ABSTRACT
Gemcitabine (GEM) is first-line therapy for pancreatic cancer but has limited efficacy in most cases. Nanoparticle-albumin bound (nab)-paclitaxel is becoming first-line therapy for pancreatic cancer, but also has limited efficacy for pancreatic cancer. Our goal was to improve the treatment outcome in patient-like models of pancreatic cancer. We previously established patient-derived orthotopic xenografts (PDOX) pancreatic cancers from two patients. The pancreatic tumor was implanted orthotopically in the pancreatic tail of nude mice to establish the PDOX models. Five weeks after implantation, 50 PDOX mouse models were randomized into five groups of 10 mice for each pancreatic cancer PDOX: untreated control; GEM (100 mg/kg, i.p., once a week for 2 weeks); GEM + nab-PTX (GEM: 100 mg/kg, i.p., once a week for 2 weeks, nab-PTX: 10 mg/kg, i.v., twice a week for 2 weeks); S. typhimurium A1-R (5 × 107 CFU/100 μl, i.v., once a week for 2 weeks); GEM + S. typhimurium A1-R (GEM: 100 mg/kg, i.p., once a week for 2 weeks, S. typhimurium A1-R; 5 × 107 CFU/100 μl, i.v., once a week for 2 weeks). GEM + nab-PTX was significantly more effective than GEM alone in one PDOX model (p = 0.0004), but there was no significant difference in the other PDOX model. The combination of GEM + S. typhimurium A1-R regressed both PDOX models. These results show S. typhimurium A1-R can overcome the ineffectiveness or partial effectiveness of GEM in patient-like models of pancreatic cancer and demonstrate clinical potential for this combination.
Introduction
Gemcitabine (GEM) is first-line chemotherapy for pancreatic cancer [Citation1] with a response rate of < 10% [Citation2,Citation3]. A Phase III clinical trial showed nab-paclitaxel (nab-PTX) plus GEM had significant improvements over GEM alone in patients with metastatic pancreatic cancer [Citation4]. GEM + nab-PTX improved the response rate to approximately 20% but still with almost all patients dying within 5 years. Transformative individualized treatment is necessary for this disease.
Our laboratory has developed clinically-relevant mouse models of cancer for discovery of transformative therapy for pancreatic cancer, the patient-derived orthotopic xenograft (PDOX) nude mouse model, with the technique of surgical orthotopic implantation (SOI). These models include breast [Citation5], ovarian [Citation6], lung [Citation7], cervical [Citation8], colon [Citation9–Citation11], and stomach cancer [Citation12], as well as sarcoma [Citation13–Citation30] and melanoma [Citation31–Citation38] and pancreatic cancer [Citation33,Citation39–Citation42]. The PDOX model, developed by our laboratory over the past 30 years, has many advantages over subcutaneous-transplant models which grow ectopically under the skin and rarely metastasize [Citation43].
A candidate for transformative therapy is tumor-targeting Salmonella typhimurium A1-R (S. typhimurium A1-R), also developed by our laboratory [Citation44]. S. typhimurium A1-R is auxotrophic for Leu-Arg, which prevents it from mounting a continuous infection in normal tissues. Tumor-targeting S. typhimurium A1-R was effective against many types of PDOX models including melanoma [Citation36–Citation38], pancreatic cancer [Citation39,Citation45] and cases of osteosarcoma [Citation20,Citation46,Citation47] and Ewing’s [Citation21] and follicular dendritic cell sarcoma [Citation15].
We preivously demonstrated the efficacy of S. typhimurium A1-R in combination with anti-vascular endothelial growth factor (VEGF) therapy on VEGF-positive human pancreatic cancer including a PDOX model [Citation39].
We preiovusly showed that tumor-targeting S. typhimurium A1-R had comparable efficacy with gemcitaine (GEM), cisplatinum (CDDP), and 5-fluorouracil (5-FU) on a pancreatic cncer PDOX models [Citation45].
It has been reported for at least two hundred years that bacterial infection can regress cancer in patients. In the late 19th and early 20th centuries, cancer patients were treated with bacteria by Dr. William B. Coley. However, after Coley’s death, bacterial therapy of cancer stopped. At the present time, there has been a resurgence of bacterial therapy of cancer and the PDOX model has been shown to be very appropriate to develop of new combination to inhibitors or regression of recalcitrant cancer [Citation48].
