ABSTRACT
Psychological stress, which exerts detrimental effects on human reproduction, may compromise the meiotic competence of oocytes. Meiotic resumption, germinal vesicle breakdown (GVBD), is the first milestone to confer meiotic competence to oocytes. In the practice of assisted reproductive technology (ART), the timing for GVBD is associated with the rates of cleavage and blastocyst formation. However, whether chronic stress compromises oocyte competence by influencing GVBD and the underlying mechanisms are unclear. In the present study, a chronic restraint stress (CRS) mouse model was used to investigate the effects of stress on oocyte meiotic resumption, as well as the mechanisms. Following a 4-week chronic restraint stress in female mice, the percentage of abnormal bipolar spindles increased and indicated compromised oocyte competence in the CRS group. Furthermore, we identified a decreased percentage of GVBD and prolonged time of GVBD in the CRS mouse oocytes compared with the control group. CRS simultaneously reduced the expression of cyclin B1 (CCNB1), which represents a regulatory subunit of M-phase/mature promoting factor (MPF). However, MG132, an inhibitor of anaphase-promoting complex/cyclosome (APC/C), could rescue the prolonged time of GVBD and increase the expression level of CCNB1 of oocytes from the CRS mice. Collectively, our results demonstrated that stress disturbed meiotic resumption through APC/C-mediated CCNB1 degradation, thus providing a novel understanding for stress-related oocyte quality decline; moreover, it may provide a non-invasive approach to select high-quality gametes and novel targets for molecular therapy to treat stress-related female infertility.
Introduction
Infertility is currently a major health issue and is increasing worldwide as a result of various adverse causes. Studies suggest that psychological stress may exert detrimental effects on reproduction. For example, epidemiological studies in humans have indicated that acute and chronic psychological stress were associated with a reduction in the rate of retrieved, fertilized, pregnancy, and live birth deliveries, as well as decreased birth weight [Citation1]. A decreased pregnancy rate was positively associated with anxiety and depression scores [Citation2,Citation3].
Restraint stress is utilized to investigate the effects of psychological stress on health and female reproduction. Psychological stress in animal models impairs reproduction in females by affecting folliculogenesis and oocyte quality. For example, 24-hour acute restraint stress could elevate the stress-related hormone Corticotropin-Releasing hormone (CRH), thus disturbing ovarian steroid biosynthesis and inducing apoptosis of granulosa cells [Citation4,Citation5]. Furthermore, CRH could decrease the rates of oocyte cleavage and blastocyst formation in vitro [Citation6]. In addition, one-time exposure to restraint stress prior to ovulation increased oocyte aneuploidy and diminished the development potential of oocytes [Citation7,Citation8]. However, ovaries responded differently to short- and long-term chronic restraint stress exposure [Citation9]. Humans are challenged not only by acute psychological stress but also by chronic stress during their daily life. Moreover, increasing evidence has demonstrated that oocyte quality depended on the events before germinal vesicle breakdown (GVBD), which suggests that the oocyte may accumulate appropriate information for meiotic resumption, fertilization and embryo development prior to chromosome condensation [Citation10,Citation11]. However, to date, it remains unclear whether long-term psychological stress during the complete process of oogenesis from primordial follicle to antral follicle poses detrimental effects on oocyte quality.
The quality of an oocyte is reflected by meiotic and developmental competence. During oocyte maturation after hormonal stimulation, the oocyte enters the GVBD stage and then serially undergoes further meiotic divisions (metaphase I [MI] and metaphase II [MII]) until secondary meiotic arrest, with a stable barrel-shaped spindle and aligned chromosome on the equatorial plate [Citation12–Citation14]. It is well documented that normal fertilization and subsequent embryonic development from the MII oocyte requires proper MII-spindle assembly and chromosome alignment, which are critical for correct sister chromatid segregation [Citation15–Citation17]. Furthermore, studies have demonstrated that oocytes with a MII-spindle deficient are more prone to lower rates of fertilization, cleavage and development to top-quality embryos [Citation18–Citation20]. Thus, the MII-spindle not only reflects oocyte meiotic competence but also determines oocyte developmental competence.
Meiotic resumption is the first milestone that confers oocyte meiotic competence. Most meiotically incompetent oocytes were arrested in the GV-stage in the process of in vitro maturation (IVM) [Citation21,Citation22]. Oocytes failed to acquire meiotic competence mainly derived from GV-stage oocytes that failed to resume meiosis [Citation23,Citation24]. Furthermore, the timing of GVBD was related to the preimplantation developmental competence [Citation25,Citation26].
In addition, previous studies have reported that the competence of oocyte meiotic resumption depended on the oocyte diameter [Citation27,Citation28], chromatin conformation [Citation29], and a series of signaling pathways [Citation30,Citation31] in the GV-stage oocyte. In mice, studies have reported that only oocyte diameters greater than 60 µm successfully underwent GVBD [Citation32]. Two main different types of chromatin organization are present in antral mouse oocytes: Surrounded Nucleolus (SN) oocytes, with a ring of Hoechst-positive chromatin that surrounds the nucleolus, and non-surrounded nucleolus (NSN) oocytes, which lack the ring and exhibit a more dispersed chromatin . A recent study reported that 17.4% of NSN oocytes were arrested at the GV-stage compared with SN oocytes, which resumed meiosis completely [Citation33].
Maturation promoting factor (MPF) is one of the most important terminal effectors in meiotic progression; it is a heterodimer composed of a catalytic Cdk1 and a regulatory CCNB1 subunit (hereafter Cdk1/CCNB1) [Citation21,Citation34]. Increasing the nuclear CCNB1 level and exogenous CCNB1 promoted G2/M transition [Citation35]. Securin is an APC/C substrate that is ubiquitinated and destroyed concomitantly with CCNB1; its degradation rate reflects the activity of APC/C as shown in previous studies [Citation36,Citation37].
In this study, we investigate the effects of CRS on meiotic resumption in mice. Following a 4-week stress, increased abnormal MII-spindles reflected yielded oocyte competence in the CRS mouse group. Moreover, time-lapse living cell imaging showed that stress delayed oocyte progression into the M-phase in the CRS mouse oocytes. Furthermore, stress increased the ratio of NSN-type oocytes and decreased the protein level of CCNB1 in the cytoplasm in CRS mouse oocytes. However, both the protein level of CCNB1 and the time of GVBD were normalized in the stressed oocytes treated with the APC/C inhibitor MG132. Therefore, our results demonstrate stress can compromise the competence of oocytes.
