 Bst cells were seeded in 96-well plates at concentration of 5 × 103 cells per well and incubated at 37 ℃ for 24 h. Then, cells were exposed to the following concentration of SSCC: 25 μg/ml, 50 μg/ml, 100 μg/ml, 200 μg/ml, 400 μg/ml, 600 μg/ml, and corresponding cells cultured for 8 – 24 h, individually. Next, 20 μl of MTT solution (5 mg/ml) was added to each well for 4 h at 37℃. Finally, the supernatant was removed and 150μl ofDMSO was added to dissolve the formazan crystals. Absorbance was measured at 490 nm in a microplate reader (Model 680; BIORAD, Hercule, CA, USA). The inhibition rates were calculated using the following formula: inhibition rate (%) = (1-OD a/OD b) × 100%, where a and b stand for the absorbance of the treatment and control group cells, respectively [Citation55].
Morphology observation
Cell morphology observation
The morphological change of cells was observed under inverted microscope. MCF-7 and BT-20 cells were seeded in 6-well plate at the concentration of 3 × 105 cells/well and incubated for 24 h. After treated with 100 μg/ml and 200 μg/ml SSCC for 8 – 24 h, cells were observed under inverted microscope, respectively. (Ts 100, Nikon, Japan).
Morphology observation and apoptosis assay
Apoptosis was evaluated by combined assessment of nuclear morphology and membrane permeability which verified using Hoechst 33,342 and PI double staining. MCF-7 and BT-20 cells were cultured in 6-well plate at concentration of 3 × 105 cells/well for 24 h. After exposed to specified concentration of SSCC for 8 – 24 h, cells were collected and washed with PBS for three times. Next, cells were stained with 5 μg/ml Hoechst 33,342 and 5 μg/ml PI at 37 ℃ for 10 min and 5 min, respectively. Finally, the stained cells were observed in the dark using a fluorescence microscope (E800, Nikon, Chiyoda-Ku, Tokyo, Japan).
Cell apoptosis assay
The apoptosis rate of cells was evaluated using Annexin V-FITC/PI apoptosis detection kit. MCF-7 cells and BT-20 cells were seeded into 6-well plate at concentration of 3 × 105 cells/well for 24 h. After treated with appointed concentration of SSCC for 8 – 24 h, the cells were harvested and washed with PBS for three times. Then cells were suspended in binding buffer and incubated in the dark for 10 min according to the manufacture’s instruction. The apoptosis rate of cells was measured by flow cytometry (BD FACS Calibur). NAC was used for a free radical scavenger. MCF-7 and BT-20 cells were pretreated with 5 mM NAC for 1 h and then cells were treated with 100 μg/ml and 200 μg/ml SSCC for 24 h, respectively. Cell apoptosis was inhibited and the percentages of apoptotic cells was measured in the same condition.
Cell cycle assay
Cell cycle was detected by PI staining. MCF-7 and BT-20 cells were seeded in 6-well plate at 3 × 105 cells/well and incubated for 24 h. After exposed to specified concentration of SSCC for 8 – 24 h, cells were collected and washed with PBS for three times. Then cells were fixed with 70% precooled ethanol (stored at −20 ℃) overnight and stained with PI (50 μg/ml) in the presence of RNase A (10 μg/ml) for 30 min at 37 ℃. The percentages of stained cells in each phase were measured by flow cytometer and analyzed by ModFit LT software.
Intracellular reactive oxygen species (ROS) assay
DCFH-DA staining kit was used to measure the generation of intracellular ROS. Cells were cultured in 6-well plate at 3 × 105 cells/well and cultured for 24 h. After incubated with appointed concentration of SSCC for 8 – 24 h, cells were harvested and washed with PBS for three times. Then cells were resuspended in PBS containing with DCFH-DA (10 μg/ml) at 37 ℃ for 30 min. Finally, cells were analyzed by flow cytometry. In the ROS inhibitor group, cells were pretreated with 5mM NAC for 1 h following treatment with 100 μg/ml and 200 μg/ml SSCC for 24 h, and the levels of ROS were detected in the same way, respectively.
Mitochondrial membrane potential (MMP) assay
The MMP assay was estimated by RH 123 staining. MCF-7 cells and BT-20 cells were seeded in 6-well plate at 3 × 105 cells/well and cultured for 24 h. After exposed to specified concentration of SSCC for 8 – 24 h, cells were collected and washed with PBS for three times. Then cells were incubated with RH123 (1mg/ml) at 37 ℃ for 30 min in the dark. Finally, the stained cells were determined by flow cytometry.
