ABSTRACT
Cell cycle progression is precisely regulated by diverse extrinsic and intrinsic cellular factors. Understanding the underlying mechanisms of cell cycle regulation is essential to address how normal development and tissue homeostasis are achieved. Here, we present a novel cell cycle regulator Caliban (Clbn), the Drosophila ortholog of human Serologically defined colon cancer antigen 1 (SDCCAG1) gene. We show that ionizing radiation induces expression of clbn, and over-expression of clbn blocks G1-to-S cell cycle transition in Drosophila, while flies loss of clbn have defective S phase checkpoint in response to irradiation. Mechanistically, induced expression of clbn suppressed E2F1 activity and down-regulates the DNA replication and expression of its downstream target cyclin E, a key regulator of G1-to-S transition. Meanwhile, clbn over-expression leads to upregulation of the CDK inhibitor Dacapo (Dap), and upregulated Dap is decreased when e2f1 is over-expressed. Furthermore, expression of clbn is down-regulated in cells with e2f1 over-expression or rbf1 knockdown, indicating that Clbn and E2F1 act antagonistically in mediating G1-to-S transition. Thus we provide genetic evidence that Clbn works together with E2F1 in regulating cell cycle progression, and Clbn is required for S phase cell cycle checkpoint in response to DNA damage.
Introduction
Tissue homeostasis is strictly maintained by a spatiotemporal regulation of cell growth, in which regulation of cell cycle machinery is a fundamental bioprocess during development in all organisms. The closely coordinated cell proliferation and cell cycle arrest are essential for proper development, and disruption of this coordination can lead to aberrant development or neoplastic diseases [Citation1].
The core cell cycle machinery is composed of multiple cyclins and cyclin-dependent kinases (CDKs) [Citation1]. During cell division, CDKs associate with specific cyclins to drive cell cycle progression. The activity of constantly expressed CDKs is modulated by the positive regulatory cyclins, which are synthesized cyclically and disrupted at specific times in a cell cycle [Citation1,Citation2]. In mammalian cells, cyclin D-CDK4/6 and cyclin E-CDK2 complexes drive the G1-to-S phase progression of the cell cycle [Citation3]. Cyclin D-CDK4/6 complexes phosphorylate retinoblastoma protein (pRB) and its family members, and then phosphorylated pRB protein dissociates from E2F proteins [Citation3,Citation4]. The released activator E2F proteins activate the transcription of cyclin E, cyclin A and other genes responsible for DNA replication, such as ribonucleotide reductase (rnr), DNA polymerase δ accessory subunit PCNA, and anaphase-promoting complex/cyclosome (APC/C) inhibitor Emi1 [Citation5,Citation6]. Furthermore, cyclin E-CDK2 complex enhances the transcription of cyclin E by phosphorylating pRB protein, and promotes its own activity in a positive feedback loop [Citation1].
Contrary to mammals, inactivation of Drosophila cyclin D-CDK4 has little effect on cyclin E expression and does not influence the initial G1 arrest [Citation7]. The Drosophila cyclin D-CDK4 complex regulates cellular growth but not G1-to-S progression [Citation8,Citation9], and the S-phase entry and progression is primarily controlled by cyclin E-CDK2 activity [Citation10,Citation11]. There are only two E2F genes, the activator dE2F1 and repressor dE2F2, one DP (dDP) and two RB-like genes (RBF1 and RBF2) in Drosophila [Citation12]. Cyclin E-CDK2 phosphorylates the RB-related proteins RBF1 and RBF2, and stimulates the expression of E2F target genes like cyclin E, rnr and PCNA [Citation13–Citation16]. Thus, in mitotic cells, cyclin E-CDK2 activity is regulated at the transcriptional level by E2F1. In addition, over-expression of cyclin A or down-regulation of the cyclin A-CDK1 inhibitor Rux can initiate the G1-to-S transition [Citation17–Citation19]. The activity of cyclin-CDK complexes is further controlled by the negative regulators-CDK inhibitors (CKIs). CKIs also modulate the cell cycle progression through components of the DNA replication machinery [Citation20]. The CKIs are degraded by the Skp, Cullin, F-box containing complex (SCF complex) [Citation21,Citation22], which leads to increased activity of cyclin-CDK complexes, therefore drives the G1-to-S transition and initiates the DNA replication. Dacapo (Dap) is the Drosophila ortholog of p21/p27 CDK inhibitor, it cooperates with RBF1 to inhibit cyclin E-CDK2 activity and prevent cell proliferation [Citation23,Citation24].