Results
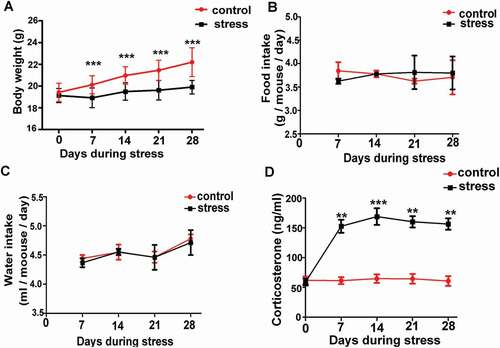
CRS decreases body weight gain and increases serum corticosterone
To establish the chronic psychological stress mouse model, CRS was conducted on mice as previously described. The serum concentration of corticosterone, which comprises a stress-related hormone, was significantly higher in the mice of the CRS group than the level of the control mice in every week during stress (, **P < 0.01, ***P < 0.001, n = 6). Thus, the chronic psychological stress mouse model was successfully established. We observed that the CRS group had a significantly lower body weight in every week during stress (, P < 0.001, n = 6). To demonstrate whether the decreased body weight was caused by decreased intake of food and water in the CRS group, we measured the intake of food and water every 24 hours per mouse; however, there was no significant difference between the control group and the CRS group (, P > 0.05, n = 6).
Figure 1. The establishment of psychological stress mouse model. A) Chronic restraint stress decreased body weight gain (n = 6). B) and C) There was no difference in average daily food or water intake per mouse between control and stress groups (n = 6). D) Chronic restraint stress significantly increased serum corticosterone in stress mice compared with control mice every week during the period of stress (n = 6). Data are presented as the mean ± SEM. **P < 0.01 vs. control. ***P < 0.001 vs. control. Control: control group; Stress: CRS group
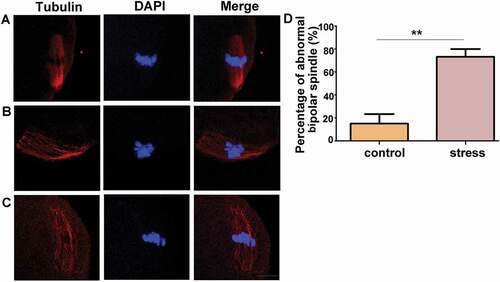
Increased abnormal bipolar spindle reflects yielded oocyte competence in CRS mice
To evaluate the oocyte competence, MII oocytes were collected, immunostained with anti-α-Tubulin antibody to observe the spindle apparatus and counterstained with DAPI to visualize the chromosome alignment. We determined that most of the control oocytes exhibited a typical barrel-shaped spindle apparatus, with a well-aligned chromosome on the equatorial plate as illustrated in . However, in the CRS group oocytes, there were more asymmetric spindles with one pole wider than the other pole as shown in . Moreover, the percentage of abnormal bipolar spindles was analyzed in oocytes from the control or CRS group mice, respectively. A significantly higher proportion of disorganized spindle morphologies was identified in the CRS mouse oocytes (, **P < 0.01, n = 54). Thus, this observation suggested that chronic restraint stress impairs oocyte competence.
Figure 2. Chronic stress increased the percentage of abnormal bipolar spindles. A) A typical barrel-shaped spindle apparatus was formed in oocytes from control group mice. B) and C) Oocytes from CRS group exhibited an asymmetric spindle with one pole wider than the other pole. D) The percentage of abnormal bipolar spindles was significantly higher in oocytes from CRS group than control group (n = 54). Data are presented as the mean ± SEM. **P < 0.01 vs. control. Control: control group; Stress: CRS group
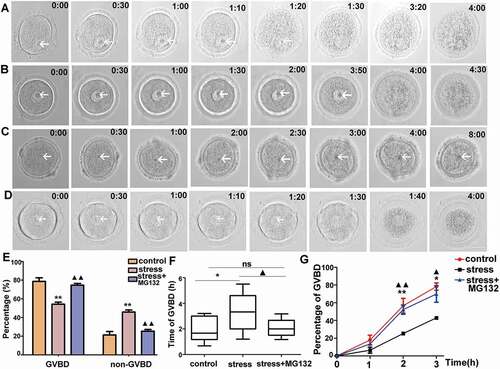
CRS delays the progression of GVBD
Because restraint stress was exerted on GV-stage oocytes in our study, we wondered whether meiotic resumption was one reason for the compromised oocyte competence. Therefore, live oocytes were collected and cultured in vitro in a living cell imaging system to explore the effects of CRS on meiotic resumption. Oocyte from the control group had a complete GVBD after 1.5 hours released from IBMX as shown in . However, the oocytes from the CRS group spent a significantly longer time for GVBD (3 hours and 50 minutes) (), and a part of the oocytes could not complete GVBD within the 8-hour observation (). The statistical data showed that the percentage of GVBD in the oocytes of the CRS group was significantly lower than that of the control group after 8 hours released from IBMX, and 42%-52% of the oocytes were arrested in the GV stage in the CRS mice compared with 13%-27% of the oocytes in the control group (, P < 0.01, n = 54). Furthermore, we analyzed the average time in which oocytes underwent GVBD, excluding oocytes that had a GV after 8 hours released from IBMX, and determined that the average time was significantly prolonged in the CRS mouse oocytes (, 1 hour and 56 minutes vs. 3 hours and 18 minutes, P < 0.05, n = 54). Although it was similar in the first one hour, the percentage of GVBD was significantly lower in the CRS group oocytes at 2 hours (, 42.86%-71.43% vs. 22.22%-28.57%, respectively, P < 0.01, n = 54) and 3 hours (, 71.11%-85.7% vs. 41.67%-44.44%, respectively, P < 0.05, n = 54) released from IBMX. Thus, the CRS mouse oocytes had a reduced capacity to resume meiosis.