Western blot analysis
The expression of proteins related to mitochondria apoptosis was detected by western blot. After treated with SSCC for 8 – 24 h, MCF-7(100 μg/ml) and BT-20 (200 μg/ml) cells with appointed concentration were harvested and wished by PBS for three times. Next, cells were lysed using RIPA and proteins were collected after centrifuging at 13,000 r/min, 4 ℃ for 20 min. The concentration of protein was determined using Bradford Protein kit [Citation56]. Protein samples were mixed with 1× reducing electrophoresis loading buffer and boiled for 5 min [Citation57]. Then protein samples were separated by 15% SDS-PAGE at 100 V and transferred into polyvinylidene fluoride (PVDF) membrane in semi-dry blotting at 15V for 30 min. Then PVDF membranes were blocked by 5% bull serum albumin (BSA) for 2 h at room temperature. Protein was incubated with the specific primary antibody at 4 ℃ overnight and secondary antibody for 1.5 h at room temperature. Protein bands were visualized using an Enhanced Chemiluminescence (ECL) reagent and exposed to X-ray photographic films in a darkroom [Citation58]. Then the bands were visualized by Quantity One software.
Statistics
All experiments were performed at least three times. Data were presented as means ± standard deviation. Significant differences were established through analysis of variance and mean comparisons were achieved by Duncan’s multiple range test. Data analysis was evaluated using SPSS software (SPSS 13.0 for Windows; SPSS, Inc, Chicago,). Difference with *P < 0.05 was considered statistically significant.
Disclosure statement
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
References
- McNamara KM, Kannai A. Possible roles for glucocorticoid signalling in breast cancer. Mol Cell Endocrinol. 2018;5:38–50.
- Vishal M, Swetha R, Thejaswini G, et al. Role of Runx2 in breast cancer-mediated bone metastasis. Int J Biol Macromol. 2017;99:608–614.
- Wang S, Li W, Fang W, et al. 36 cases adenoid cystic carcinoma of the breast in China: comparison with matched grade one invasive ductal carcinoma-not otherwise specified. Pathol Res Pract. 2017;213(4):310–315.
- Wakeam E, Acuna SA, Leighl NB, et al. Surgery versus chemotherapy and radiotherapy for early and locally advanced small cell lung cancer: a propensity-matched analysis of survival. Lung Cancer. 2017;109:78–88.
- Saltz LB, Clarke S, Diazrubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–2019.
- Tebbutt NC, Murphy F, Zannino D, et al. Risk of arterial thromboembolic events in patients with advanced colorectal cancer receiving bevacizumab. Ann Oncol. 2011;22(8):1834–1838.
- Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20(11):1842–1847.
- Curigliano G, Criscitiello C, Esposito A, et al. Over-using chemotherapy in the adjuvant setting. Breast. 2017;31:303–308.
- Yang X, Tang S, Dai C, et al. Quinocetone induces mitochondrial apoptosis in HepG2 cells through ROS-dependent promotion of VDAC1 oligomerization and suppression of Wnt1/β-catenin signaling pathway. Food Chem Toxic. 2017;105:161–176.
- Mieyal JJ, Gallogly MM, Qanungo S, et al. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal. 2008;10(11):1941–1988.
- Diebold L, Chandel NS. Mitochondrial ROS regulation of proliferating cells. Free Radical Bio Med. 2016;100:86–93.
- Crompton M, Barksby E, Johnson N, et al. Mitochondrial Intermembrane Junctional Complexes and their Involvement in Cell Death. Biochimie. 2002;84:143–152.
- Ma WD, Zou YP, Wang P, et al. Chimaphilin induces apoptosis in human breast cancer MCF-7 cells through a ROS-mediated mitochondrial pathway. Food Chem Toxicol. 2014;70:1–8.
- Wang S, Shen P, Zhou J, et al. Diet phytochemicals and cutaneous carcinoma chemoprevention: A review. Pharmacol Res. 2017;119:327–346.
- Weinstein IB. Cancer Prevention: recent Progress and Future Opportunities. Cancer Res. 1991;51:5080–5085.
- Corre LL, Chalabi N, Delort L, et al. Resveratrol and breast cancer chemoprevention: molecular mechanisms. Mol Nutr Food Res. 2005;49(5):462–471.
- Shu L, Cheung KL, Khor TO, et al. Phytochemicals: cancer chemoprevention and suppression of tumor onset and metastasis. Cancer Metast Rev. 2010;29(3):483–502.
