ABSTRACT
Triple negative breast cancer (TNBC) is a heterogeneous disease that has no available targeted therapies. Previously, we have shown that caloric restriction (CR) can augment the effects of radiation therapy in a TNBC mouse model. To build upon this, we now present data regarding the combination of chemotherapy and CR in the same 4T1 model. Chemotherapy can induce inflammation that breeds resistance to therapy. We propose CR as a mechanism to decrease chemotherapy-induced inflammation and increase efficacy of therapy. 12-week old Balb/c mice were orthotopically injected with a syngeneic triple negative breast cancer cell line (4T1) and were treated in one of six cohorts: ad lib fed (AL), 30% reduction in calorie intake (CR), cisplatin or docetaxol alone or a combination CR+cisplatin/docetaxol. Mice in the cohorts receiving chemotherapy+CR had longer overall survival (12 ± 2 days) as compared to the AL group. These mice also demonstrated less lung metastases at the final time point of in vivo imaging. In addition, downregulation of the IGF-1R and IRS signaling pathways were noted most significantly in those mice receiving combination therapy. Lastly, serum from these mice demonstrated an increase in inflammatory cytokines TNF-α and IL-1β in response to chemotherapy alone. This increase was dampened by the addition of CR. Taken together, these data suggest that while chemotherapy is effective in TNBC, it can cause inflammation, which can be a driver of resistance to therapy. This chemotherapy-induced inflammation can be reversed with the use of CR as a nontoxic adjunct to treatment.
Introduction
Triple-negative breast cancer (TNBC) is a heterogeneous group of breast cancers (BCa) that do not express the estrogen receptor (ER) or progesterone receptor (PR), and they exhibit normal expression of the human epidermal growth factor receptor (HER-2). Therefore, no targeted therapies exist for these patients and chemotherapy remains standard of care. TNBCs are associated with a poor prognosis, characterized by early relapse and a significantly shorter survival compared to non-triple-negative cancers.[Citation1] Efforts to improve outcomes have focused on maximizing their response to neoadjuvant chemotherapy (NAC).[Citation2] TNBC patients who achieve pathologic complete response (pCR) after NAC have similar overall survival (OS) as patients with non-TNBC.[Citation2] While TNBC patients have higher rates of pCR to NAC (20–40%), the patients who do not achieve pCR have significantly worse outcomes.[Citation2,Citation3] Therefore, interventions which can increase response to chemotherapy are needed to improve outcomes in TNBC.
Recent findings indicate that many cancers, including BCa, develop resistance to standard therapies via chemotherapy-induced inflammation. Patients who are exposed to chemotherapies such as doxorubicin, cisplatin and 5-fluorouracil exhibit aberrant expression of multiple inflammatory pathways including nuclear factor kappa B (NFκB) and tumor necrosis factor alpha (TNF-α).[Citation4,Citation5] Exposure to these agents has shown to upregulate these pathways causing induction of angiogenesis, proliferation and metastasis. Currently, there are no proven interventions to counteract this inflammation; however, there is a need to increase response to chemotherapy and reduce chemotherapy-induced inflammation.
Dietary interventions such as caloric restriction (CR), have been shown to slow primary tumor growth and metastases in the preclinical setting and have been used in survivorship to decrease recurrence rates.[Citation6–Citation8] Multiple studies have shown diet to enhance standard cancer therapies such as radiation [Citation8,Citation9] and other cytotoxic anti-cancer therapies.[Citation10,Citation11] CR has also been shown to decrease inflammatory markers and adipokines.[Citation12,Citation13] However, CR has yet to demonstrate the ability to decrease inflammation in an oncologic setting. Here, we present novel preclinical data regarding the use of CR in a TNBC murine model alongside chemotherapeutic agents to decrease inflammation and improve therapeutic response.