We have previously shown that Drosophila Caliban (Clbn), the ortholog of human Serologically defined colon cancer antigen 1 gene (SDCCAG1), can act as a nuclear exporting mediator and performs a tumor suppressor function in the NSCLC human lung cancer cell [Citation25]. The clbn knock-out flies are hypersensitive to ionizing radiation, and the clbn gene displays a two-stage transcriptional upregulation after irradiation and is involved into both p53-dependent and -independent apoptosis in response to DNA damage [Citation26]. However, the precise role of Clbn in cell cycle regulation and DNA damage response (DDR) remains poorly understood. When NSCLC cells were treated with methyl 4-methoxy-3-(3-methyl-2-butenoyl) benzoate (VT1), a chemical blocking cell cycle progression at G1 phase, SDCCAG1 has an increased expression [Citation27], and the autologous antibody of SDCCAG1 was found in colon cancer patients [Citation28]. In the present study, we carried out a series of genetic analysis, and provided the direct evidence that Clbn regulates G1-to-S cell cycle transition by antagonizing the E2F1 activity to regulate cyclin E and Dacapo expression and DNA replication, and plays an important role in regulating the S phase checkpoint in response to DNA damage.
Results
clbn knock-out flies have defective S phase checkpoint after ionizing radiation
We have previously shown that the clbn knock-out flies are hypersensitive to ionizing radiation, and Clbn plays a role in both p53-dependent and – independent apoptosis [Citation26]. We further investigated whether it is required for the DNA damage induced cell cycle checkpoint. Third instar larvae (L3) of wild-type (w1118) flies and clbn KO alleles were treated with 10 Gy ionizing radiation, and BrdU incorporation assay was performed to detect the S phase checkpoint in the brain lobes. The amount of incorporated BrdU in brain lobes in wild-type flies after irradiation was decreased by 87.5% (-aʹ,c)), indicating an intact S phase checkpoint. In clbn KO alleles, the incorporated BrdU were greatly increased after irradiation, and there is no significant difference of BrdU signals before and after irradiation, suggesting a defective S phase checkpoint in clbn KO mutant (-bʹ,c)). The significantly increased number of S phase cells in clbn knock-out flies after irradiation suggests that Clbn regulates S phase checkpoint in response to DNA damage.
Figure 1. clbn knock-out allele has defective S phase checkpoint and intact G2/M checkpoint in response to DNA damage.
The third instar larvae of wild-type fly (w1118) and clbn knock-out mutant were irradiated with 10 Gy. Brains from untreated or irradiated flies were stained with anti-BrdU antibody to detect S phase cells. Representative brains (a-aʹ, b-bʹ) were shown. Quantitative analysis of BrdU labeling cells was shown in (c). Wing discs from w1118 flies (d-dʹ), clbn mutant (e-eʹ) and mei-41 mutant (mei-41 −/-) (f-fʹ) were stained with anti-PH3 antibody to detect mitotic cells. Representative wing discs were shown. Quantitative analysis of mitotic cells was shown in (g). Data was derived from at least five brains or discs for each genotype and three independent experiments. Error bar represents the standard error of mean. *P < 0.001, n.s. not significant.
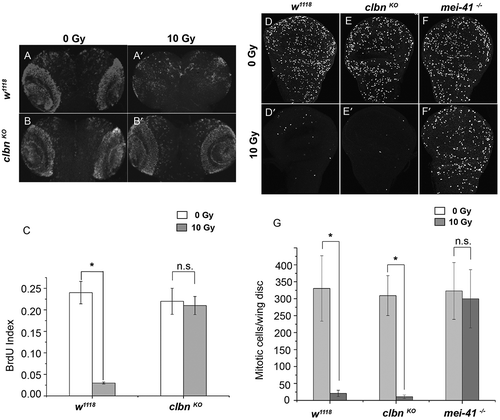
Clbn is dispensable for DNA damage induced G2/M checkpoint
Next we analyzed whether Clbn is required for DNA damage induced G2/M checkpoint. Wing discs from wild-type fly, clbn KO allele, and mei-41 allele, the Drosophila homolog of ATR, were stained with the mitotic marker anti-phospho-Histone H3 (PH3) antibody, and the number of mitotic cells was quantified. Mitotic cells were almost totally vanished in wild-type flies and clbn KO mutant following irradiation (-dʹ,e-eʹ,g)), indicating a normal G2/M checkpoint existed in clbn KO mutant. As a positive control, wing discs from strong mei-41 allele, mei-41 RT1(mei-41 −/-), showed a much larger number of mitotic cells following irradiation similar as no treatment (-fʹ,g)).