In the present study, we utilized two PDOX nude mouse models of pancreatic cancer from two different patients and determined whether S. typhimurium A1-R in combination with GEM could overcome GEM-resistance.
Results and discussion
For pancreatic cancer PDOX model #1, all treatments inhibited tumor growth compared to untreated control to a different degree: GEM: p < 0.0001; GEM + nab-PTX: p < 0.0001; S. typhimurium A1-R: p < 0.0001; GEM + S. typhimurium A1-R: p < 0.0001 on day 14 after treatment initiation. GEM + nab-PTX and GEM + S. typhimurium A1-R significantly inhibited tumor growth more than GEM alone (p = 0.0004 and p < 0.0001, respectively). However, GEM + S. typhimurium A1-R significantly inhibited tumor growth more compared to other treatments (GEM: p < 0.0001; GEM + nab-PTX: p = 0.0372; S. typhimurium A1-R: p < 0.0001). Only the GEM + S. typhimurium A1-R combination could regress the tumor (.
Figure 1. Quantitative treatment efficacy.
Bar graphs show relative tumor volume before and after treatment for each treatment condition. (a) Pancreatic cancer PDOX #1. (b) Pancreatic cancer PDOX #2. Error bars: ± SD. Relative tumor volume is the ratio of tumor volume after treatment to the tumor volume before the start of the treatment period.
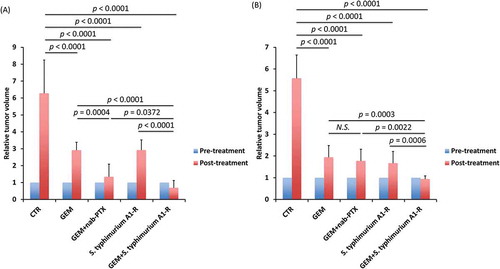
For PDOX model #2, all treatments inhibited tumor growth compared to untreated control to a different degree: GEM: p < 0.0001; GEM + nab-PTX: p < 0.0001; S. typhimurium A1-R: p < 0.0001; GEM + S. typhimurium A1-R: p < 0.0001 on day 14. GEM + S. typhimurium A1-R significantly inhibited tumor growth more than GEM alone (p = 0.0003). However, there was no significant difference between GEM + nab-PTX and GEM alone, unlike pancreatic cancer PDOX #1. GEM + S. typhimurium A1-R significantly inhibited tumor growth more compared to other treatments (GEM: p = 0.0003; GEM + nab-PTX: p = 0.0022; S. typhimurium A1-R: p = 0.0006). Only the GEM + S. typhimurium A1-R combination therapy could regress the tumor ().
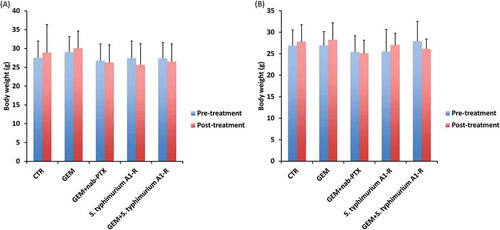
Body weight on day 14 of treatment, compared with day 0, did not significantly differ between any treatment group (). There were no animal deaths in any group.
Figure 2. Effect of each treatment on mouse body weight. Bar graphs show mouse body weight in each treatment group at pre- and post-treatment timepoints. (a) Pancreatic cancer PDOX #1. (b) Pancreatic cancer PDOX #2. Error bars: ± SD.
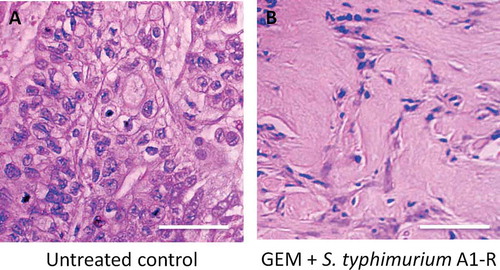
Histologically, the untreated control pancreatic cancer PDOX tumors were mainly comprised of viable cells. In contrast, the pancreatic cancer PDOX tumors treated with the combination therapy of GEM + S. typhimurium A1-R showed extensive necrosis ().
Figure 3. Tumor histology. (a). Untreated control. (b). Tumor treated with GEM+ S. typhimurium A1-R). Scale bars: 100 μm.