Materials and methods
Mouse experiments and tissue collection
All animal experiments were approved by the Institutional Animal Care and Use Committee at Thomas Jefferson University. A total of 68 female 10 week-old BALB/c mice were acquired from Charles River Laboratories. Mice were randomized to treatment groups (10 per group) as follows: 1) ad libitum fed (AL), 2) 30% reduction in calorie intake (CR), 3) cisplatin alone (Cis), 4) docetaxel alone (Tax), 5) CR and cisplatin (Cis+CR) or 5) Tax+CR. Additionally, 8 mice without tumors were randomized to AL or CR for serum analysis. Treatments have been previously described.[Citation8] Briefly, CR mice were individually housed and baseline food intake was measured in order to calculate reduction. At 12 weeks, 50,000 4T1 luciferase-tagged cells (gift from Patricia Steeg) were orthotopically injected into the #4 mammary fat pad. Once tumor was palpable (~50mm3), mice began assigned therapy. Mice were fed a complete life cycle diet (#5010, LabDiet, St. Louis, MO), which is formulated as 58% calories from carbohydrates, 13% calories from fat and 28% calories from protein. Nutrient restriction was achieved in a global reduction of calories and no manipulations to individual dietary components were done. CR mice were stepped down to 90% of their individual baseline intake for 5 days for the CR diet, followed by 10% decreases every 5 days until a 30% total reduction was achieved. Mice in the cisplatin treatment groups received IP injections of Cisplatin (SellekChem, Houston, TX) at 5mg/kg on days 7, 12, 17 and 21 post-injection. Docetaxol (Active BioChem, Maplewood, NJ) was delivered at 15mg/kg on days 7, 14 and 21 post-injection.
Mice were weighed and tumor measurements taken three times weekly. The longest dimension (L) of tumor was measured and at a right angle to that axis (W). Tumor volume was calculated by L × W × W × π/6. Mice were euthanized when the primary tumor reached 2500 mm3 in size or earlier if required by humane endpoints. Three mice from each treatment group were euthanized 6 weeks after treatment initiation for serum analysis. Remaining mice were euthanized per protocol and were grossly examined for extent of disease. Primary tumors and lung metastases were isolated and either fixed in formalin for histologic evaluation, snap frozen for protein evaluation or preserved in RNAlater (Ambion, Life Technologies, Grand Island, NY) to assess RNA.
Blood was collected via intra-cardiac puncture at time of death in mice designated for serum analysis. Blood was separated into serum and plasma for ELISA measurements and stored at –80°C. Measurements of IGF-1, IGF-BP3, insulin, adiponectin, leptin, TNF-α and IL-1β were done. Analytes were measured using sandwich ELISAs (R&D Systems, Minneapolis, MN, USA) and insulin was measured using the Ultra-Sensitive Insulin Elisa Kit (Crystal Chem, Elk Grove Village, IL, USA). ELISAs were run according to manufacturer’s instructions using 10ul of sample unless otherwise specified.
Live bioluminescent imaging
Tumor cells were luciferase-tagged, allowing for real-time bioluminescent imaging of tumors, which was completed twice weekly throughout the experiment. Supine and prone images were obtained 15-minutes post-injection with 100 µl of D-luciferin at a concentration of 5 ng/µl using the IVIS Lumina XR System and Live Imaging Software (PerkinElmer, Waltham, MA). Focal point was 12.5 cm and filter parameters were set to auto-detect appropriate exposure times depending on a minimum photon flux threshold. Metastatic foci within the lungs were defined as areas that met at least a 15% fluorescence threshold.[Citation9] Time to metastasis development, size and number of metastases were documented.
Protein analysis and Western blotting
Primary tumor lysates were homogenized in RIPA buffer (50 mm Tris-Cl, 150 mm NaCl, 1% NP40, 0.25% Sodium deoxycholate, 1 mm PMSF) with proteinase inhibitor cocktail (Genentech, San Francisco, CA). Protein concentration was quantified with BSA assay, diluted in 6x SDS buffer, and run on a NuPAGE 4–12% Bis-Tris gels (Life Technologies, Philadelphia, PA). Western blots were performed to assess the expression of actin (Sigma Aldrich, St. Louis, MO), Akt1, pAkt, and PI3KCa (Cell Signaling Technology, Danvers, MA) according to the manufacturer’s instructions and quantified using ImageJ software (NIH, Bethesda, MD).
