ABSTRACT
Apatinib is a novel tyrosine kinase inhibitor that targets VEGFR2 signal and exhibits potent anti-tumor effects in human cancers. In this study, we aim to investigate the efficacy of Apatinib in cervical cancer. The protein expression of VEGFR2 and its relationships with clinical parameters were investigated in a panel of cervical cancer patients. In vitro, a series of experiments were performed to detect the effects of Apatinib on the proliferation, apoptosis and cell cycle in cervical cancer cells. Both the immortalized cell lines and primary cultured tissues were used to investigate the synergy between Apatinib and chemotherapeutic drugs. The in vivo effects of Apatinib were validated in a nude mouse model. Compared to that in normal cervix, VEGFR2 protein was significantly upregulated in cervical cancer tissues (P < 0.001); this was positively correlated with advanced tumor stage, lymph node metastasis, and a poor prognosis. In vitro, Apatinib markedly induced apoptosis and G1-phase arrest, suppressed cell growth, and decreased colony formation ability. We also found that primary cancer tissues with higher level of VEGFR2 were much more sensitive to Apatinib. Further, we proved that Apatinib significantly increased the sensitivity to Paclitaxel in cervical cancer cells and the mouse model. Collectively, we firstly report the anti-tumor efficacy of Apatinib in cervical cancer. Moreover, Apatinib synergized with Paclitaxel to achieve more significant suppression on tumor growth, proposing that Apatinib might be a potent drug for cervical cancer.
Introduction
In China, cervical cancer is the leading malignancy originating from the female reproductive system, with an estimated 98,900 new cases and 30,500 deaths in 2015 [Citation1]. In certain areas where early screening (HPV test+TCT) is not available, numerous cervical cancer patients present with already advanced stages when first diagnosed [Citation2]. Previous studies have demonstrated that about 1/3 of advanced cervical cancer manifest local or distant recurrence/metastasis after surgery or radiation therapy, where currently available drugs did not show strong therapeutic efficacy [Citation3].
Apatinib is a novel and highly selective VEGFR2 inhibitor that can suppress the phosphorylation of VEGFR2 and block its downstream targets such as PI3K/mTOR/AKT, MAPK/ERK and FAK/STAT3 [Citation4]. A compelling body of evidence indicates that Apatinib exerts potent anti-tumor effects in various human cancers, including gastric cancer, breast cancer, non-small cell lung cancer (NSCLC), and cholangio-carcinoma [Citation5–Citation8]. Considering its profound efficacy, Apatinib has been recently approved to treat patients with advanced metastatic gastric cancer by the China Food and Drug Administration (CFDA), and a series of clinical trials are currently underway to test its efficacy in other malignancies. In the present study, we aim to investigate the effects of Apatinib in human cervical cancer.
Results
VEGFR2 is overexpressed in cervical cancer and predicts a poor prognosis
As the IHC showed, positive VEGFR2 staining was detected in 19.1% of normal tissues (4/21, with none manifesting high VEGFR2 expression), and in 71.5% (108/151, with 58 cases showing high VEGFR2 expression) of cervical cancer cases (P < 0.001, ). Overexpression of VEGFR2 was not correlated with histological differentiation (P = 0.391, ), but was significantly correlated with advanced tumor stage (P = 0.026, ). There was no relationship between VEGFR2 expression and pathologic subtype (P = 0.513, ) or with patient age (P = 0.285, ). Among patients who accepted the surgery (n = 128), a high level of VEGFR2 was notably associated with lymph node metastasis (P = 0.012, ), but not lymph vascular space invasion (P = 0.33, ).