In the present study, first-line therapy for pancreatic cancer, GEM, could not arrest or regress tumor growth in either of the 2 PDOX models. GEM showed some tumor inhibition compared to the untreated control, similar to the situation in the clinic.
Recently, a randomized Phase III clinical trial showed promising efficacy in metastatic pancreatic cancer treated with the combination of GEM + nab-PTX [Citation4]. In the present study, GEM + nab-PTX was significantly more effective than GEM alone in pancreatic cancer PDOX #1 (), but there was no significantly difference in pancreatic cancer PDOX #2 (). Neither pancreatic cancer PDOX #1 or #2 could be regressed by GEM + nab-PTX. In contrast, the combination of S. typhimurium A1-R + GEM could regress both PDOX models.
The tumor-targeting S. typhimurium A1-R, developed by our laboratory [Citation44], is auxotrophic for Leu-Arg, which prevents it from mounting a continuous infection in normal tissues. In the present study, S. typhimurium A1-R could increase the partial response of GEM of the pancreatic cancer PDOX #1 and #2 to cause regression, suggesting the generality of this treatment.
The present study has important implications since it showed the strong additional efficacy of S. typhimurium A1-R combined with GEM, in contrast to nab-PTX which showed benefit with GEM in only one of the two PDOX models, suggesting clinical potential of the GEM+ S. typhimurium A1-R combination. The PDOX model enables precise, individualized therapy, especially for recalcitrant disease such as pancreatic cancer [Citation35].
S. typhimurium A1-R can directly target and kill cancer cells [Citation49] as well as decoy the cancer cells from the chemoresistant G0/G1 phase to the chemosensitive S-phase of the cell cycle, making them more susceptible to GEM [Citation50–Citation52]. A similar type of cell-cycle decoy occurred when cancer cells were injected with an adenovirus [Citation53]. The cell cycle decoy from G0/G1 to S may be a general response to cancer-cell infection. The decoy has important implications since the majority of cancer cells in a mature tumor are in G0/G1 and chemoresistant and may account for drug resistance found so often in solid tumors in the clinic [Citation50,Citation51,Citation54].
Previously-developed concepts and strategies of highly-selective tumor targeting can take advantage of molecular targeting of tumors, including tissue-selective therapy which focuses on unique differences between normal and tumor tissues [Citation55–Citation60].
Materials and methods
Mice
Athymic nu/nu male nude mice (AntiCancer, Inc., San Diego, CA), 4–6 weeks old, were used in this study. All mice were kept in a barrier facility on a high efficiency particulate arrestance (HEPA)-filtered rack under standard conditions of 12-hour light/dark cycles. The animals were fed an autoclaved laboratory rodent diet. All animal experiments were performed with an AntiCancer Institutional Animal Care and Use Committee (IACUC)-protocol specifically approved for this study and in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Animals under Assurance Number A3873-1. Anesthesia and analgesics were used for all surgical experiments to avoid unnecessary suffering of the mice. Subcutaneous injection of a ketamine mixture (a 0.02 ml solution of 20 mg/kg ketamine, 15.2 mg/kg xylazine, and 0.48 mg/kg acepromazine maleate) was used for mice. The response of animals during surgery was monitored carefully to maintain adequate depth of anesthesia. The animals were observed daily and humanely sacrificed by CO2 inhalation when they met the following criteria: severe tumor burden (more than 20 mm in diameter), prostration, significant body weight loss, difficulty breathing, rotational motion and body-temperature drop.
Patient-derived tumor
The tumor from a pancreatic cancer patient #1 was previously resected in the MD Anderson Cancer Center. Written informed consent was provided by the patient and the Institutional Review Board (IRB) of MD Anderson Cancer Center approved this experiment.
The tumor from pancreatic cancer patient #2 was previously resected in the University of California, San Diego (UCSD). Written informed consent was provided by the patient, and the Institutional Review Board (IRB #140046CX) of UCSD approved this experiment.
Establishment of PDOX models of pancreatic cancer by surgical orthotopic implantation (SOI)
After nude mice were anesthetized with the ketamine solution described above, a 1–1.5 cm skin incision was made on the left-side abdomen through the skin, fascia and peritoneum and pancreas was exposed. Surgical sutures (8–0 nylon) were used to implant tumor fragments onto the tail of the pancreas to establish the PDOX model. The wound was closed with a 6–0 nylon suture (Ethilon, Ethicon, Inc., NJ) [Citation33,Citation39,Citation41,Citation42,Citation45,Citation61].