Statistics
Two-way ANOVA was performed for tumor regression curves and one-way ANOVA with Tukey’s post-hoc HSD was performed for metastases and serum analyzes to compare multiple treatment groups with a p ≤ 0.025 (Bonferroni correction). Results are expressed as mean plus or minus SEM unless otherwise stated. Mantel-Cox log-rank test was performed for survival and time to metastases analysis. Analysis was completed using GraphPad Prism commercially available software (GraphPad Software, La Jolla, CA).
Results
CR augments chemotherapy and decreases disease progression
The combination of CR+chemotherapy had an additive effect on tumor growth delay of primary tumor (). Tumor regrowth at a reference value of 1000 mm3 was decreased by 13.1% in cisplatin alone mice and 37.5% in Cis+CR mice compared to ad lib control mice (p = 0.005 for Cis+CR). While mice treated with docetaxol did not demonstrate tumor growth delay by caliper measurements, in vivo imaging demonstrated a decrease in metabolic activity in the primary tumor ()). Mice in the Tax+CR group demonstrated a similar growth delay as mice treated with CR alone (data not shown). Bioluminescent imaging revealed changes in disease progression ()). The benefit of CR+chemotherapy is evident with an apparent reduction in intensity of primary tumor and decreased metastases at late stages of disease.
Figure 1. Caloric restriction in combination with chemotherapy decreases primary tumor growth as well as delays metastases and increases survival. Primary tumors were measured and the volume of each tumor is plotted over time according to intervention (A). CR alone reduced primary tumor growth at a reference point of 1000mm3by 32% as compared with the ad lib (AL) group, similarly the cisplatin (Cis) alone group experienced a 16% reduction in primary tumor growth. The CR combination group (Cis+CR) demonstrates an additive response with respect to primary tumor growth (64% reduction at 1000 mm3reference). *** denotes significance of p < 0.05 as compared to AL. Mice were monitored to identify lung metastases. The number of lesions visible on in vivo imaging was significantly lower in the arms receiving CR, chemotherapy alone and chemotherapy with CR as compared to AL (B and C). At 33-days post injection, the AL mice had an average of 3 lung metastases visible on imaging and the CR group had 1.5, cisplatin alone had 0.67 and Cis+CR had 0 (B; p < 0.05) docetaxol had 1.0, Tax+CR had 0.67 (p < 0.05) (C). Time to metastases was prolonged in all treatment groups, but most significantly in those groups receiving combination therapy (D). For panels B and C, ** denotes significance of p < 0.05 as compared with the AL group and *** denotes p < 0.025 as compared to the CR alone group
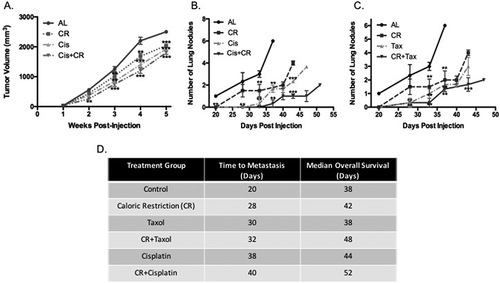
Figure 2. The addition of CR to chemotherapy alters metabolic signaling within the primary tumor as well as metastases. Representative images of fluorescent imaging demonstrate the differences in primary tumor growth and metastases in the chemotherapy and CR+chemotherapy groups (A). Key signaling nodes in the Akt/PI3K pathway were detected by western blot in primary tumor tissue (C). Quantification was normalized to the AL control group for PI3K and the ratio of pAkt to Akt was calculated and plotted for all groups. Mice receiving CR alone had a significant reduction in PI3K by 50% (p = 0.018), whereas the other groups had a more modest reduction, the most notable at 30% in the Cis+CR arm (p = 0.034). All treatment groups demonstrated reduction in the pAkt/Akt ratio, the effect of CR appeared to be additive as compared to either CR alone or chemotherapy alone. * denotes significance level of p < 0.05 for the PI3K and ** denotes p < 0.05 for pAkt/Akt ratio. Serum IGF-1 and its major binding protein IGF-BP3 were measured and the ratio of free IGF-1 was calculated and plotted (D). The effect of adding CR to chemotherapy was additive in terms of free IGF-1 with 52% reduction compared to AL in the Tax+CR arm and 65% reduction compared to AL in the Cis+CR arm. * denotes p < 0.05 and ** denotes p < 0.01.]