Figure 1. VEGFR2 was overexpressed in cervical cancer and predicted poor prognosis. (a and b) As the IHC showed, positive VEGFR2 staining was detected in 19.1% normal cervix (4/21, none was VEGFR2 high expression) while in 71.5% (108/151, 58 cases were VEGFR2 high expression) cervical cancer (P < 0.001); (c and d) Overexpression of VEGFR2 was not correlated with histological differentiation (P = 0.391), but significantly indicated advanced tumor stage (P = 0.026); (e and f) There was no relationship between VEGFR2 expression and pathological subtype (P = 0.513) or with patient age (P = 0.285); (g and h) Among patients who accepted the surgery (n = 128), high level of VEGFR2 was notably associated with lympho node metastasis (P = 0.012), but not lymph vascular space invasion (P = 0.330); (i) Patients with high expression of VEGFR2 obtained a much shorter overall survival (P = 0.025)
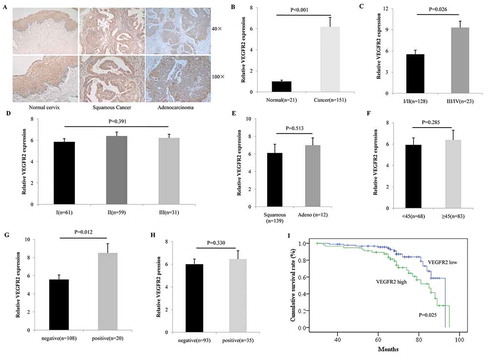
We further investigated the relationship between VEGFR2 levels and patient overall survival, and the Kaplan-Meier analysis indicated that patients with a high expression of VEGFR2 exhibited a much shorter overall survival (P = 0.025, ).
Apatinib suppresses cellular proliferation and arrests cell cycle progression
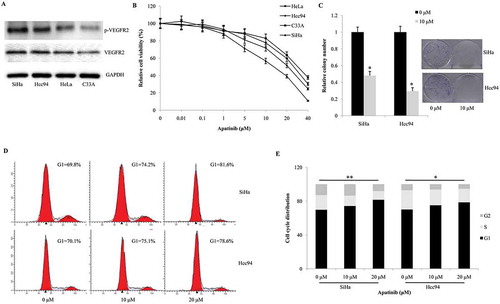
Then we tested the protein expression of VEGFR2 and phosphorylated VEGFR2 in cervical cancer cells. According with western blot, VEGFR2 protein was positive in all the four cervical cancer cell lines. SiHa and Hcc94 cells presented the highest level of phosphorylated VEGFR2, while C33A and HeLa cells contained moderate and mild level of phosphorylated VEGFR2 (). Next, we screened the cytotoxicity of Apatinib using CCK-8 assay. As our data shown, SiHa and HCC94 cells were more sensitive to Apatinib than C33A and HeLa cells (IC50 = 13.9 μM, 21.8 μM, 26.6 μM, 31.2 μM, respectively, ). Next, we explored the effects of Apatinib on colony-formation. Compared these to the negative control, Apatinib significantly decreased the number of colonies formed after a 2-week culture in both SiHa and Hcc94 cells (P = 0.037 and 0.023, ). Further, the 24-hour treatment of Apatinib induced significant G1-phase arrest in a dose-dependent manner in both SiHa and Hcc94 cells (P = 0.007 and 0.016, ).
Figure 2. Apatinib suppresses cellular proliferation by arresting the cell cycle and inducing apoptosis. (a) As western blot shown, the protein expression of VEGFR2 were positive in all the four cervical cancer cell lines. SiHa and Hcc94 cells presented the highest level of phosphorylated VEGFR2, while C33A and HeLa cells contained moderate and mild level of phosphorylated VEGFR2; (b) In CCK-8 assay, SiHa and HCC94 cells were more sensitive to Apatinib than C33A and HeLa cells (IC50 = 13.9 μM, 21.8 μM, 26.6 μM, 31.2 μM, respectively); (c) Compared these to the negative control, Apatinib significantly decreased the number of colonies formed after a 2- week culture in both SiHa and Hcc94 cells (P = 0.037 and 0.023, respectively); (d and e) the 24-hour treatment of Apatinib induced significant G1-phase arrest in a dose-dependent manner in both SiHa and Hcc94 cells (P = 0.007 and 0.016, respectively)
Apatinib promotes apoptosis and induces cellular stress in cervical cancer cells
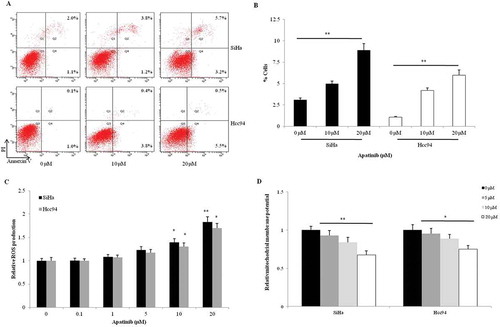
Compared to the control group, we found that both 10 μM and 20 μM Apatinib could direct more SiHa and Hcc94 cells toward apoptosis (P = 0.005 and 0.001, respectively, ). The elevation of ROS commonly indicates cellular response to stress like chemotherapy and radiotherapy. In the two cervical cancer cells, Apatinib significantly enhanced the production of ROS in a dose-dependent manner, especially in the 10 μM and 20 μM groups (). Then we detected the effects of Apatinib on cellular mitochondrial membrane potential (an apoptotic marker). Compared to the control, the mitochondrial membrane potential in SiHa and Hcc94 cells were notably downregulated by the 2-hour treatment with Apatinib (P = 0.003 and 0.023, respectively, ).