- Lee JS, Hong EK. Hericium erinaceus enhances doxorubicin-induced apoptosis in human hepatocellular carcinoma cells. Cancer Lett. 2010;297(2):144–154.
- Stoica A, Pentecost E, Martin MB. Effects of selenite on estrogen receptor-alpha expression and activity in MCF-7 breast cancer cells. J Cell Biochem. 2000;79:282–292.
- Jablonska E, Socha K, Reszka E, et al. Cadmium, arsenic, selenium and iron- Implications for tumor progression in breast cancer. Environ Toxicol Pharmacol. 2017;53:151–157.
- Monnot GC, Romero P. Rationale for immunological approaches to breast cancer therapy. Breast. 2018;2:187–195.
- Guo CH, Hsia S, Shih MY, et al. Effects of selenium yeast on oxidative stress, growth inhibition, and apoptosis in human breast cancer cells. Int J Med Sci. 2015;12(9):748–758.
- He N, Shi X, Zhao Y, et al. Inhibitory effects and molecular mechanisms of selenium-containing tea polysaccharides on human breast cancer MCF-7 cells. J Agric Food Chem. 2013;61(3):579–588.
- Pi J, Jin H, Liu R, et al. Pathway of cytotoxicity induced by folic acid modified selenium nanoparticles in MCF-7 cells. Appl Microbiol Biotechnol. 2013;97(3):1051–1062.
- Li S, Zhou Y, Wang R, et al. Selenium sensitizes MCF-7 breast cancer cells to doxorubicin-induced apoptosis through modulation of phospho-Akt and its downstream substrates. Mol Cancer Ther. 2007;6(3):1031–1038.
- Wang C, Liang F, Su J, et al. Polysaccharides from Epimedium koreanum Nakai with immunomodulatory activity and inhibitory effect on tumor growth in LLC-bearing mice. J Ethnopharmacol. 2017;207:8–18.
- Hu Y, Zhang J, Zou L, et al. Chemical characterization, antioxidant, immune-regulating and anticancer activities of a novel bioactive polysaccharide from Chenopodium quinoa seeds. Int J Biol Macromol. 2017;99:622–629.
- Liu A, Song W, Cao D, et al. Growth inhibition and apoptosis of human leukemia K562 cells induced by seleno-short-chain chitosan. Method Find Exp Clin. 2008;30:181–186.
- Wei X, Li L, Wang Y, et al. Design and evaluation of a novel potential carrier for a hydrophilic antitumor drug: auricularia auricular polysaccharide-chitosan nanoparticles as a delivery system for doxorubicin hydrochloride. Int J Pharm. 2016;511(1):267–275.
- Gupta U, Sharma S, Khan I, et al. Enhanced apoptotic and anticancer potential of paclitaxel loaded biodegradable nanoparticles based on chitosan. Int J Biol Macromol. 2017;98:810–819.
- Nappo M, Berkov S, Massucco C, et al. Apoptotic activity of the marine diatom and eicosapentaenoic acid in BT20 cells. Pharm Biol. 2012;50(4):529–535.
- Zhao Y, Zhang S, Wang P, et al. Seleno-short-chain chitosan induces apoptosis in human non-small-cell lung cancer A549 cells through ROS-mediated mitochondrial pathway. Cytotechnology. 2017;69:851–863.
- Xiao Y, Hua W, Ni HM, et al. Inhibition of Drp1 protects against senecionine-induced mitochondria-mediated apoptosis in primary hepatocytes and in mice. Redox Biol. 2017;12:264–273.
- Niu J, Li C, Wu H, et al. Propidium iodide (PI) stains Nissl bodies and may serve as a quick marker for total neuronal cell count. Acta Histochem. 2015;117(2):182–187.
- Giordano G, Hong S, E M F, et al. Measurements of cell death in neuronal and glial cells. Humana Press. 2011;758(758):171.
- Nikolova K, Kaloyanova S, Mihaylova N, et al. New fluorogenic dyes for analysis of cellular processes by flow cytometry and confocal microscopy. J Photochem Photobiol B. 2013;129:125–134.
- Mahdavi M, Lavi M, Yekta R, et al. Evaluation of the cytotoxic, apoptosis inducing activity and molecular docking of spiroquinazolinone benzamide derivatives in MCF-7 breast cancer cells. Chem Biol Interact. 2016;260:232–242.