Increased expression of Clbn protein after irradiation
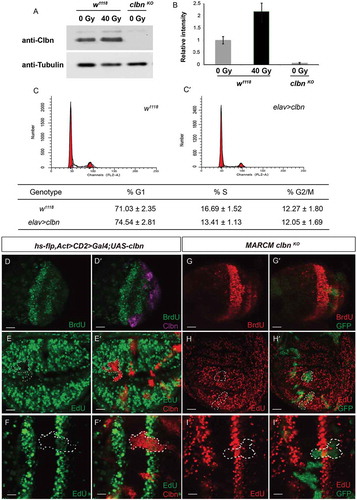
We previously demonstrated that clbn transcription is upregulated in response to DNA damage [Citation26]. To study whether DNA damage can influence clbn expression not only at transcription level, but also at protein level, we detected the level of Clbn protein before and after irradiation using samples from third instar larvae. We observed a 2.2 folds increase of Clbn protein level 4 h after irradiation (,)), indicating that irradiation also increased protein expression of Clbn.
Figure 2. Clbn expression is induced by irradiation and over-expression of clbn attenuates G1-to-S progression.
The lysate from untreated or irradiated flies were subjected to western-blot analysis. The sample from clbn knock-out mutant was used as a negative control (a). Relative intensity of Clbn western blot band was calculated and shown in (b). The cell cycle profiles of larval brain cells from wild-type w1118 (c), elav-clbn (Cʹ) were detected by flow cytometry. Representative flow cytometry profiles of the cell cycle phases distribution were shown. Quantitative data were presented as mean ± standard error from three experiments. (d–dʹ) Flp-out clones expressing clbn in the brain hemisphere from larval stage, with decreased incorporation of BrdU (green, D) and over-lapping in clbn over-expressing cells (magenta, Dʹ). Flp-out clones expressing clbn in the eye disc (e-eʹ) or wing disc (f-fʹ), with decreased incorporation of EdU (green, E and F) and over-lapping in white dashed circle (Eʹ and Fʹ). MARCM clones of clbn KO in brain lobes, eye disc and wing disc (GFP, Gʹ, Hʹ and Iʹ). Mutant clones lacking clbn exhibited normal BrdU (red, G) and EdU (red, H and I) incorporation. Scale bars, 30 um (D-Dʹ, E-Eʹ, G-Gʹ, H-Hʹ), 10 um (F-Fʹ, I-Iʹ).
Over-expression of clbn attenuates G1-to-S progression and DNA replication in brain and imaginal disc
To determine the biological effect of increased expression of Clbn on cell cycle progression, and provide more evidence that Clbn can mediate the G1-to-S transition, we used elav-Gal4 to drive the over-expression of clbn in brain cells and detected cellular DNA content by FACS analysis. Over-expression of clbn in brain cells induced a statistically significant decrease in the S phase population at 94 ± 2 hrs AEL when compared to that of wild-type flies (13.41 ± 1.13% S phase cells in elav-clbn vs 16.69 ± 1.52% S phase cells in w1118, P < 0.01, -cʹ)), indicating Clbn is involved into regulation of the G1-to-S cell cycle transition, and over-expression of clbn partially block the G1-S progression. Moreover, the percentage of cells at G2/M phase is comparable between clbn over-expression flies and wild-type flies (-cʹ)), indicating that Clbn is not required for the G2-to-M cell cycle progression.
Furthermore, we performed BrdU incorporation assay in L3 brains and EdU incorporation assay in L3 wing discs and eye discs respectively to analyze whether Clbn can regulate the DNA replication. Clones over-expressing clbn showed greatly reduced BrdU signals in larval brains (-dʹ)) and EdU signals in wing discs and eye discs (-fʹ)). However, we did not observe the significant change of BrdU or EdU signals in clbn mutant clones (-iʹ)), suggesting that loss of clbn alone does not influence the G1-to-S transition, and Clbn might play a supportive role to maintain the cell cycle progression under physiological condition.