Figure 3. Apatinib promotes apoptosis and induces cellular stress in cervical cancer cells. (a and b) Apatinib directed more SiHa and Hcc94 cells toward apoptosis compared to the control group (P = 0.005 and 0.001, respectively); (c) In the two cervical cancer cells, Apatinib significantly promoted the production of ROS in a dose-dependent manner, especially in the 10 μM and 20 μM groups; (d) Compared to the control, the mitochondrial membrane potential in SiHa and Hcc94 cells were notably downregulated by the 2-hour treatment with Apatinib (P = 0.003 and 0.023, respectively)
Apatinib affected the gene expression profile in SiHa cells
To further reveal the underlying mechanisms, we treated SiHa cells with 20 μM Apatinib for 24 hours and performed microarray analysis to investigate alterations in gene expression profile. As shown in , many genes involved in cell cycle progression (CDK1, CCNC and CCND3), apoptosis (DAD1 and BAG1), and proliferation (PCNA) were significantly downregulated. Moreover, several key elements in MAPK/ERK (MAPK3, MAPK8 and MAPK3K1) and TGF (TGFB1) signaling pathways were also suppressed. Using signaling pathway analysis (KEGG analysis), we observed that p53, proteasome, TGF-beta, cell cycle, homologous recombination and mismatch repair signaling pathways were the most significantly regulated by Apatinib (). In addition, Gene Ontology analysis showed that the genes involved in mitotic cell cycle (both G1/S transition and G2/M transition), cell division, DNA repair, and cell proliferation were under the precise regulation of Apatinib (). Interestingly, the expressions of VEGF-A and IL-6 were notably upregulated after the Apatinib treatment, which might be due to the negative feedback effects of blocking VEGFR2 activities (). Using real time PCR, we confirmed the increased mRNA levels of VEGF-A, IL-6 (), and the decreased expressions of CDK1, CCNC, CCND3 (). Further, the activities of VEGFR2 and its known downstream targets were detected by western blot. In both SiHa and Hcc94 cells, the 24-hour treatment with Apatinib significantly inhibited the phosphorylation of VEGFR2, ERK and AKT, indicating its highly selective targeting and efficiency (). Consistent with the cell cycle alterations and microarray analysis, we detected the significant downregulation of cyclin D3 protein and upregulation of p27 ().