Figure 3. Time-lapse microscope observation of live oocytes that underwent GVBD in each group in vitro. A) Oocytes from control group cultured in basic culture medium. B) and C) Oocytes from stress group cultured in basic culture medium. D) Oocyte from stress group cultured in basic culture medium plus MG132. E) Percentages of GVBD and non-GVBD among control, stress, and stress+MG132 groups, respectively, after 8 hours released from IBMX (n = 54). F) The average time required for oocytes from control, stress, and stress+MG132 groups, excluding GV-arrested oocytes for 8 h (n = 54). G) The percentage of GVBD varied from time to time in oocytes among control, stress, and stress+MG132 groups (n = 54). Arrows indicate germinal vesicle in oocytes. Data are presented as the mean ± SEM. Bar = 25 µm. *P < 0.05 vs. control group; **P < 0.01 vs. control group; ▲P < 0.05 vs. stress group; ▲▲P < 0.01 vs. stress group. Control: oocytes from control mice cultured in basic culture medium; stress: oocytes from CRS mice cultured in basic culture medium; stress+MG132: oocytes from CRS group cultured in basic culture medium plus MG132. (GVBD, germinal vesicle breakdown)
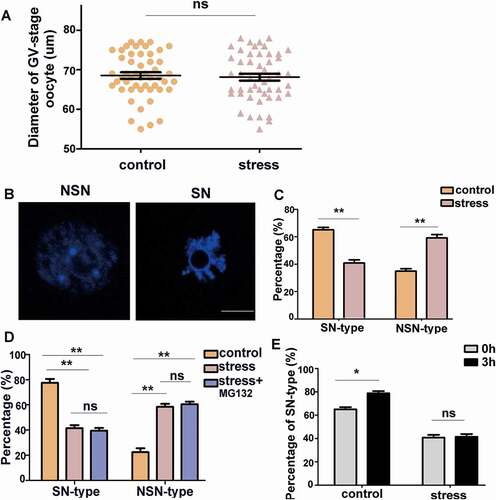
CRS changes the chromatin configuration in GV oocytes
Previous studies have demonstrated that the diameters of immature oocytes were associated with meiotic competence in many species [Citation27,Citation38,Citation39]. We measured the oocyte diameter of the control and CRS group mice. However, there was no significant difference between the two groups (, P > 0.05, n = 50). Previous studies have reported that oocytes with the SN-type nucleus completed meiotic maturation at a high frequency, whereas the frequency of maturation of oocytes with the NSN-type nucleus was low [Citation40,Citation41]. Therefore, the oocyte chromatin configuration was examined in the control and CRS mice. The chromatin configuration pattern was classified into two subgroups as shown in : NSN-type and SN-type oocytes. We determined that the proportions of the two chromatin patterns significantly differed between the oocytes from the control and CRS groups. Stress caused a significantly increasing number of NSN-type oocytes (, P < 0.01, n = 80) and a decreasing number of SN oocytes (, P < 0.01, n = 80). These results demonstrate that psychological stress did not affect the size of oocytes; however, it increased the ratio of oocytes with dispersed chromatin, which may affect the progression of GVBD.
Figure 4. Psychological stress increased the percentage of NSN-type oocytes. A) There was no difference in oocyte diameter between control and CRS groups (n = 50). B) NSN-type and SN-type oocytes are shown (Bar = 25 µm). C) The percentage of NSN-type oocytes increased in CRS group (n = 80). D) The percentage of SN-type and NSN-type oocytes among different groups after three-hour culture in vitro (n = 80). E) After three-hour culture, the percentage of SN-type oocytes increased in control group, whereas there was no difference in CRS group mouse oocytes (n = 80). Data are presented as the mean ± SEM. * P < 0.01, **P < 0.01, ns: non-significant difference
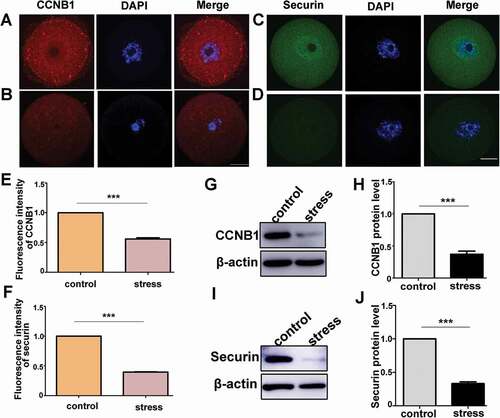
CRS decreases the level of CCNB1 and securin in oocytes
Studies have indicated that the accumulation of CCNB1 in oocytes accelerated GVBD [Citation30,Citation42]. To investigate whether CCNB1 participated in the process of GVBD delay caused by psychological stress, the protein level was evaluated in oocytes. As illustrated in , CCNB1 was distributed throughout the cytoplasm in the control mouse oocytes. However, in the oocytes from the CRS group, the fluorescence signal of CCNB1 was significantly decreased (, P < 0.001, n = 54). In addition, the protein level of CCNB1 was evaluated through western blotting, and there was a significantly decreased protein level in the CRS group mouse oocytes (, P < 0.001, n = 3). The accumulation of CCNB1 in the G2/M transition was mainly maintained by transient low activity of APC/C, which is an E3 ligase that regulates mitotic and meiotic progression through the ubiquitylation and subsequent degradation of key sets of substrates [Citation37,Citation43,Citation44]. Previous studies have indicated that the securin degradation rate could reflect the activity of APC/C [Citation36]. We subsequently assessed the level of securin to measure the APC/C activity. We determined that securin was distributed throughout the cytoplasm and enriched in GV in the oocytes of the control group (). However, there was almost no detectable securin in the oocytes of the CRS group (). The average fluorescence intensity of securin in the control oocytes was significantly higher than that of the CRS group (, P < 0.001, n = 54). Furthermore, the protein level of securin in the CRS group oocytes was significantly decreased in the western blot plane (, P < 0.001, n = 3). Isotype IgGs for the primary antibody were used as the negative controls (supplementary Figure 1). These data indicated the important regulator of meiosis progression, CCNB1, decreased in the oocytes from CRS mice, which may be the cause of the prolonged time of GVBD.
Figure 5. Psychological stress decreased the protein levels of CCNB1 and securin. A) CCNB1 expression in control mouse oocytes. B) CCNB1 expression in stress mouse oocytes. C) Securin distribution in oocytes from control group. D) Securin distribution in oocytes from CRS group. E) The average fluorescence intensity of CCNB1 significantly decreased in CRS mouse oocytes (n = 54). F) The fluorescence intensity of securin in oocytes from CRS group mice significantly decreased compared with control group mice (n = 54). G) and H) CCNB1 protein level decreased in CRS group (150 oocytes per lane, n = 3). I) and J) Securin protein level significantly decreased in oocytes from CRS group (50 oocytes per lane, n = 3). Data are presented as the mean ± SEM. Bar = 25 µm. ***P < 0.001. CCNB1: cyclin B1
MG132 rescues the delayed progression into m-phase of oocytes in CRS mice
MG132, a highly specific and reversible proteasome inhibitor, was used to inhibit APC/C-mediated CCNB1 and securin degradation [Citation45,Citation46]. As shown in , the GVBD occurrence of oocytes from the CRS group cultured with MG132 was identified 1.5 hours after release from IBMX. Moreover, the percentage of GVBD cultured for 8 hours was significantly increased compared with the oocytes of the CRS group (, P < 0.05, n = 54), and there was no significant difference between the oocytes from the control group and the CRS group treated with MG132 (, 1 hour and 55 minutes vs. 2 hours and 4 minutes, respectively, P > 0.05, n = 54). Furthermore, the average time for GVBD occurrence significantly decreased in the CRS mouse oocytes cultured with MG132 compared with the CRS mouse oocytes cultured in basic culture medium (, 2 hours and 4 minutes vs. 3 hours and 18 minutes, respectively, P < 0.05, n = 54). Interestingly, MG132 increased the percentage of GVBD at 2 or 3 hours released from IBMX in the CRS oocytes, respectively, compared with that in the oocytes from the CRS mice (, ▲P < 0.05, ▲▲P < 0.01, n = 54), and there was no significant difference between the MG132 group and the control group (, P > 0.05, n = 54). These data indicated that high APC/C activity in GV stage oocytes may be responsible for the delayed progression into the M-phase.