![Figure 2. The addition of CR to chemotherapy alters metabolic signaling within the primary tumor as well as metastases. Representative images of fluorescent imaging demonstrate the differences in primary tumor growth and metastases in the chemotherapy and CR+chemotherapy groups (A). Key signaling nodes in the Akt/PI3K pathway were detected by western blot in primary tumor tissue (C). Quantification was normalized to the AL control group for PI3K and the ratio of pAkt to Akt was calculated and plotted for all groups. Mice receiving CR alone had a significant reduction in PI3K by 50% (p = 0.018), whereas the other groups had a more modest reduction, the most notable at 30% in the Cis+CR arm (p = 0.034). All treatment groups demonstrated reduction in the pAkt/Akt ratio, the effect of CR appeared to be additive as compared to either CR alone or chemotherapy alone. * denotes significance level of p < 0.05 for the PI3K and ** denotes p < 0.05 for pAkt/Akt ratio. Serum IGF-1 and its major binding protein IGF-BP3 were measured and the ratio of free IGF-1 was calculated and plotted (D). The effect of adding CR to chemotherapy was additive in terms of free IGF-1 with 52% reduction compared to AL in the Tax+CR arm and 65% reduction compared to AL in the Cis+CR arm. * denotes p < 0.05 and ** denotes p < 0.01.]](/cms/asset/38760455-0c84-4976-9c69-ca7ff9a05b67/kccy_a_1471314_f0002_c.jpg)
Metastatic disease was significantly delayed with administration of chemotherapy both with and without CR (Figure 1(d)). We have previously demonstrated the synergistic effects of CR with cytotoxic therapy on metastatic disease at both the anatomic and histologic levels.[Citation9] Lung metastases developed at a median of 20 days in the control group, 28 days in the CR group, 30 days in the docetaxel group, 38 days in the cisplatin group, 32 days in the Tax+CR group and 40 days in the Cis+CR group (p = 0.002; Kaplan-Meier curve in Supplementary Data). In addition to delaying metastatic disease, CR+chemotherapy prolonged OS in this TNBC model (Figure 1(d); Kaplan-Meier curve in Supplementary Data). Mice in the Tax+CR lived 10 days longer than the AL group on average (p = 0.002) and 14 days longer in the Cis+CR group (p < 0.001).
Combination therapy results in decreased IGF-1R and IRS signaling
IGF-1 and its binding protein IGF-BP3 were measured in serum and plasma after 6 weeks of dietary intervention. Groups receiving chemotherapy showed a significant decrease in free serum IGF-1 levels compared to control mice (no tumors) ()). CR+chemotherapy resulted in a further decrease in IGF-1 levels (52% decrease in the Tax+CR arm; 65% in the Cis+CR arm).
Downstream signaling nodes in the IGF-1R pathway were measured including PI3K, Akt and pAkt ()). The Tax arm had a significant decrease in both PI3K and the ratio of pAkt/Akt and the Cis arm had a 20% decrease in PI3K and 32% decrease in pAkt/Akt ratio compared to control (p = 0.051 and p = 0.0.03 respectively). This was compounded by the addition of CR to each; PI3K and pAkt/Akt ratio decreased by 30% and 49% in the Tax+CR arm and 35% and 51% in the Cis+CR arm (p < 0.05 for all).