Figure 4. Apatinib affects the gene expression profiles in cervical cancer cells. (a) According with the gene expression profile in SiHa, many genes involved in cell cycle progression (CDK1, CCNC and CCND3), apoptosis (DAD1 and BAG1), and proliferation (PCNA) were significantly downregulated by the treatment of Apatinib; (b) Using KEGG analysis, we observed that p53, proteasome, TGF-beta, cell cycle, homologous recombination and mismatch repair signaling pathways were the most significantly regulated by Apatinib; (c) Gene Ontology analysis showed that the genes involved in mitotic cell cycle (both G1/S transition and G2/M transition), cell division, DNA repair, and cell proliferation were under the precise regulation of Apatinib; (d and e) Using real time PCR, we confirmed the increased mRNA levels of VEGF-A, IL-6, and the decreased expressions of CDK1, CCNC, CCND3; (f) In both SiHa and Hcc94 cells, the 24-hour treatment with Apatinib significantly inhibited the phosphorylation of VEGFR2, ERK and AKT
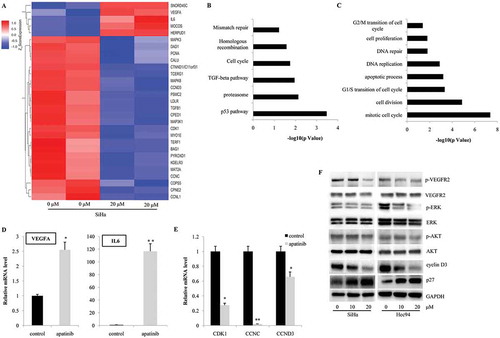
Apatinib synergizes with Paclitaxel in cervical cancer cells
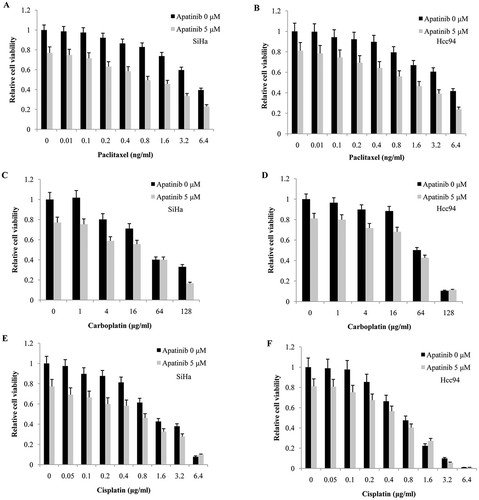
We then tested whether Apatinib could synergize with routine chemotherapy in cervical cancer. The combination index (CI) was calculated using the Chou-Talalay equation and CI<1 indicated a synergistic effect between the two drugs. As our data show, a low dose of Apatinib (5 μM) allowed for significant synergistic effects with Paclitaxel in both SiHa (CI ranged from 0.351 to 0.629, , ) and Hcc94 cells (CI ranged from 0.159 to 0.552, , ). However, we found no synergy between Apatinib and cisplatin (CI ranged from 1.149 to 5.647 in SiHa, CI ranged from 0.85 to 1.682 in Hcc94, , ) or carboplatin (CI ranged from 0.669 to 1.402 in SiHa and from 0.955 to 1.206 in Hcc94, , ).
Table 1. Combination indexes (CI) of Apatinib with Paclitaxel, Cisplatin or carboplatin
Table 2. The clinico-pathological parameters of cervical cancer patients and normal control
Table 3. Primer sequences for real time PCR
Apatinib suppresses the growth of SiHa xenografts in vivo
To better mimic the biologic characteristics of cervical cancer, we used 9 primary cultured tissues to test the anti-tumor efficacy of Apatinib. Among these, 4 cases showed significant sensitivity to Apatinib (IC50 < 20 μM, ). Then we investigated the correlation between Apatinib efficacy and VEGFR2 expression. As shown in , cancer tissues with higher level of VEGFR2 were more sensitive to the treatment with Apatinib (R2 = 0.4067, P = 0.035).
Figure 6. Apatinib suppresses the growth of SiHa xenografts in vivo. (a) As the CCK-8 assay shown, 5 of 11 primary cervical cancer tissues showed significant sensitivity to Apatinib (IC50 < 20 μM); (b) these cancer tissues with higher level of VEGFR2 were more sensitive to Apatinib (R2 = 0.4067, P = 0.035); (c) In a nude mouse model, the single use of Apatinib or Paclitaxel significantly suppressed the growth of SiHa xenografts, and the combination with the 2 drugs further augmented this effect (P = 0.043, 0.031 and <0.001, respectively); (d) Compared to the control group, tumor weights in the Apatinib group and Paclitaxel group were significant lower (P = 0.05 and 0.024, respectively), and the tumor weights in the combination group were the lowest (P = 0.007); (e and f) As IHC shown, the phosphorylation of VEGFR2 was positive in the control group and Paclitaxel group (P = 0.033 and 0.019, respectively), but was notably inhibited in Apatinib group and combination group. In addition, Ki-67 (a proliferative marker) was suppressed by the single treatment with Apatinib or Paclitaxel, and was further decreased in tumors treated with Apatinib plus Paclitaxel (P = 0.041, 0.029 and 0.005, respectively)
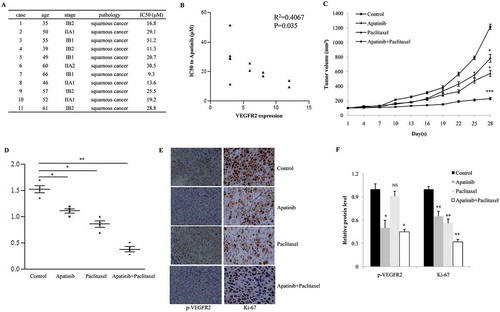