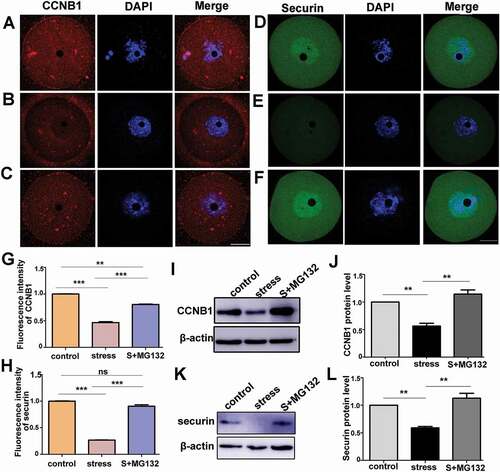
MG132 increases the expression level of CCNB1 and securin in oocytes from CRS group
We subsequently investigated whether the changes in the progression into the M-phase induced by MG132 were accompanied by an upregulation of the CCNB1 and securin protein level. MG132 could reverse the excessive degraded CCNB1 significantly in the CRS mouse oocytes (, P < 0.001, n = 54). However, there was a significantly lower fluorescence intensity of CCNB1 in the CRS+MG132 group compared to the control group (, P < 0.01, n = 54). Moreover, it was verified that the protein level of CCNB1 in the CRS+MG132 group was significantly increased compared with the CRS group (, P < 0.01, n = 3), and there was no significant difference between the control and CRS+MG132 groups by western blotting (,j), P > 0.05, n = 3). As illustrated in , CRS significantly decreased the level of securin and the accumulation in GV (P < 0.001, n = 54). However, in the CRS+MG132 group, as shown in , securin was increased and enriched in GV. The decreased fluorescence intensity of the CRS mouse oocytes was significantly normalized in the CRS+MG132 group (, P < 0.001, n = 54). Moreover, there was no significant difference between the control group and the CRS+MG132 group (, P > 0.05, n = 54). Furthermore, the protein level of securin in the CRS group was normalized by MG132 as shown in (P < 0.01, n = 3), and there was no significant difference between the control and CRS+MG132 groups (P > 0.05, n = 3). These data indicated that MG132 could accumulate the level of CCNB1 and securin in CRS group mice, which may contribute to meiotic resumption.
Figure 6. APC/C inhibitor, MG132, rescued the decreases in the protein levels of CCNB1 and securin in CRS mouse oocytes. A) and D) Immunostaining of CCNB1 and securin in control oocytes cultured in basic culture medium for three hours. B) and E) Immunostaining of CCNB1 and securin in stress oocytes cultured in basic culture medium. C) and F) Immunostaining of CCNB1 and securin in stress group oocytes cultured in basic culture medium + MG132. G) and H) The fluorescence intensity of CCNB1 or securin among three groups was statistically analyzed; MG132 increased the proteins level of CCNB1 and securin in oocytes from CRS group mice (n = 54). I) and J) The protein level of CCNB1 in different groups was analyzed by Western blot; MG132 significantly increased the protein level of CCNB1 in oocytes from CRS group mice (150 oocytes per lane, n = 3). K) and L) Western blotting showing the reduced expression of securin was rescued by MG132 in CRS group mouse oocytes (50 oocytes per lane, n = 3). Data are presented as the mean ± SEM. Bar = 25 µm. **P < 0.01, ***P < 0.001. Control: control group oocytes cultured in basic culture medium; Stress: CRS group oocytes cultured in basic culture medium; S+MG132: CRS group oocytes cultured in basic culture medium + MG132
MG132 fails to rescue the decreased percentage of sn-type oocytes in CRS mouse oocyte in vitro
As previously indicated, the CRS group mouse oocytes treated with MG132 successfully resumed meiosis and the proteins levels of CCNB1 and securin in the CRS mouse oocytes. To investigate whether MG132 could rescue the dispersed chromatin of the CRS group oocyte, oocytes were stained with Hoechst after three hours of culture in basic culture medium with or without MG132, respectively. As shown in , compared with the oocytes from the control group, the percentage of SN-type oocytes was decreased and NSN-type oocytes was increased significantly in the CRS mouse oocytes cultured in culture medium with or without MG132 (P < 0.05, n = 80), whereas there was no difference between the oocytes from the CRS group cultured with or without MG132 (P > 0.05, n = 80). Moreover, we determined that transient GV-arrest significantly changed the chromatin organization in the control mouse oocytes, whereas it did not affect the chromatin configuration of the oocytes from the CRS group as illustrated in . Specifically, after three hours of GV-arrest, the percentage of SN-oocytes significantly increased in the control group (61.54%-67% vs. 75%-82.5%, respectively, P < 0.05, n = 80); however, there was no difference between freshly collected and three-hour arrested oocytes from the CRS mice (36.36%-44.4% vs. 38.46%-46.5%, respectively, P > 0.05, n = 80). Therefore, MG132 failed to condense chromatin in oocytes from the CRS group mice. Transient GV-arrest condensed the chromatin in the control group mouse oocytes and not the CRS mouse oocytes.
Discussion
We utilized a mouse model, in which female BALB/c mice were exposed to restraint stress for 4 weeks, which covers the complete duration of oogenesis in mice [Citation47]. The decreased body weight gain and significantly increased serum corticosterone level demonstrated that the mice underwent experimental stress. Previous studies have reported that 24-hour acute restraint stress diminished the developmental potential of oocytes and the mechanisms were complicated, including CRH-induced granulosa apoptosis and increased aneuploidy [Citation4,Citation8,Citation48]. However, whether the effects of chronic restraint stress on the entire process of oogenesis compromised oocyte competence and its effects on oocyte meiotic resumption are not clear.
After ovulation, oocytes directly enter the metaphase II (MII) stage, waiting for fertilization, in which stable barrel-shaped spindle formation reflects meiotic progression and competence [Citation49]. Furthermore, maternal age-associated meiotic defects have been related to an abnormal spindle apparatus [Citation50]. Moreover, a disarranged spindle was responsible for a lower fertilization rate in obese females [Citation51,Citation52]. It has been reported that benzo[a]pyrene(BaP)-exposure yielded oocyte quality as a result of a prominently defective spindle assembly [Citation53,Citation54]. In our study, 4-week stress significantly increased the percentage of abnormal bipolar spindles; thus, oocyte competence was compromised by chronic restraint stress.
One of the first events that occur during in vitro maturation (IVM), which has an important impact on subsequent developmental events, is meiotic resumption, evidenced by GVBD. Oocytes that failed to acquire meiotic competence were mainly blocked at the GV-stage [Citation21,Citation24]. Furthermore, one study suggested that early GVBD was a predictor of high implantation of developmentally competent oocytes IVM for preselecting high quality oocytes [Citation25]. Psychological stress exerts detrimental effects on the developmental competence of oocytes [Citation7,Citation55]. In the present study, the percentage of oocytes from the CRS mice that underwent GVBD within 8 hours was significantly decreased and the average time for GVBD was prolonged. Our results suggested that psychological stress-induced abnormal meiotic resumption may be responsible for the compromised competence of oocytes.