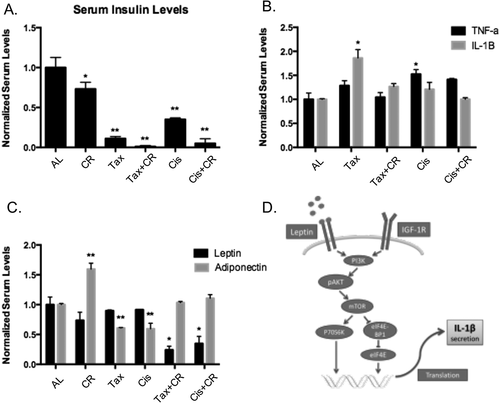
Serum insulin was assessed as it relates to TNF-α signaling and IGF-1R signaling through the IRS pathway. Insulin levels in control CR mice (without tumors) decreased significantly (27%) at 6 weeks on treatment compared to the AL no tumor group ()). Because of the potential metabolic effects of active malignancy, values in the experimental groups were normalized to the AL fed mouse without tumor. Serum insulin in mice with tumors receiving CR alone decreased by 40% compared to AL control mice at 6 weeks of diet (p < 0.01). Mice receiving CR+chemotherapy had the most significant decrease in serum insulin across all treatment groups; 87% reduction in the Tax+CR arm (p = 0.001) and 82% in the Cis+CR arm (p = 0.003) ()).
Figure 3. Chemotherapy-induced inflammation is blunted by the addition of CR. Serum insulin levels were measured across groups and normalized to the AL control, CR alone decreased this by 25%, chemotherapy alone altered serum insulin and the combination therapy again had an additive effect with 87% reduction in the Tax+CR arm (p =0.001) and 82% in the Cis+CR arm (p = 0.003) (A). * denotes p < 0.05 and ** denotes p < 0.01. Inflammatory cytokines TNF-αand IL-1βwere both measured and increased with the use of chemotherapy alone as compared to AL controls; Tax increased TNF-αby 1.6-fold (p = 0.042) and Cis increased TNF-αby 1.2-fold. The addition of CR dampened this increase to 1.2-fold in the Tax+CR arm and 1.0 (equivalent) in the Cis+CR arm (B). * denotes p < 0.05. Adipokines fluctuate with chemotherapy treatment and leptin significantly decreases in the Tax+CR and Cis+CR arms (75% reduction and 62% reduction; p = 0.021, p = 0.037) (C). While adiponectin is decreased with systemic treatment alone, this response is dampened by the addition of CR, the Tax+CR arm had equivalent levels as compared to the AL control and the Cis+CR arm had a 1.2-fold increase, though nonsignificant. * denotes p < 0.05 for leptin, ** denotes p < 0.05 for adiponectin. Proposed mechanism by which leptin can affect tumor growth and the IGF-1R pathway (D)
CR dampens inflammation caused by cytotoxic therapies
CR was associated with decreased insulin levels and therefore a global anti-inflammatory response. A potential mechanism for this is through reduction in TNF-α and IL-1β signaling via the IRS pathway. TNF-α was measured across treatment groups and there was a statistically significant increase in the cisplatin alone group (1.4-fold increase), and an increase in the docetaxol alone arm. The addition of CR to chemotherapy decreased TNF-α levels when compared against mice receiving chemotherapy alone ()). IL-1β, however, demonstrated a significant reduction across all cytotoxic therapies when combined with CR ()). Similarly, mice receiving CR+chemotherapy, experienced a significant decrease in IL-1β compared to chemotherapy alone (34% decrease Tax+CR vs. Tax; 42% decrease Cis+CR vs. Cis).
Adipokines can affect tumor growth via IGF-1R and inflammatory pathways ()). In serum, adiponectin decreased with systemic treatment alone and dampened with CR ()). The Tax+CR arm had equivalent levels compared to the AL control and the Cis+CR arm had a 1.2-fold increase. Although not significantly changed in the chemotherapy alone groups, leptin was significantly decreased in the Tax+CR and Cis+CR arms (75% reduction and 62% reduction; p = 0.021, p = 0.037) ()).