A nude mouse model bearing SiHa xenografts was established to test the anti-tumor effects of Apatinib. According to the growth curve, the single use of Apatinib or Paclitaxel significantly suppressed the growth of SiHa xenografts, and the combination with the 2 drugs further augmented this effect (P = 0.043, 0.031 and <0.001, respectively, ). At the end of treatment, all tumors were collected and weighted. Compared to the control group, tumor weights in the Apatinib group and Paclitaxel group were significant lower (P = 0.05 and 0.024, respectively, ), and the tumor weights in the combination group were the lowest (P = 0.007, ). As shown in , the phosphorylation of VEGFR2 was positive in the control group and Paclitaxel group (P = 0.033 and 0.019, respectively), but was notably inhibited in Apatinib group and combination group; in addition, Ki-67 (a proliferative marker) was suppressed by the single treatment with Apatinib or Paclitaxel, and was further decreased in tumors treated with Apatinib plus Paclitaxel (P = 0.041, 0.029 and 0.005, respectively).
Discussion
VEGFR2 is the main receptor that interacts with VEGF-A to promote angiogenesis. Beside its functions during angiogenesis, the VEGF/VEGFR2 signal also played a role in tumor proliferation, migration, invasion and immunity, through regulating the downstream targets such as PI3K/AKT, MAPK/ERK, PLCγ, c-SRC and STAT3 [Citation9–Citation13]. A large body of evidence has demonstrated the high expression of VEGFs and VEGFR2 in cervical pre-cancerous lesions and invasive cancer, which significantly correlated with worsening clinico-pathologic parameters and a poor prognosis [Citation14–Citation17]. Thus, targeting VEGF/VEGFR2 signal was proposed to be a potent strategy for treating cervical cancer.
In the present study, we first investigated the efficacy of Apatinib in cervical cancer, and our data showed that Apatinib suppressed cervical cancer growth in a dose-dependent manner by arresting cell cycle, inducing apoptosis and promoting ROS production. Similar findings have been reported by a large number of researchers. Tian et al. reported that Apatinib suppressed the growth of lung cancer and stomach cancer by inactivating VEGFR2, c-kit, and c-src; moreover, Apatinib also prevented the budding of the rat aortic ring [Citation4]. In osteosarcoma, Apatinib can inhibit the growth of osteosarcoma cells by inducing autophagy in addition to cell cycle arrest and apoptosis. Blocking VEGFR2 with Apatinib significantly suppressed the activity of STAT3 and BCL-2 and subsequently suppressed angiogenesis and tumor growth of the osteosarcoma, suggesting that co-targeting VEGFR2 and STAT3 might constitute a potent therapeutic strategy [Citation18]. Furthermore, Apatinib showed definitive efficacy in RET-rearranged lung adenocarcinoma. The abnormal KIF5B-RET fusion gene enhanced cellular invasion and migration via the Src signaling pathway, and this was significantly suppressed by Apatinib [Citation19]. Interestingly, we found that the mRNA expression of VEGF-A and IL6 were markedly upregulated after the treatment with Apatinib, which might suggest a feedback effect by blocking VEGFR2 and warrant further investigations. Collectively, our findings strongly support Apatinib as a valid drug for treating human cervical cancer.
Apatinib has been found to improve cellular sensitivity to routine chemotherapy. In 2010, Mi et al. first reported that Apatinib significantly increased the cytotoxicity of Doxorubicin in a large panel of human cancer cell lines. They then proved that Apatinib could reverse multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters such as ABCB1 and ABCG2 [Citation20]. Moreover, Li et al. found that Apatinib notably enhanced the anti-tumor activity of gefitinib in NSCLC cells and xenografts carrying T790M mutations and demonstrated resistance to EGFR-TKIs [Citation7]. Herein, we herein reported that Apatinib synergizes with Paclitaxel (but not Cisplatin or Carboplatin) and exhibits a powerful ability to suppress the growth of cervical cancer in vitro and in vivo. This findings warrant the clinical trials to investigate whether the combination of Paclitaxel and Apatinib could achieve more efficacy in cervical cancer patients.