The nuclear entry of CCNB1 during prophase has been observed in many species using a CCNB1-GFP fusion protein [Citation56,Citation57]. It has been suggested that the accumulation of CCNB1 was accompanied by GVBD, whose degradation was mediated by APC/C [Citation58]. The present study indicated that oocytes from psychological stress mice had a significantly lower protein level of CCNB1 than the control mouse oocytes. Furthermore, oocytes from psychological stress mice had a decreased level of CCNB1, which was correlated with the decreased ratio of GVBD and delayed GVBD. Because the translation of CCNB1 mRNA at the GV stage was repressed [Citation59], we proposed that excessive degradation of CCNB1, which was mainly mediated by APC/C, was induced in the CRS group mouse oocytes. Therefore, we measured the protein level of securin, which may reflect the activity of APC/C [Citation37]. The expression level of securin was significantly lower in the oocytes of the CRS group mice. Further evidence showed that an APC/C inhibitor, MG132, could normalize the percentage of GVBD and the average time for GVBD induced by psychological stress. Moreover, the distribution and intensity of securin were completely repaired, which suggested that APC/C was over activated in oocytes of CRS mice. However, the fluorescence intensity of CCNB1 was only increased to 79%-85% of the level in control oocytes by MG132. In western blotting, comparable CCNB1 protein levels were identified in the oocytes of the CRS group cultured in basic culture medium with MG132 and the control group. In addition, our finding shown that the protein level of securin almost could not be detected in oocyte from CRS group cultured in vitro without MG132 by western blotting. This result indicated high activity APC/C degraded securin in vitro continuously. However, the protein level of CCNB1 in the same condition was detected by western blotting. Consistently, previous studies demonstrated that securin could inhibit APC/C activity, thereby regulating CCNB1 accumulation in GV stage oocytes to promote M-phase entrance [Citation60]. These results suggested that psychological stress disturbed meiotic resumption through APC/C-mediated CCNB1 excessive degradation in mouse oocytes.
In addition to the accumulation of CCNB1, the diameter and chromatin configuration of oocytes were important to confer oocyte meiotic resumption competence. The threshold diameter of the mouse oocyte for GVBD was 60 µm when grown in vitro [Citation32]. In the present study, the oocytes collected were larger than 60 µm and there was no difference between the control and CRS groups. It is implicated that oocyte size is not responsible for the delayed meiotic resumption under a psychological stress condition. In our study, psychological stress increased the composition of NSN-type oocytes and significantly decreased SN-type oocytes. However, MG132, which rescued the delay of meiotic resumption, did not increase the ratio of SN-type oocytes in the CRS group. These results indicated that chromatin configuration may be a mature result of oocytes; however, it could not determine meiotic competence. Consistent with these results, Ogushi et al. reported that enucleated porcine oocytes could mature successfully [Citation61]. Furthermore, through a nuclear transfer technique, Azusa et al. reported that 88% of oocytes with cytoplasm from SN-type oocytes were matured regardless of the nuclear type compared with 20–26% of oocytes with cytoplasm from NSN-type oocytes. It is implied that the meiotic competence was determined by materials in the cytoplasm but not the chromatin configuration [Citation62]. In addition, a previous study reported that the time for GVBD was shortened by acute restraint stress before ovulation, which had limited effects on cytoplasm factors [Citation8]. Therefore, cytoplasmic factors appear to determine meiotic competence; however, a correlation between chromatin configuration and meiotic competence has been reported. NSN-type chromatin becomes SN-type oocytes when antral follicles form [Citation29,Citation63]. Thus, oocytes may acquire the cytoplasmic factors involved in meiotic competence accompanied by the transition of chromatin configuration, thus resulting in an indirect correlation between chromatin configuration and meiotic competence. In addition, previous studies have reported that temporary GV-arrest improved oocyte competence in the presence of GVBD inhibitors [Citation64,Citation65]. Previous studies have reported that IBMX arrested the meiosis promotion of the NSN to SN configuration transition [Citation66]. In our study, after 3 hours of incubation with IBMX, the percentage of SN-type oocytes was significantly increased in the oocytes of the control group mice. However, chromatin organization responded differently to transient IBMX arrest between the control and CRS group mouse oocytes in vitro in the present study. The mechanisms that underlie this differential response require further investigation.
In conclusion, our findings demonstrated that psychological stress yielded oocyte competence reflected by an increased abnormal bipolar spindle formation. The disturbance in meiosis resumption in mouse oocytes, including a decreased percentage of GVBD and a delay of progression into the M-phase, may contribute to compromised oocyte competence. Furthermore, the expression protein levels of CCNB1 and securin were decreased in chronic restraint, which could be rescued by the APC/C inhibitor MG132. Our study implies that psychological stress may pose detrimental effects on oocyte quality, thereby decreasing the oocyte maturation rate and in vitro fertilization (IVF) rate; these results shed new light on the understanding of oocyte quality decline induced by stress. Moreover, these findings indicate a novel approach to select high-quality mature oocytes in vitro. We also provide evidence that an APC/C inhibitor or exogenous CCNB1/securin supplement could serve as a strategy for improving the meiotic activation of poor quality oocytes in the practice of in vitro maturation.
Methods and materials
Animal experiments
Female BALB/c mice aged 6 weeks were purchased from Shanghai SLAC Laboratory Animal Co. Ltd. and were housed in the Department of animal experiments, the Medical school of Shanghai JiaoTong University. The mice were housed with a constant temperature (25°C) and a 10L:14D photoperiod (lights on from 8:00 AM to 6:00 PM). The mice were randomly separated into control and CRS groups. The CRS group mice were placed in 50 ml centrifuge tubes from 9:00 AM to 3:00 PM, which were multi-punctured to maintain sufficient ventilation. The mice in the tubes could breathe freely and move back and forth to some extent; however, they could not turn around. The procedure was randomly conducted for 6 days in a week for continuous 4 weeks. The control mice were placed in their original cages; however, food and water were not provided from 9:00 AM to 3:00 PM. The body weight, intake of food and water of the mice were recorded once per week.
All procedures for animals were approved by the Institutional Animal Care and Use Committee of Shanghai and were performed in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals. Efforts were made to minimize animal suffering and limit the number of animals used in this study.
Serum corticosterone measurement
Mice were euthanized after anesthesia via the inhalation of isoflurane (RWD Life Science Co., Ltd, R510-22). The blood samples of the control and CRS groups were collected each week during stress and were subsequently centrifuged (3000 rpm, 15 min) to separate serum after standing for 2 hours at room temperature (RT). The serum collected was stored at −80°C until further use. The serum concentration of corticosterone was assayed using the enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, KGE009) and procedures provided by the manufacturer. The minimum detectable dose (MDD) is 0.028 ng/ml, and the percent coefficient of variation (%CV) is <8%.