Discussion
Here, we demonstrate that CR is a viable, non-toxic method to increase the efficacy of chemotherapy in BCa. This is particularly relevant for patients undergoing NAC. Patients who achieve pCR have far better outcomes than those who do not and therefore response rates require improvement. CR+chemotherapy in this model demonstrated delayed primary tumor growth and development of metastatic disease, which is likely the explanation for the survival benefit. Additional preclinical models have demonstrated that energy restriction can increase the effectiveness of systemic anticancer therapies.[Citation10,Citation11] In humans, fasting prior to chemotherapy can decrease toxicity and protect normal tissues.[Citation14] While there are no randomized trials demonstrating the advantage of dietary restriction combined with chemotherapy, there are a number of single arm trials that confirm the “differential stress response” exhibited by normal cells undergoing nutrient restriction.[Citation10,Citation14,Citation15] Our previous results [Citation9] as well as the current data presented corroborate these findings. One proposed mechanism by which CR inhibits tumor growth is by its modulation of circulating levels of free IGF-1 in the serum.[Citation16–Citation19] Our data suggest a significant decrease in free IGF-1 with combination treatment. We also noted that AL fed mice with tumor implants demonstrated a mild decrease in free serum IGF-1, though not significant from the control. We postulate that this decrease could be due to usage of IGF-1 by tumor cells in growth pathway signaling. Concerning the use of chemotherapy, we noted that free IGF-1 decreased in all chemotherapy groups, though significant decreases were apparent only in groups receiving CR+chemotherapy. Further research should focus on the significance of IGF-1 with respect to the efficacy of chemotherapy.
Our data demonstrate that the presence of tumor can alter expression of both metabolic markers and inflammatory cytokines. This concept of a tumor-driven “metabolic syndrome” is novel and cytotoxic therapies aimed at cancer cells may compound this phenomenon leading to drug resistance.[Citation4] Insulin is directly correlated with TNF-α signaling and is altered in metabolic syndrome along with adipokines. Therefore, insulin, adiponectin and leptin may work together to deter or potentiate inflammation based on available nutrients in the tumor microenvironment such as glucose. Studies have shown that insulin can be modulated via dietary intervention.[Citation20] In our model, the decreased insulin resulting from CR is associated with improved response to chemotherapy. Similarly, both leptin and adiponectin have been linked to breast cancer risk and tumor progression.[Citation21,Citation22] Increased adiponectin levels confer anti-tumor characteristics to the tumor microenvironment and opposes the pro-tumor effects of leptin. Adiponectin receptor expression on tumor cells may have a role in tumor suppression as well as decreased TNF-α production.[Citation23] The level of receptor expression is affected by insulin levels.[Citation23] The leptin receptor (OB-R) is expressed not only on adipocytes, but also on BCa cells and macrophages.[Citation24] The activation of OB-R on BCa cells leads to activation of PI3K and stimulation of proliferation pathways. In this report, we demonstrate that chemotherapy reduces adiponectin levels in serum, which then allows for unopposed leptin action. The implementation of CR can mitigate this decrease in adiponectin levels, thereby providing anti-leptin activity, which is proposed to counteract growth signaling in tumor cells. On macrophages, activation of OB-R leads to release of inflammatory cytokines, namely IL-1β, which binds its receptor on BCa cells, leading to proliferation. While chemotherapy alone does not change leptin levels, the use of CR in our BCa model significantly reduces leptin levels, providing a potential mechanism by which CR improves response to chemotherapy.