- Min W, Zhou A, Tao A, et al. N-Hydroxyphthalimide exhibits antitumor activity by suppressing mTOR signaling pathway in BT-20 and LoVo cells. J Exp Clin Cancer Res. 2016;35(1):1–12.
- Zhao Y, Guo C, Wang L, et al. A novel fluorinated thiosemicarbazone derivative- 2-(3,4-difluorobenzylidene) hydrazinecarbothioamide induces apoptosis in human A549 lung cancer cells via ROS-mediated mitochondria-dependent pathway. Biochem Biophys Res Commun. 2017;491:65–71.
- Wang W, Wang D, Wang Z, et al. Synthesis of new sarsasapogenin derivatives with cytotoxicity and apoptosis-inducing activities in human breast cancer MCF-7 cells. Eur J Med Chem. 2017;127:62–71.
- Chang HC, Chen ST, Chien SY, et al. Capsaicin may induce breast cancer cell death through apoptosis-inducing factor involving mitochondrial dysfunction. Hum Exp Toxicol. 2011;30:1657–1665.
- Xu H, Hao S, Gan F, et al. In vitro immune toxicity of ochratoxin A in porcine alveolar macrophages: a role for the ROS-relative TLR4/MyD88 signaling pathway. Chem Biol Interact. 2017;272:107–116.
- Lu P, Lin H, Gu Y, et al. Antitumor effects of crocin on human breast cancer cells. Int J Clin Exp Med. 2015;8(11):20316.
- Jin S, Zhang QY, Kang XM, et al. Daidzein induces MCF-7 breast cancer cell apoptosis via the mitochondrial pathway. Ann Oncol. 2010;21(2):263.
- Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci. 2009;122(4):437–441.
- Kuwana T, Newmeyer DD. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin in Cell Biolk. 2003;15(6):691–699.
- Uzu M, Sato H, Shimizu A, et al. Connexin 43 enhances Bax activation via JNK activation in sunitinib-induced apoptosis in mesothelioma cells. J Pharmacol Sci. 2017;134:101–107.
- Peng W, J G W, Y B J, et al. Antitumor activity of 4-O-(2ʹ’-O-acetyl-6ʹ’-O-p-coumaroyl-beta-D-glucopyranosyl)-p-coumaric acid against lung cancers via mitochondrial-mediated apoptosis. Chem Biol Interact. 2015;233:8–13.
- Lan T, Zhao H, Xiang B, et al. Suture compression induced midpalatal suture chondrocyte apoptosis with increased caspase-3, caspase-9, Bad, Bak, Bax and Bid expression. Biochem Biophysical Res Communications. 2017;489(2):179.
- W B L, Xie F, H Q S, et al. Anti-tumor effect of polysaccharide from Hirsutella sinensis on human non-small cell lung cancer and nude mice through intrinsic mitochondrial pathway. Int J Bio Macromol. 2017;99:258.
- Eskes R, Desagher S, Antonsson B, et al. Bid Induces the Oligomerization and Insertion of Bax into the Outer Mitochondrial Membrane. Mol Cell Biol. 2000;20(3):929.
- Ou YW, Zhao ZT, Wu CY, et al. Mig-2 attenuates cisplatin-induced apoptosis of human glioma cells in vitro through AKT/JNK and AKT/p38 signaling pathways. Acta Pharmacol Sin. 2014;35(9):1199–1206.
- Yan MY, Chien SY, Kuo SJ, et al. Tanshinone IIA inhibits BT-20 human breast cancer cell proliferation, through increasing caspase 12, GADD153 and phospho-p38 protein expression. Int J Mol Med. 2012;29(5):855.
- Ponraj T, Paulpandi M, Vivek R, et al. Protein regulation and Apoptotic induction in human breast carcinoma cells (MCF-7) through lectin from G. beauts. Int J Bio Macromol. 2017;95:1235–1245.
- Seidl K, Zinkernagel AS. The MTT assay is a rapid and reliable quantitative method to assess Staphylococcus aureus induced endothelial cell damage. J Microbio Methods. 2013;92(3):307–309.
- O’Sullivan J, McMahon HE. Development of a heat-mediated protein blotting method. Anal Biochem. 2016;499:66–70.
- Holzmüller W, Kulozik U. Protein quantification by means of a stain-free SDS-PAGE technology without the need for analytical standards: verification and validation of the method. J Food Compos Anal. 2016;48:128–134.
- Pan C, Lan X, Chen H, et al. An economical single-sided antibody incubation method for Western blotting. J Virol Methods. 2010;169(2):409–411.