Oocyte collection
For GV oocytes, mice were super-ovulated via an intraperitoneal injection of 10 IU of Pregnant Mares Serum Gonadotropin (PMSG, Ningbo Sansheng Pharmaceutical Co., Ltd, S170705). The mice were sacrificed 46–48 h after PMSG injection. The ovaries were removed and immediately transferred to dissection medium, which consisted of M2 medium (sigma, M7167) supplemented with 200 µM 3-Isobutyl-1-methylxanthine (IBMX, Beyotime Institute of Biotechnology, SC0195) to maintain the oocytes arrested at the GV stage. The cumulus-enclosed oocytes were isolated by mechanical perforation of the ovaries with a 27-gauge needle. The cumulus cells were removed by repeated mouth pipetting, using narrow-bore glass Pasteur pipettes. To obtain MII-stage oocytes, the mice were initially injected with 10 IU PMSG (Ningbo Sansheng Pharmaceutical Co., Ltd, S170705), followed by 10 IU of human chorionic gonadotrophin (hCG, Ningbo Sansheng Pharmaceutical Co., Ltd, S170892) after 48 h. Fourteen h after hCG injection, the mice were euthanized, and the oviducts were dissected and transferred into M2 medium at 37°C. Masses of cumulus-enclosed MII oocytes were released from oviducts using forceps. Moreover, 0.03% hyaluronidase (sigma, H1115000) was added to the medium to remove the cumulus cells.
Meiotic resumption and inhibitor treatment
For the in vitro culture, three groups were classified as follows: a) oocytes from the control group with basic culture medium; b) oocytes from the CRS group with basic culture medium; and c) oocytes from the CRS group with conditional culture medium. The basic culture medium was M16 (sigma, M7292), whereas the conditional culture medium was M16 (sigma, M7292) supplemented with 5 µM MG132 (MedChemExpress, MCE, HY-13,259). The APC/C inhibitor MG132 was resolved in PBS with a concentration of 5 mM and stored at −80°C.
Live oocytes of the three groups were transferred to a 3-µl drop of culture medium covered by mineral oil (sigma, M8410). Images of the live oocytes were acquired with a 20x objective on a spinning disk confocal microscope (cellvis, 170,328) with a Leica confocal microscope (Leica TCS SP8, Wetzlar, Germany) and were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD).
For the oocytes cultured in vitro, 200 μM IBMX were added to the culture medium. Oocytes were subsequently cultured in groups (n = 30) in 90-µl drops of culture medium, respectively, at 37.5°C in a humidified atmosphere of 5% CO2 in air. After three hours, oocytes were collected to stain with Hoechst-33,342 (Beyotime Institute of Biotechnology, SC0042), immunofluorescence or western blotting of CCNB1 and securin.
Evaluation of oocyte diameter and chromatin configuration
The oocyte size was calculated as the maximum oocyte diameter excluding the zona pellucida. In each group, chromatin was identified after staining with 10 µg/ml Hoechst-33,342. The nuclear chromatin conformation was imaged with a Leica Application Suite X (LASX) confocal microscope (Leica TCS SP8, Wetzlar, Germany). Classification of the chromatin configuration was performed based on previous studies [Citation67,Citation68]. In brief, the chromatin configuration was classified into three groups according to its condensation status and its distribution around the nucleolus (or nucleolar-like body, NLB): SN oocytes, with a ring of Hoechst-positive chromatin surrounding the nucleolus, and NSN oocytes, which lacked the ring and exhibited a more dispersed chromatin.
Immunofluorescence
GV-stage and MII oocytes were washed in pre-warmed PBS three times and were subsequently fixed in 4% paraformaldehyde for 5 min at room temperature, followed by being washed in PBS that contained 0.05% Tween-20 and 0.1% BSA for 5 min. The oocytes were permeabilized in PBS that contained 0.2% Triton X-100 and 0.1% BSA for 15 min at room temperature and were subsequently blocked in blocking buffer that contained 1% goat-serum, 1% BSA and 0.05% Tween-20 in PBS overnight at 4°C. The blocked oocytes were incubated for 90 min at room temperature with rabbit anti-securin (1:50,18,040–1-AP, Proteintech), mouse anti-cyclin B1 (1:100, ab72, Abcam), mouse anti-α-tubulin (1:200, T5168, sigma) or rabbit IgG (1:100, 31,235, Life Technologies), mouse IgG (1:100, 31,903, Life Technologies) as negative controls. Oocytes were labeled with secondary antibody immunoglobulin (IgG) (A11005, A11034, Life Technologies). Spread oocytes after staining were mounted in mounting medium that contained DAPI (Vector Laboratories, H1200) and were imaged by a 63X, 1.4 NA oil differential interference contrast objective and a Leica Application Suite X (LASX) confocal microscope (Leica TCS SP8, Wetzlar, Germany) with the following bandpass emission filters (nm): 385–470 (Hoechst 33,342), 505–530 (Alexa Fluor 488) and 585–615 (Alexa Fluor 594). All images were obtained using fixed microscopic parameters, and the fluorescence intensity from each oocyte was analyzed using ImageJ software (version 1.50i, NIH, Bethesda, MD, USA).
Western blotting
Oocytes were washed twice in PBS with 200 μM IBMX, lysed in cold RIPA buffer (P0013B, Beyotime Institute of Biotechnology) supplemented with protease inhibitor cocktail (DI111-01, TransGen Biotech) and phosphatase inhibitor (TransGen Biotech, DI201-01) at 1:100 and then heated at 95°C for 5 minutes. Proteins were separated via 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and were transferred to a polyvinyldifloridine membrane (Millipore, USA). The membranes were blocked with 5% non-fat milk and probed with the specific primary antibodies anti-beta actin (1:5000, HRP-60,008, Proteintech Group, Inc.), rabbit anti-securin (1:500,18,040–1-AP, Proteintech), and mouse anti-cyclin B1 (1:400, ab72, Abcam) at 4°C overnight. After washing with TBS that contained 0.1% Tween-20 (TBST), the membranes were incubated with the appropriate HRP-conjugated anti-rabbit or anti-mouse IgG (1:3000, CST) for 2 h at room temperature. The membranes were washed three times for 10 min with TBST, and the signals were measured using an enhanced chemiluminescence (ECL) detection kit (Millipore, USA). The level of actin was used as an internal standard. The immunoreactive band intensities in Western blotting were quantified by ImageJ software (version 1.50i, NIH, Bethesda, MD, USA). For the blots of CCNB1 and securin, oocytes were collected for each sample in 20 µl of sample buffer, respectively.