This report is one of the first on CR’s effects on chemotherapy-induced inflammation. Given the link between inflammatory cytokine production and insulin and adipokines, we investigated expression levels of TNF-α and IL-1β. As expected, TNF-α was increased in all groups when compared to control mice without tumors. This is corroborated by other studies demonstrating an increase in TNF-α in organisms with established tumors and suggests that it may be a marker of the extent of disease.[Citation25–Citation27] It is also known that chemotherapy can induce TNF-α production, which can signal proapoptotic or mitogenic pathways.[Citation4,Citation5] However, in the presence of chemotherapy, it appears that CR decreases circulating levels of TNF-α which may be a sign of decreased systemic disease progression as seen in a pilot clinical trial in BCa patients.[Citation28] Serum IL-1β is significantly increased in those mice with tumors that were fed an AL diet and it appeared to decrease across groups that received CR as an intervention. IL-1β’s role in pro-survival pathways is through the activation of mitogenic signaling involving NFκB and therefore this decrease may be a crucial way to reverse resistance to chemotherapies.
We present several important findings regarding the physiologic and molecular changes caused by combining CR with chemotherapy. In conclusion, CR augments chemotherapy and may counteract chemotherapy-induced inflammation, thereby reducing risk of treatment resistance. In order to move CR to the clinical space, it will be important to establish an appropriate threshold in these biomarkers in an oncologic population. Future research should focus on defining what constitutes a meaningful change in the biomarkers associated with CR. Additionally, treatments could be tailored to each patient based on the response in biomarkers, which is a less invasive way to monitor response and would allow for all patients to achieve maximum benefit from therapy.
Supplemental Material
Download PDF (53.9 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Jitariu AA, Cimpean AM, Ribatti D, et al. Triple negative breast cancer: the kiss of death. Oncotarget. 2017 Jul 11;8(28):46652–46662. PubMed PMID: 28445140; PubMed Central PMCID: PMCPMC5542300.
- Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008 Mar 10;26(8):1275–1281. PubMed PMID: 18250347.
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014 Jul 12;384(9938):164–172. PubMed PMID: 24529560.
- Vyas D, Laput G, Vyas AK. Chemotherapy-enhanced inflammation may lead to the failure of therapy and metastasis. Onco Targets Ther. 2014;7:1015–1023. PubMed PMID: 24959088; PubMed Central PMCID: PMCPMC4061164.
- Torres MA, Pace TW, Liu T, et al. Predictors of depression in breast cancer patients treated with radiation: role of prior chemotherapy and nuclear factor kappa B. Cancer. 2013 Jun 01;119(11):1951–1959. PubMed PMID: 23512358; PubMed Central PMCID: PMCPMC3663885.
- De Lorenzo MS, Baljinnyam E, Vatner DE, et al. Caloric restriction reduces growth of mammary tumors and metastases [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Carcinogenesis. 2011 Sep;32(9):1381–1387. PubMed PMID: 21665891; PubMed Central PMCID: PMC3165123. eng.
- Hursting SD, Lavigne JA, Berrigan D, et al. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans [Review]. Annu Rev Med. 2003;54:131–152. PubMed PMID: 12525670; eng.
- Saleh AD, Simone BA, Palazzo J, et al. Caloric restriction augments radiation efficacy in breast cancer. Cell Cycle. 2013 May 21;12(12). PubMed PMID: 23708519; Eng. DOI:https://doi.org/10.4161/cc.25016
- Simone BA, Dan T, Palagani A, et al. Caloric restriction coupled with radiation decreases metastatic burden in triple negative breast cancer. Cell Cycle. 2016 Sep;15(17):2265–2274. PubMed PMID: 27027731; PubMed Central PMCID: PMCPMC5004690.
- Lee C, Raffaghello L, Brandhorst S, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. 2012 Mar 07;4(124):124ra27. PubMed PMID: 22323820; PubMed Central PMCID: PMCPMC3608686.
- Safdie F, Brandhorst S, Wei M, et al. Fasting enhances the response of glioma to chemo- and radiotherapy. PLoS One. 2012;7(9):e44603. PubMed PMID: 22984531; PubMed Central PMCID: PMCPMC3439413.
- Imayama I, Ulrich CM, Alfano CM, et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: a randomized controlled trial. Cancer Res. 2012 May 01;72(9):2314–2326. PubMed PMID: 22549948; PubMed Central PMCID: PMCPMC3342840.