In conclusion, we are the first group to report the significant anti-tumor effects of Apatinib in cervical cancer, which could inhibit VEGFR2 phosphorylation and its downstream targets. Further, we demonstrated that Apatinib synergized with Paclitaxel to achieve more significant suppression both in vitro and vivo. We propose that Apatinib be used as a potent drug for cervical cancer.
Materials and methods
Samples collection
From June 2010 to December 2013, 151 cervical cancer tissues were collected from the First Affiliated Hospital of Zhengzhou University. Twenty-one age-matched normal cervical tissues were used as normal controls. Written consent was obtained from all patients. Clinico-pathologic information is provided in and diagnosis was confirmed by two experienced pathologists. Tumor staging was carried out according to FIGO 2009 (International Federation of Gynecology and Obstetrics) criteria. Our study was approved by the Ethical Committee of the First Affiliated Hospital of Zhengzhou University.
Cell lines and reagents
Four human cervical cancer cell lines (HeLa, SiHa, HCC 94, and C33A) were purchased from the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). The media used were as follows: RPMI 1640 (Hyclone, Logan, UT, USA) for SiHa and HCC 94, DMEM (Hyclone) for HeLa, and MEM (Hyclone) for C33A. Ten percent fetal bovine serum (FBS, ExCell Bio, Shanghai, China) was used to supplement the medium.
We purchased Apatinib, Paclitaxel, Cisplatin and Carboplatin from Abmole Bioscience (Shanghai, China).
Immunohistochemical staining
Slides containing sections at a thickness of 4 μm were dewaxed and hydrated, and heated antigen retrieval was performed in citrate buffer (10 mM, pH = 6.0). We washed the slides 3 times with PBS and incubated them with 5% BSA at 37°C for 10 minutes. After the BSA was rinsed away, the slides were further incubated overnight with primary antibodies at 4°C. The secondary antibody was applied the next day and the staining was developed using DAB chromogenic reagent kit (Boster Biocompany, Wuhan, China) following the manufacturer’s instructions. The results were scored by 2 experienced pathologists with respect to staining intensity and the percentage of immunoreactive cancer cells. When the proportion of cells showing brown granules was <10%, we provided a score of 0; at 11% to 25%, a score of 1; at 26% to 50%, 2; 51% to 75%, 3; and 76% to 100%, 4. Regarding staining intensity, negative staining was given a score of 0; mild positivity, 1; moderate positivity, 2; and strong positivity, 3. The staining positivity was determined using the positive percentage score multiplied by the intensity score. A score of 0 to ≤ 1 was defined as “negative”, and >1 as “positive”. Total scores of 6 or more were categorized as high VEGFR2 expression; otherwise, they were categorized as low VEGFR2 expression.
Reactive oxygen species (ROS) assay
The production of ROS was detected using a commercial kit purchased from Beyotime (Shanghai, China). Briefly, 1 × 104 cells/well were routinely cultured in a 96-well plate and treated with different doses of Apatinib for 6 hours. Then the DCFH-DA solution (10 μM) was added into each well and reacted for 20 minutes. The fluorescence (excitation wavelength = 488 nm, emission wavelength = 525 nm) was measured on a plate reader. This procedure was repeated three times.
Mitochondrial membrane potential measurement
The mitochondrial membrane potential assay kit with JC-1 (Beyotime) was used for this experiment. In brief, 1 × 106 cervical cancer cells were treated with Apatinib for 2 hours and then the cells were stained with JC-1 for 15 minutes in the dark. Finally, the cells were analyzed on a FACS Calibur (BD Biosciences, CA, USA) and data was analyzed using the FCS4 express software. This procedure was repeated three times.
Real time PCR
Total RNA was extracted using the Trizol buffer (Beyotime) and the mRNA expression of target genes was determined by real time PCR using the BeyoFast™ SYBR Green qPCR Mix (Beyotime). The primer sequences were listed in . β-actin was used as the internal control.