Preparation and administration of S. typhimurium A1-R
GFP-expressing S. typhimurium A1-R (AntiCancer Inc., San Diego, CA) was grown overnight on LB medium (Fisher Sci., Hanover Park, IL) and then diluted 1:10 in LB medium. Bacteria were harvested at late-log phase, washed with PBS, and then diluted in PBS. S. typhimurium A1-R was injected intravenously. A total of 5 × 107 CFU S. typhimurium A1-R in 100 μl PBS was administered to each mouse [Citation49,Citation62,Citation63].
Treatment study design for the PDOX models of pancreatic cancer
Pancreatic cancer PDOX mouse models were randomized into five groups of 10 mice each: untreated control; GEM (100 mg/kg, i.p., once a week for 2 weeks); GEM + nab-PTX (GEM: 100 mg/kg, i.p., once a week for 2 weeks, nab-PTX: 10 mg/kg, i.v., twice a week for 2 weeks); S. typhimurium A1-R (5 × 107 CFU/100 μl, i.v., once a week for 2 weeks); GEM + S. typhimurium A1-R (GEM: 100 mg/kg, i.p., once a week for 2 weeks; S. typhimurium A1-R, 5 × 107 CFU/100 μl, i.v., once a week for 2 weeks). Treatments started when the PDOX tumors reached 50 mm3. Tumor length and width were measured on day 0 and 14. Tumor volume was calculated with the following formula: Tumor volume (mm3) = length (mm) × width (mm) × width (mm) × 1/2. Data are presented as mean ± SD. The tumor volume ratio is defined at the tumor volume at day 14 to day 0. Relative tumor volume enables tumor growth or inhibition to be visualized more clearly. Tumor size at the initiation and end of treatment was measured with calipers during laparotomy.
Histological examination
Fresh tumor samples were fixed in 10% formalin and embedded in paraffin before sectioning and staining. Tissue sections (5 μm) were deparaffinized in xylene and rehydrated in an ethanol series. Hematoxylin and eosin (H&E) staining was performed according to standard protocols. Histological examination was performed with a BHS System Microscope (Olympus Corporation, Tokyo, Japan). Images were acquired with INFINITY ANALYZE software (Lumenera Corporation, Ottawa, Canada) [Citation33].
Statistical analysis
JMP version 11.0 was used for all statistical analyzes. Significant differences for continuous variables were determined using the Mann-Whitney U test. Line graphs expressed average values and error bar show SD. A probability value of P ≤ 0.05 is considered statistically significant.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413.
- Jenks S. AACR highlights: promise for treating pancreatic cancer. J Natl Cancer Inst. 2011;103:786–787.
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825.
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703.
- Fu X, Le P, Hoffma RM. A metastatic-orthotopic transplant nude-mouse model of human patient breast cancer. Anticancer Res. 1993;13:901–904.
- Fu X, Hoffman RM. Human ovarian carcinoma metastatic models constructed in nude mice by orthotopic transplantation of histologically-intact patient specimens. Anticancer Res. 1993;13:283–286.
- Wang X, Fu X, Hoffman RM. A new patient-like metastatic model of human lung cancer constructed orthotopically with intact tissue via thoracotomy in immunodeficient mice. Int J Cancer. 1992;51:992–995.
- Hiroshima Y, Zhang Y, Zhang N, et al. Establishment of a patient-derived orthotopic xenograph (PDOX) model of HER-2-positive cervical cancer expressing the clinical metastatic pattern. PLoS One. 2015;10:e0117417.
- Fu X, Besterman JM, Monosov A, et al. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc Natl Acad Sci USA. 1991;88:9345–9349.
- Metildi CA, Kaushal S, Luiken GA, et al. Fluorescently-labeled chimeric anti-CEA antibody improves detection and resection of human colon cancer in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J Surg Oncol. 2014;109:451–458.
- Hiroshima Y, Maawy A, Metildi CA, et al. Successful fluorescence-guided surgery on human colon cancer patient-derived orthotopic xenograft mouse models using a fluorophore-conjugated anti-CEA antibody and a portable imaging system. J Laparoendosc Adv Surg Tech A. 2014;24:241–247.