Statistical analysis
Statistical significance was determined by GraphPad Prism software (GraphPad Software Inc., California, USA). Data regarding the body weight, food and water intake, serum corticosterone concentration and rate of GVBD in vitro between the control and CRS groups were evaluated using Repeated Measures ANOVA. Moreover, the differences in the intensity of fluorescence and band intensity of western blotting between the control and stress groups were analyzed using Student’s t test following Welch’s correction if necessary. The percentage of GVBD, average time of GVBD, fluorescence and western blotting band intensities among the three groups were analyzed via one-way analysis of variance (ANOVA) followed by the Tukey post hoc test. All experiments were repeated independently at least three times and at least 10 oocytes from 2 respectively different mice were used in each single experiment.
Declaration of interest
The authors declare that there is no conflict of financial interest or benefit that could be perceived as prejudicing the impartiality of the research reported.
Supplemental Material
Download MS Word (869.5 KB)Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Klonoff-Cohen H, Chu E, Natarajan L, et al. A prospective study of stress among women undergoing in vitro fertilization or gamete intrafallopian transfer. Fertil Steril. 2001;76:675–687.
- Schroder AK, Katalinic A, Diedrich K, et al. Cumulative pregnancy rates and drop-out rates in a German IVF programme: 4102 cycles in 2130 patients. Reproductive Biomedicine Online. 2004;8:600–606.
- Terzioglu F, Turk R, Yucel C, et al. The effect of anxiety and depression scores of couples who underwent assisted reproductive techniques on the pregnancy outcomes. Afr Health Sci. 2016;16:441–450.
- Liang B, Wei DL, Cheng YN, et al. Restraint stress impairs oocyte developmental potential in mice: role of CRH-induced apoptosis of ovarian cells. Biol Reprod. 2013;89:64.
- Ghizzoni L, Mastorakos G, Vottero A, et al. Corticotropin-releasing hormone (CRH) inhibits steroid biosynthesis by cultured human granulosa-lutein cells in a CRH and interleukin-1 receptor-mediated fashion. Endocrinology. 1997;138:4806–4811.
- Dinopoulou V, Partsinevelos GA, Mavrogianni D, et al. The effect of CRH and its inhibitor, antalarmin, on in vitro growth of preantral mouse follicles, early embryo development, and steroidogenesis. Endocrinology. 2013;154:222–231.
- Zhang SY, Wang JZ, Li JJ, et al. Maternal restraint stress diminishes the developmental potential of oocytes. Biol Reprod. 2011;84:672–681.
- Zhou P, Lian HY, Cui W, et al. Maternal-restraint stress increases oocyte aneuploidy by impairing metaphase I spindle assembly and reducing spindle assembly checkpoint proteins in mice. Biol Reprod. 2012;86:83.
- Divyashree S, Yajurvedi HN. Long-term chronic stress exposure induces PCO phenotype in rat. Reproduction. 2016;152:765–774.
- Sirard MA, Desrosier S, Assidi M. In vivo and in vitro effects of FSH on oocyte maturation and developmental competence. Theriogenology. 2007;68 Suppl 1:S71–6.
- Blondin P, Bousquet D, Twagiramungu H, et al. Manipulation of follicular development to produce developmentally competent bovine oocytes. Biol Reprod. 2002;66:38–43.
- Homer H, Gui L, Carroll J. A spindle assembly checkpoint protein functions in prophase I arrest and prometaphase progression. Science. 2009;326:991–994.
- Manandhar G, Schatten H, Sutovsky P. Centrosome reduction during gametogenesis and its significance. Biol Reprod. 2005;72:2–13.
- Schuh M, Ellenberg J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell. 2007;130:484–498.
- Keefe D, Liu L, Wang W, et al. Imaging meiotic spindles by polarization light microscopy: principles and applications to IVF. Reproductive Biomedicine Online. 2003;7:24–29.
- Wang WH, Keefe DL. Spindle observation in living mammalian oocytes with the polarization microscope and its practical use. Cloning and Stem Cells. 2002;4:269–276.
- Chen SU, Lien YR, Chao KH, et al. Effects of cryopreservation on meiotic spindles of oocytes and its dynamics after thawing: clinical implications in oocyte freezing–a review article. Mol Cell Endocrinol. 2003;202:101–107.
- Wang WH, Meng L, Hackett RJ, et al. Limited recovery of meiotic spindles in living human oocytes after cooling-rewarming observed using polarized light microscopy. Hum Reproduction. 2001;16:2374–2378.
- Moon JH, Hyun CS, Lee SW, et al. Visualization of the metaphase II meiotic spindle in living human oocytes using the Polscope enables the prediction of embryonic developmental competence after ICSI. Hum Reproduction. 2003;18:817–820.
- Cristina Magli M, Capoti A, Resta S, et al. Prolonged absence of meiotic spindles by birefringence imaging negatively affects normal fertilization and embryo development. Reproductive Biomedicine Online. 2011;23:747–754.
- Lenart P, Ellenberg J. Nuclear envelope dynamics in oocytes: from germinal vesicle breakdown to mitosis. Curr Opin Cell Biol. 2003;15:88–95.
- Sanchez F, Romero S, De Vos M, et al. Human cumulus-enclosed germinal vesicle oocytes from early antral follicles reveal heterogeneous cellular and molecular features associated with in vitro maturation capacity. Hum Reproduction. 2015;30:1396–1409.
- Luciano AM, Franciosi F, Modina SC, et al. Gap junction-mediated communications regulate chromatin remodeling during bovine oocyte growth and differentiation through cAMP-dependent mechanism(s). Biol Reprod. 2011;85:1252–1259.
- Gall L, De Smedt V, Crozet N, et al. Meiotically incompetent and competent goat oocytes: timing of nuclear events and protein phosphorylation. Theriogenology. 1996;46:825–835.
- Higaki S, Kishi M, Koyama K, et al. Early germinal vesicle breakdown is a predictor of high preimplantation developmental competent oocytes in mice. Zygote. 2017;25:41–48.
- Zeng HT, Ren Z, Guzman L, et al. Heparin and cAMP modulators interact during pre-in vitro maturation to affect mouse and human oocyte meiosis and developmental competence. Hum Reproduction. 2013;28:1536–1545.
- Silva GM, Brito IR, Sales AD, et al. In vitro growth and maturation of isolated caprine preantral follicles: influence of insulin and FSH concentration, culture dish, coculture, and oocyte size on meiotic resumption. Theriogenology. 2017;90:32–41.
- Eppig JJ, Schroeder AC. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol Reprod. 1989;41:268–276.
- Zuccotti M, Giorgi Rossi P, Martinez A, et al. Meiotic and developmental competence of mouse antral oocytes. Biol Reprod. 1998;58:700–704.
- Holt JE, Tran SM, Stewart JL, et al. The APC/C activator FZR1 coordinates the timing of meiotic resumption during prophase I arrest in mammalian oocytes. Development. 2011;138:905–913.
- Dedieu T, Gall L, Crozet N, et al. Mitogen-activated protein kinase activity during goat oocyte maturation and the acquisition of meiotic competence. Mol Reprod Dev. 1996;45:351–358.
- Hirao Y, Miyano T, Kato S. Acquisition of maturational competence in in vitro grown mouse oocytes. J Exp Zool. 1993;267:543–547.