Western blot
Total protein was extracted from cells using the RIPA buffer (Beyotime). Then the samples were separated on SDS-PAGE gels and transferred to PVDF membranes for the incubation with primary antibodies. On the next day, appropriate secondary antibodies were added on the membranes and specific protein bands were developed using a commercial ECL chemiluminescence kit (Beyotime). The primary antibodies were all purchased from Cell Signaling Technology (Danvers, MA, USA).
Cell viability assay
A cell counting kit 8 (CCK-8, Abmole Bioscience) was used to evaluate cellular viability. In brief, 2 × 103 cells per well were seeded in 96-well plates and routinely cultured overnight. Then the cells were treated with different doses of Apatinib for 72 hours. The absorbance at 450 nm at indicated time points was measured on a microplate reader. This experiment was repeated three times.
Colony formation assay
One hundred cells per well were plated into 6-well plates and cultured overnight. Then Apatinib was added and these cells were further cultured for 2 weeks. Finally, the clones were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Colonies containing more than 50 cells were counted on a microscope. This procedure was repeated three times.
Apoptosis assay
Apoptosis was detected using an Annexin V-FITC/PI Apoptosis Detection Kit (CoWin Biosciences, Beijing, China) according to the manufacturer’s instructions. Briefly, 1 × 106 cells were plated into a 60-mm dish and incubated overnight. Apatinib was added to treat the cells for another 24 hours; and then cells were harvested and stained, and the apoptotic status was analyzed on a FACS caliber system (BD Biosciences, CA, USA). This procedure was repeated three times.
Cell cycle analysis
To assess cell cycle, 1 × 106 cells were plated into a 60-mm dish and treated with different dose of Apatinib for 24 hours. Then cells were harvested and stained with PI (Cell Cycle and Apoptosis Detection Kit, CoWin Biosciences) and cell cycle status was determined on the FACS caliber system. This procedure was repeated three times.
Gene expression profiling by microarray
The Affymetrix Human oelncRNA (Oebiotech, Shanghai, China) was used to compare gene-expression profiles between SiHa cells treated with Apatinib or not. Total RNAs was quantified by the NanoDrop 2000 (Thermo Scientific, CA, United States) and the RNAs integrity was assessed using Agilent Bioanalyzer 2100 (Agilent Technologies, CA, United States).The sample labeling, microarray hybridization and washing were performed based on the manufacturer’s standard protocols. Gene Chip Command Console (Affymetrix, CA, United States) software was used to extract raw data and Expression Console (Affymetrix) software offered RNA normalization for both gene and exon level analysis. Genesrping software (Agilent Technologies) was used to finish the basic analysis. Differentially expressed genes were then identified through fold-change as well as P value calculated with t-test. The threshold set for up- and down-regulated genes was a fold change ≥ 2.0 and a P value ≤ 0.05. Afterwards, GO analysis and KEGG analysis were applied to determine the roles of these differentially expressed mRNAs played in these GO terms or pathways. Finally, Hierarchical Clustering was performed to display the distinguishable genes’ expression pattern among samples.
Primary culture of cervical cancer tissues
Fresh cervical cancer tissues were obtained during surgery (with specimens larger than 1 cm3) and immediately washed with Hank’s Buffered Salt Solution (HBSS), minced into tiny pieces with a scalpel, and then digested in a solution containing 0.2% collagenase IA for 1 hour at 37°C. After a short centrifugation (1000g, 5 minutes), the cells were re-suspended with DMEM medium and seeded into appropriate plates.
Tumor xenografts growth assay
Our protocol was approved by the Institutional Authority for Laboratory Animal Care of Jiangnan University. The 4–5-week-old female Balb/c nude mice were purchased from Slac biotechnology (Shanghai, China), and 5 × 106 SiHa cells were subcutaneously injected into the right axilla of the mice. Different treatments were then administered when the average tumor volume reached 100 mm3. The mice were divided into four groups (n = 6 for each): control group, Apatinib group, Paclitaxel group, and Apatinib + Paclitaxel group. We administered Apatinib by gavage from days 1 to 14 at a dose of 150 mg/kg/day, and Paclitaxel by intraperitoneal injection twice a week at a dose of 15 mg/kg (8 times in total). At the end of the fourth week, all mice were sacrificed and tumors were collected for further analysis. Tumor diameters were measured every 3 days and tumor volume was calculated as 0.5 × length × width2.