- Furukawa T, Kubota T, Watanabe M, et al. Orthotopic transplantation of histologically intact clinical specimens of stomach cancer to nude mice: correlation of metastatic sites in mouse and individual patient donors. Int J Cancer. 1993;53:608–612.
- Murakami T, DeLong J, Eilber FC, et al. Tumor-targeting Salmonella typhimurium A1-R in combination with doxorubicin eradicate soft tissue sarcoma in a patient-derived orthotopic xenograft PDOX model. Oncotarget. 2016;7:12783–12790.
- Hiroshima Y, Zhao M, Zhang Y, et al. Tumor-targeting Salmonella typhimurium A1-R arrests a chemo-resistant patient soft-tissue sarcoma in nude mice. PLoS One. 2015;10:e0134324.
- Kiyuna T, Murakami T, Tome Y, et al. High efficacy of tumor-targeting Salmonella typhimurium A1-R on a doxorubicin- and dactolisib-resistant follicular dendritic-cell sarcoma in a patient-derived orthotopic xenograft PDOX nude mouse model. Oncotarget. 2016;7:33046–33054.
- Murakami T, Singh AS, Kiyuna T, et al. Effective molecular targeting of CDK4/6 and IGF-1R in a rare FUS-ERG fusion CDKN2A-deletion doxorubicin-resistant Ewing’s sarcoma in a patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget. 2016;7:47556–47564.
- Hiroshima Y, Zhang Y, Zhang N, et al. Patient-derived orthotopic xenograft (PDOX) nude mouse model of soft-tissue sarcoma more closely mimics the patient behavior in contrast to the subcutaneous ectopic model. Anticancer Res. 2015;35:697–701.
- Igarashi K, Murakami T, Kawaguchi K, et al. A patient-derived orthotopic xenograft (PDOX) mouse model of an cisplatinum-resistant osteosarcoma lung metastasis that was sensitive to temozolomide and trabectedin: implications for precision oncology. Oncotarget. 2017;8:62111–62119.
- Igarashi K, Kawaguchi K, Kiyuna T, et al. Temozolomide combined with irinotecan caused regression in an adult pleomorphic rhabdomyosarcoma patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget. 2017;8:75874–75880.
- Igarashi K, Kawaguchi K, Murakami T, et al. Intra-arterial administration of tumor-targeting Salmonella typhimurium A1-R regresses a cisplatin-resistant relapsed osteosarcoma in a patient-derived orthotopic xenograft (PDOX) mouse model. Cell Cycle. 2017;16:1164–1170.
- Murakami T, Kiyuna T, Kawaguchi K, et al. The irony of highly-effective bacterial therapy of a patient-derived orthotopic xenograft (PDOX) model of Ewing’s sarcoma, which was blocked by Ewing himself 80 years ago. Cell Cycle. 2017;16:1046–1052.
- Igarashi K, Kawaguchi K, Murakami T, et al. High efficacy of pazopanib on an undifferentiated spindle-cell sarcoma resistant to first-line therapy is identified with a patient-derived orthotopic xenograft (PDOX) nude mouse model. J Cell Biochem. 2017;118:2739–3743.
- Kiyuna T, Murakami T, Tome Y, et al. Analysis of stroma labeling during multiple passage of a sarcoma imageable patient-derived orthotopic xenograft (iPDOX) in red fluorescent protein transgenic nude mice. J Cell Biochem. 2017;118:3367–3371.
- Igarashi K, Murakami T, Kawaguchi K, et al. A patient-derived orthotopic xenograft (PDOX) mouse model of an cisplatinum-resistant osteosarcoma lung metastasis that was sensitive to temozolomide and trabectedin: implications for precision oncology. Oncotarget. 2017;8:62111–62119.
- Igarashi K, Kawaguchi K, Murakami T, et al. A novel anionic-phosphate-platinum complex effectively targets an undifferentiated pleomorphic sarcoma better than cisplatinum and doxorubicin in a patient-derived orthotopic xenograft (PDOX). Oncotarget. 2017;8:63353–63359.