Over-expression of clbn reduced expression of cyclin E
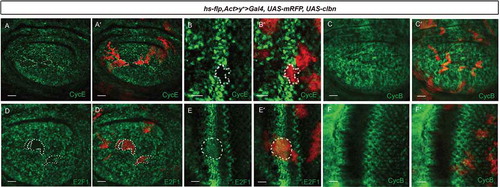
The G1-to-S transition in Drosophila relies mainly on cyclin E-CDK2 activity. Next we addressed whether the blocked G1-to-S transition by clbn over-expression is via regulating cyclin E expression. We generated Flp-out clones over-expressing clbn in wing discs and eye discs respectively. Clones with clbn over-expression showed greatly reduced level of cyclin E protein (-aʹ,b-bʹ)). On the other hand, expression level of cyclin E in clbn knock-out clones was comparable with that of control clones (Figure S1(a-bʹ)). We also checked the expression level of cyclin B, which plays an important role at G2-M transition, in clbn over-expressing clones. We did not observe the significant change of cyclin B level in clbn over-expression clones (-cʹ,f-fʹ)) or in the posterior region with clbn over-expression driven by hh > Gal4 (Figure S2(a-aʹ)), which is consistent with the data above that Clbn is dispensable for the G2-M transition (-cʹ)).
Figure 3. Over-expression of clbn reduces expression of cyclin E and E2F1.
Flp-out clones over-expressing clbn in the wing disc (a-aʹ,c-cʹ,d-dʹ) or eye disc (b-bʹ,e-eʹ,f-fʹ) from larval stage are marked by mRFP (red, (aʹ-fʹ)), stained with anti-cyclin E (green, A and B), anti-cyclin B (green, C and F) and anti-E2F1 antibodies (green, D and E). Clones over-expressing clbn exhibit decreased expression of cyclin E and E2F1, and normal expression of cyclin B. Scale bars, 30 um (a-aʹ,c-cʹ,d-dʹ), 10 um (b-bʹ,e-eʹ,f-fʹ).
Antagonistic acts between Clbn and E2F1
As cyclin E is a direct target of E2F1 transcription factor, we wish to know whether decreased expression of cyclin E in clbn over-expressing cells is via regulated activity of E2F1. We detected the protein level of E2F1 in Flp-out clones over-expressing clbn in wing discs and eye discs respectively. A strongly reduced expression of E2F1 was observed in clbn clones (-dʹ,e-eʹ)), and in the posterior region of wing discs with clbn over-expression driven by hh > Gal4 (Figure S3). Interestingly, the expression of Clbn is severely repressed by E2F1. The Flp-out clones over-expressing e2f1 or the posterior region of wing discs with e2f1 over-expression driven by hh > Gal4 had greatly reduced expression of clbn (-aʹ,b-bʹ)), and greatly reduced expression of Clbn was also observed in clones with rbf1 knock-down (-cʹ)).
Figure 4. Expression of Clbn is suppressed by E2F1.
(a-aʹ) Wing disc showing expression of Clbn in the posterior region with e2f1 over-expression driven by hh>Gal4. Clbn staining is shown in green, and E2F1 expression is shown in red. Expression of clbn is suppressed by e2f1 over-expression. Flp-out clones over-expressing e2f1 (b-bʹ) or knocking down rbf (c-cʹ) in the wing disc from larval stage are marked by mRFP, and stained with anti-Clbn antibody (green). Clones over-expressing e2f1 or knocking down rbf exhibit decreased expression of Clbn. Wing disc showing expression of Clbn and E2F1 in w1118 (d-dʹʹ). Knockdown of e2f1 (e-eʹʹ,f-fʹʹ) or dp1 (g-gʹ) by hh > Gal4 increases expression level of Clbn. Scale bars, 30 um.