Statistical analysis
SPSS software (version 21.0, SPSS Inc., Chicago, IL, USA) was used for statistical analyzes. The χ 2 -test was used to assess the correlations between VEGFR2 expression and clinico-pathologic parameters, the Kaplan–Meier method was performed for the survival analysis, and other data were assessed using Student’s two-tailed t tests or ANOVA appropriately. P < 0.05 was considered to be statistically significant.
Author contributions
JL and YW designed and planned all the experiments. HFQ and QLL performed the in vitro experiments, MT performed the in vivo experiments. JL analyzed the data and wrote the manuscript.
Acknowledgments
We thanks the Letpub company for the English-writing service.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. PubMed PMID: 26808342.
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. PubMed PMID: 25651787.
- Mountzios G, Soultati A, Pectasides D, et al. Developments in the systemic treatment of metastatic cervical cancer. Cancer Treat Rev. 2013;39(5):430–443. PubMed PMID: 22727690.
- Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102(7):1374–1380. PubMed PMID: 21443688.
- Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34(13):1448–1454. PubMed PMID: 26884585.
- Hu X, Zhang J, Xu B, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer. 2014;135(8):1961–1969. PubMed PMID: 24604288.
- Li F, Zhu T, Cao B, et al. Apatinib enhances antitumour activity of EGFR-TKIs in non-small cell lung cancer with EGFR-TKI resistance. Eur J Cancer. 2017;84:184–192. PubMed PMID: 28822888.
- Peng H, Zhang Q, Li J, et al. Apatinib inhibits VEGF signaling and promotes apoptosis in intrahepatic cholangiocarcinoma. Oncotarget. 2016;7(13):17220–17229. PubMed PMID: 26967384.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. PubMed PMID: 21376230.
- Zhu P, Hu C, Hui K, et al. The role and significance of VEGFR2(+) regulatory T cells in tumor immunity. Onco Targets Ther. 2017;10:4315–4319. PubMed PMID: 28919780.
- Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23(5):1011–1027. PubMed PMID: 15585754.
- Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13(12):871–882. PubMed PMID: 24263190.
- Smith GA, Fearnley GW, Abdul-Zani I, et al. Ubiquitination of basal VEGFR2 regulates signal transduction and endothelial function. Biol Open. 2017;6(10):1404–1415. PubMed PMID: 28798148.
- Branca M, Giorgi C, Santini D, et al. Aberrant expression of VEGF-C is related to grade of cervical intraepithelial neoplasia (CIN) and high risk HPV, but does not predict virus clearance after treatment of CIN or prognosis of cervical cancer. J Clin Pathol. 2006;59(1):40–47. PubMed PMID: 16394279.
- Chen B, Zhang C, Dong P, et al. Molecular regulation of cervical cancer growth and invasion by VEGFa. Tumour Biol. 2014;35(11):11587–11593. PubMed PMID: 25135429.
- Goncharuk IV, Vorobjova LI, Lukyanova NY, et al. Vascular endothelial growth factor exression in uterine cervical cancer: correlation with clinicopathologic characteristics and survival. Exp Oncol. 2009;31(3):179–181. PubMed PMID: 19783962.
- Hammes LS, Tekmal RR, Naud P, et al. Up-regulation of VEGF, c-fms and COX-2 expression correlates with severity of cervical cancer precursor (CIN) lesions and invasive disease. Gynecol Oncol. 2008;110(3):445–451. PubMed PMID: 18565574.
- Liu K, Ren T, Huang Y, et al. Apatinib promotes autophagy and apoptosis through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death Dis. 2017;8(8):e3015. PubMed PMID: 28837148.
- Lin C, Wang S, Xie W, et al. Apatinib inhibits cellular invasion and migration by fusion kinase KIF5B-RET via suppressing RET/Src signaling pathway. Oncotarget. 2016;7(37):59236–59244. PubMed PMID: 27494860.
- Mi YJ, Liang YJ, Huang HB, et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res. 2010;70(20):7981–7991. PubMed PMID: 20876799.