- Miyake K, Murakami T, Kiyuna T, et al. The combination of temozolomide-irinotecan regresses a doxorubicin-resistant patient-derived orthotopic xenograft (PDOX) nude-mouse model of recurrent Ewing’s sarcoma with a FUS-ERG fusion and CDKN2A deletion: direction for third-line patient therapy. Oncotarget. 2017;8:103129–103136.
- Murakami T, Li S, Han Q, et al. Recombinant methioninase effectively targets a Ewing’s sarcoma in a patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget. 2017;8:35630–35638.
- Igarashi K, Li S, Han Q, et al. Growth of a doxorubicin-resistant undifferentiated spindle-cell sarcoma PDOX is arrested by metabolic targeting with recombinant methioninase. J Cell Biochem. Forthcoming.
- Igarashi K, Kawaguchi K, Li S, et al. Recombinant methioninase in combination with DOX overcomes first-line DOX resistance in a patient-derived orthotopic xenograft nude-mouse model of undifferentiated spindle-cell sarcoma. Cancer Lett. submitted.
- Igarashi K, Kawaguchi K, Kiyuna T, et al. Tumor-targeting Salmonella typhimurium A1-R combined with recombinant methioninase and cisplatinum eradicates an osteosarcoma cisplatinum-resistant lung metastasis in a patient-derived orthotopic xenograft (PDOX) mouse model: decoy, trap and kill chemotherapy moves toward the clinic. Cell Cycle. submitted.
- Kawaguchi K, Igarashi K, Li S, et al. Combination treatment with recombinant methioninase enables temozolomide to arrest a BRAF V600E melanoma growth in a patient-derived orthotopic xenograft. Oncotarget. 2017;8:85516–85525.
- Kawaguchi K, Igarashi K, Li S, et al. Recombinant methioninase (rMETase) is an effective therapeutic for BRAF-V600E-negative as well as -positive melanoma in patient-derived orthotopic xenograft (PDOX) mouse models. Oncotarget. 2018;9:915–923.
- Kawaguchi K, Han Q, Li S, et al. Intra-tumor L-methionine level highly correlates with tumor size in both pancreatic cancer and melanoma patient-derived orthotopic xenograft (PDOX) nude-mouse models. Oncotarget. Forthcoming.
- Kawaguchi K, Han Q, Li S, et al. Targeting methionine with oral recombinant methioninase (o-rMETase) arrests a patient-derived orthotopic xenograft (PDOX) model of BRAF-V600E mutant melanoma: implications for clinical cancer therapy and prevention. Cell Cycle. Forthcoming.
- Kawaguchi K, Murakami T, Chmielowski B, et al. Vemurafenib-resistant BRAF-V600E mutated melanoma is regressed by MEK targeting drug trametinib, but not cobimetinib in a patient-derived orthotopic xenograft (PDOX) mouse model. Oncotarget. 2016;7:71737–71743.
- Kawaguchi K, Igarashi K, Murakami T, et al. Tumor-targeting Salmonella typhimurium A1-R combined with temozolomide regresses malignant melanoma with a BRAF-V600 mutation in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget. 2016;7:85929–85936.
- Kawaguchi K, Igarashi K, Murakami T, et al. Tumor-targeting Salmonella typhimurium A1-R sensitizes melanoma with a BRAF-V600E mutation to vemurafenib in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J Cell Biochem. 2017;118:2314–2319.
- Yamamoto M, Zhao M, Hiroshima Y, et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R on a melanoma patient-derived orthotopic xenograft (PDOX) nude-mouse model. PLoS One. 2016;11:e0160882.
- Hiroshima Y, Zhang Y, Murakami T, et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R in combination with anti-angiogenesis therapy on a pancreatic cancer patient-derived orthotopic xenograph (PDOX) and cell line mouse models. Oncotarget. 2014;5:12346–12357.
- Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci USA. 1992;89:5645–5649.
- Hiroshima Y, Maawy A, Zhang Y, et al. Metastatic recurrence in a pancreatic cancer patient derived orthotopic xenograft (PDOX) nude mouse model is inhibited by neoadjuvant chemotherapy in combination with fluorescence-guided surgery with an anti-CA 19–9-conjugated fluorophore. PLoS One. 2014;9:e114310.
- Hiroshima Y, Maawy AA, Katz MH, et al. Selective efficacy of zoledronic acid on metastasis in a patient-derived orthotopic xenograph (PDOX) nude-mouse model of human pancreatic cancer. J Surg Oncol. 2015;111:311–315.