- Bui TTH, Belli M, Fassina L, et al. Cytoplasmic movement profiles of mouse surrounding nucleolus and not-surrounding nucleolus antral oocytes during meiotic resumption. Mol Reprod Dev. 2017;84:356–362.
- Han SJ, Chen R, Paronetto MP, et al. Wee1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr Biol. 2005;15:1670–1676.
- Holt JE, Weaver J, Jones KT. Spatial regulation of APCCdh1-induced cyclin B1 degradation maintains G2 arrest in mouse oocytes. Development. 2010;137:1297–1304.
- Sivakumar S, Gorbsky GJ. Spatiotemporal regulation of the anaphase-promoting complex in mitosis. Nat Rev Mol Cell Biol. 2015;16:82–94.
- Herbert M, Levasseur M, Homer H, et al. Homologue disjunction in mouse oocytes requires proteolysis of securin and cyclin B1. Nat Cell Biol. 2003;5:1023–1025.
- Durinzi KL, Saniga EM, Lanzendorf SE. The relationship between size and maturation in vitro in the unstimulated human oocyte. Fertil Steril. 1995;63:404–406.
- Fair T, Hyttel P, Greve T. Bovine oocyte diameter in relation to maturational competence and transcriptional activity. Mol Reprod Dev. 1995;42:437–442.
- Liu H, Aoki F. Transcriptional activity associated with meiotic competence in fully grown mouse GV oocytes. Zygote. 2002;10:327–332.
- Zuccotti M, Ponce RH, Boiani M, et al. The analysis of chromatin organisation allows selection of mouse antral oocytes competent for development to blastocyst. Zygote. 2002;10:73–78.
- Holt JE, Lane SI, Jennings P, et al. APC(FZR1) prevents nondisjunction in mouse oocytes by controlling meiotic spindle assembly timing. Mol Biol Cell. 2012;23:3970–3981.
- Yang Y, Yang CR, Han SJ, et al. Maternal mRNAs with distinct 3ʹ UTRs define the temporal pattern of Ccnb1 synthesis during mouse oocyte meiotic maturation. Genes Dev. 2017;31:1302–1307.
- Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656.
- Josefsberg LB, Galiani D, Dantes A, et al. The proteasome is involved in the first metaphase-to-anaphase transition of meiosis in rat oocytes. Biol Reprod. 2000;62:1270–1277.
- Terret ME, Wassmann K, Waizenegger I, et al. The meiosis I-to-meiosis II transition in mouse oocytes requires separase activity. Curr Biol. 2003;13:1797–1802.
- Kalich-Philosoph L, Roness H, Carmely A, et al. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci Transl Med. 2013;5:185ra62.
- Wu XF, Yuan HJ, Li H, et al. Restraint stress on female mice diminishes the developmental potential of oocytes: roles of chromatin configuration and histone modification in germinal vesicle stage oocytes. Biol Reprod. 2015;92:13.
- Watanabe Y. Geometry and force behind kinetochore orientation: lessons from meiosis. Nat Rev Mol Cell Biol. 2012;13:370–382.
- Qiu D, Hou X, Han L, et al. Sirt2-BubR1 acetylation pathway mediates the effects of advanced maternal age on oocyte quality. Aging Cell. 2018;17.
- Han L, Wang H, Li L, et al. Melatonin protects against maternal obesity-associated oxidative stress and meiotic defects in oocytes via the SIRT3-SOD2-dependent pathway. J Pineal Res. 2017;63:e12431.
- Machtinger R, Combelles CM, Missmer SA, et al. The association between severe obesity and characteristics of failed fertilized oocytes. Hum Reproduction. 2012;27:3198–3207.
- Miao Y, Zhou C, Bai Q, et al. The protective role of melatonin in porcine oocyte meiotic failure caused by the exposure to benzo(a)pyrene. Hum Reproduction. 2018;33:116–127.
- Zhang M, Miao Y, Chen Q, et al. BaP exposure causes oocyte meiotic arrest and fertilization failure to weaken female fertility. FASEB J. 2018;32:342–352.
- Liu YX, Cheng YN, Miao YL, et al. Psychological stress on female mice diminishes the developmental potential of oocytes: a study using the predatory stress model. PloS One. 2012;7:e48083.
- Marangos P, Carroll J. The dynamics of cyclin B1 distribution during meiosis I in mouse oocytes. Reproduction. 2004;128:153–162.
- Hinchcliffe EH, Thompson EA, Miller FJ, et al. Nucleo-cytoplasmic interactions that control nuclear envelope breakdown and entry into mitosis in the sea urchin zygote. J Cell Sci. 1999;112(Pt 8):1139–1148.
- Takizawa CG, Morgan DO. Control of mitosis by changes in the subcellular location of cyclin-B1-Cdk1 and Cdc25C. Curr Opin Cell Biol. 2000;12:658–665.
- Reis A, Chang HY, Levasseur M, et al. APCcdh1 activity in mouse oocytes prevents entry into the first meiotic division. Nat Cell Biol. 2006;8:539–540.
- Marangos P, Carroll J. Securin regulates entry into M-phase by modulating the stability of cyclin B. Nat Cell Biol. 2008;10:445–451.
- Ogushi S, Fulka J Jr., Miyano T. Germinal vesicle materials are requisite for male pronucleus formation but not for change in the activities of CDK1 and MAP kinase during maturation and fertilization of pig oocytes. Dev Biol. 2005;286:287–298.
- Inoue A, Nakajima R, Nagata M, et al. Contribution of the oocyte nucleus and cytoplasm to the determination of meiotic and developmental competence in mice. Hum Reproduction. 2008;23:1377–1384.
- Lee HS, Yin XJ, Jin YX, et al. Germinal vesicle chromatin configuration and meiotic competence is related to the oocyte source in canine. Anim Reprod Sci. 2008;103:336–347.
- Kumar S, Kumar M, Dholpuria S, et al. Transient arrest of germinal vesicle breakdown improved in vitro development potential of buffalo (Bubalus Bubalis) oocytes. J Cell Biochem. 2018;119:278–289.
- Shu YM, Zeng HT, Ren Z, et al. Effects of cilostamide and forskolin on the meiotic resumption and embryonic development of immature human oocytes. Hum Reproduction. 2008;23:504–513.
- Ola SI, Wang Q, Ai JS, et al. Meiotic competence and acetylation pattern of UV light classified mouse antral oocytes after meiotic arrest with isobutylmethylxanthine. Mol Reprod Dev. 2007;74:591–599.
- Monti M, Redi CA. Isolation and characterization of mouse antral oocytes based on nucleolar chromatin organization. J Vis Exp. 2016;107:e53616.
- Ami D, Mereghetti P, Natalello A, et al. FTIR spectral signatures of mouse antral oocytes: molecular markers of oocyte maturation and developmental competence. Biochim Biophys Acta. 2011;1813:1220–1229.