- Xydakis AM, Case CC, Jones PH, et al. Adiponectin, inflammation, and the expression of the metabolic syndrome in obese individuals: the impact of rapid weight loss through caloric restriction. J Clin Endocrinol Metab. 2004 Jun;89(6):2697–2703. PubMed PMID: 15181044.
- Safdie FM, Dorff T, Quinn D, et al. Fasting and cancer treatment in humans: A case series report. Aging (Albany NY). 2009 Dec 31;1(12):988–1007. PubMed PMID: 20157582; PubMed Central PMCID: PMCPMC2815756.
- Raffaghello L, Safdie F, Bianchi G, et al. Fasting and differential chemotherapy protection in patients. Cell Cycle. 2010 Nov 15;9(22):4474–4476. PubMed PMID: 21088487; PubMed Central PMCID: PMCPMC3048045.
- Fontana L, Weiss EP, Villareal DT, et al. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans [Clinical Tria Comparative Study Randomized Controlled Trial Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Aging Cell. 2008 Oct;7(5):681–687. PubMed PMID: 18843793; PubMed Central PMCID: PMC2673798. eng.
- Harvie M, Howell A. Energy restriction and the prevention of breast cancer [Research Support, Non-U.S. Gov’t Review]. Proc Nutr Soc. 2012 May;71(2):263–275. PubMed PMID: 22414375; eng. .
- Fairey AS, Courneya KS, Field CJ, et al. Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial [Clinical Trial Randomized Controlled Trial Research Support, Non-U.S. Gov’t]. Cancer Epidemiology, Biomarkers & Prevention: a Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology. 2003 Aug;12(8):721–727. PubMed PMID: 12917202; eng.
- Allen NE, Appleby PN, Davey GK, et al. The associations of diet with serum insulin-like growth factor I and its main binding proteins in 292 women meat-eaters, vegetarians, and vegans [Comparative Study Research Support, Non-U.S. Gov’t]. Cancer Epidemiology, Biomarkers & Prevention: a Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology. 2002 Nov;11(11):1441–1448. PubMed PMID: 12433724; eng.
- Lee C, Longo VD. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene. 2011 Jul 28;30(30):3305–3316. PubMed PMID: 21516129. .
- Mantzoros C, Petridou E, Dessypris N, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004 Mar;89(3):1102–1107. PubMed PMID: 15001594.
- Miyoshi Y, Funahashi T, Kihara S, et al. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003 Nov 15;9(15):5699–5704. PubMed PMID: 14654554.
- Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. 2012 Aug;33(4):547–594. PubMed PMID: 22547160; PubMed Central PMCID: PMCPMC3410224. .
- Newman G, Gonzalez-Perez RR. Leptin-cytokine crosstalk in breast cancer. Mol Cell Endocrinol. 2014 Jan 25;382(1):570–582. PubMed PMID: 23562747; PubMed Central PMCID: PMCPMC3844060. .
- Rodriguez-Berriguete G, Sanchez-Espiridion B, Cansino JR, et al. Clinical significance of both tumor and stromal expression of components of the IL-1 and TNF-alpha signaling pathways in prostate cancer. Cytokine. 2013 Nov;64(2):555–563. PubMed PMID: 24063999; Eng.
- Fontana L. Neuroendocrine factors in the regulation of inflammation: excessive adiposity and calorie restriction [Research Support, N.I.H., ExtramuralResearch Support, Non-U.S. Gov’t Review]. Exp Gerontol. 2009 Jan-Feb;44(1–2):41–45. PubMed PMID: 18502597; PubMed Central PMCID: PMC2652518. eng. .
- Waters JP, Pober JS, Bradley JR. Tumour necrosis factor and cancer [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review]. J Pathol. 2013 Jul;230(3):241–248. PubMed PMID: 23460481; eng. .
- Zhang GJ, Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma [Research Support, Non-U.S. Gov’t]. Anticancer Res. 1999 Mar-Apr;19(2B):1427–1432. PubMed PMID: 10365118; eng.