- Hoffman RM. Patient-derived orthotopic xenografts: better mimic of metastasis than subcutaneous xenografts. Nat Rev Cancer. 2015;15:451–452.
- Hoffman RM, Zhao M. Methods for the development of tumor-targeting bacteria. Expert Opin Drug Discov. 2014;9:741–750.
- Hiroshima Y, Zhao M, Maawy A, et al. Efficacy of Salmonella typhimurium A1-R versus chemotherapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX). J Cell Biochem. 2014;115:1254–1261.
- Hayashi K, Zhao M, Yamauchi K, et al. Systemic targeting of primary bone tumor and lung metastasis of high-grade osteosarcoma in nude mice with a tumor-selective strain of Salmonella typhimurium. Cell Cycle. 2009;8:870–875.
- Murakami T, Igarashi K, Kawaguchi K, et al. Tumor-targeting Salmonella typhimurium A1-R regresses an osteosarcoma in a patient-derived xenograph model resistant to a molecular-targeting drug. Oncotarget. 2017;8:8035–8042.
- Hoffman RM, editor. Bacterial therapy of cancer: methods and protocols. Methods in molecular biology 1409. Walker, John M., series ed. New York (NY): Humana Press (Springer Science+Business Media); 2016.
- Zhao M, Yang M, Li X-M, et al. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci USA. 2005;102:755–760.
- Yano S, Zhang Y, Zhao M, et al. Tumor-targeting Salmonella typhimurium A1-R decoys quiescent cancer cells to cycle as visualized by FUCCI imaging and become sensitive to chemotherapy. Cell Cycle. 2014;13:3958–3963.
- Yano S, Takehara K, Zhao M, et al. Tumor-specific cell-cycle decoy by Salmonella typhimurium A1-R combined with tumor-selective cell-cycle trap by methioninase overcome tumor intrinsic chemoresistance as visualized by FUCCI imaging. Cell Cycle. 2016;15:1715–1723.
- Igarashi K, Kawaguchi K, Kiyuna T, et al. Tumor-targeting Salmonella typhimurium A1-R combined with recombinant methioninase and cisplatinum eradicates an osteosarcoma cisplatinum-resistant lung metastasis in a patient-derived orthotopic xenograft (PDOX) mouse model: decoy, trap and kill chemotherapy moves toward the clinic. Cell Cycle. Forthcoming.
- Yano S, Tazawa H, Hashimoto Y, et al. A genetically engineered oncolytic adenovirus decoys and lethally traps quiescent cancer stem-like cells into S/G2/M phases. Clin Cancer Res. 2013;19:6495–6505.
- Yano S, Zhang Y, Miwa S, et al. Spatial-temporal FUCCI imaging of each cell in a tumor demonstrates locational dependence of cell cycle dynamics and chemoresponsiveness. Cell Cycle. 2014;13:2110–2119.
- Blagosklonny MV. Matching targets for selective cancer therapy. Drug Discov Today. 2003;8:1104–1107.
- Blagosklonny MV. Teratogens as anti-cancer drugs. Cell Cycle. 2005;4:1518–1521.
- Blagosklonny MV. Treatment with inhibitors of caspases, that are substrates of drug transporters, selectively permits chemotherapy-induced apoptosis in multidrug-resistant cells but protects normal cells. Leukemia. 2001;15:936–941.
- Blagosklonny MV. Target for cancer therapy: proliferating cells or stem cells. Leukemia. 2006;20:385–391.
- Apontes P, Leontieva OV, Demidenko ZN, et al. Exploring long-term protection of normal human fibroblasts and epithelial cells from chemotherapy in cell culture. Oncotarget. 2011;2:222–233.
- Blagosklonny MV. Tissue-selective therapy of cancer. Br J Cancer. 2003;89:1147–1151.
- Matsuo Y, Raimondo M, Woodward TA, et al. CXC-chemokine/CXCR2 biological axis promotes angiogenesis in vitro and in vivo in pancreatic cancer. Int J Cancer. 2009;125:1027–1037.
- Zhao M, Yang M, Ma H, et al. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res. 2006;66:7647–7652.
- Zhao M, Geller J, Ma H, et al. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc Natl Acad Sci USA. 2007;104:10170–10174.