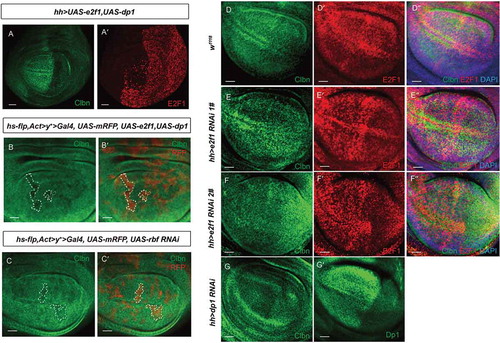
In the wing disc, the expression level of Clbn and E2F1 is comparable between anterior compartment and posterior compartment in wild-type flies (-dʹ)). When e2f1 or dp1 expression was knocked down in the posterior region of developing wing discs by RNAi, clbn expression was elevated compared to its expression in the anterior region (-eʹʹ,f-fʹʹ,g-gʹ)). Moreover, over-expression of e2f1 can increase the expression of its downstream gene cyclin E (-aʹ,b-bʹ)) and promote the DNA replication (-cʹ)), while over-expression of clbn simultaneously largely reversed the expression level of cyclin E (-dʹ,e-eʹ)) and EdU signals incorporated in e2f1 over-expressing clones (-fʹ)). These data indicates that Clbn and E2F1 work antagonistically to modulate G1-to-S cell cycle progression.
Figure 5. Clbn antagonizes E2F1 activity to regulate cyclin E expression and DNA replication.
Flp-out clones over-expressing e2f1 in the wing disc (a-aʹ,c-cʹ) or eye disc (b-bʹ) from larval stage are marked by mRFP, and stained with anti-cyclin E antibody (green, a-aʹ,b-bʹ) and EdU (green, c-cʹ). Clones over-expressing e2f1 exhibit elevated expression of cyclin E and incorporated EdU, and clbn over-expression simultaneously suppresses the induced expression of cyclin E (d-dʹ and e-eʹ) and incorporated EdU (f-fʹ). Scale bars, 30 um.
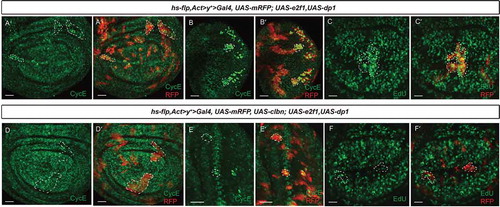
E2F1 and Clbn regulate dap expression reversely
We further investigated the effect of clbn over-expression on Dap expression, the mammalian homolog of p21/p27 CDK inhibitor. The level of Dap protein was determined by immunostaining in the posterior region of wing discs with clbn over-expression and in wing discs and eye discs containing clbn over-expressing clones. An increased level of Dap protein was observed in the posterior region of wing discs (-aʹ)) and clbn over-expressing clones (-bʹ,c-cʹ)). Therefore, the cell cycle arrest caused by clbn over-expression correlated with up-regulation of Dap expression, which can inhibit cyclin E-CDK2 activity.
Figure 6. Dap expression is reversely regulated by Clbn and E2F1.
(a-aʹ) Over-expression of clbn driven by hh > Gal4 increases expression of Dap in the posterior compartment. Flp-out clones over-expressing clbn in the wing disc (b-bʹ) or eye disc (c-cʹ) from larval stage are marked by mRFP, and stained with anti-Dap antibody (green). Clones over-expressing clbn exhibit elevated expression of Dap. (d-dʹ) Co-expression of clbn and e2f1 driven by hh > Gal4 decreases expression of Dap in the posterior compartment caused by clbn over-expression. Flp-out clones co-expressing clbn and e2f1 in the wing disc (e-eʹ) or eye disc (f-fʹ) from larval stage are marked by mRFP, and stained with anti-Dap antibody (green). Over-expressing e2f1 reduced the enhanced expression of Dap driven by clbn over-expression. Scale bars, 30 um.
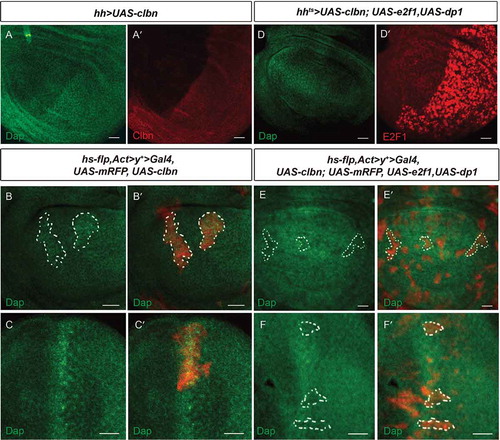
Moreover, over-expression of e2f1 simultaneously with clbn by hh > Gal4 decreased the expression of Dap in the posterior region in wing discs (-dʹ)) and in Flp-out clones in wing discs (-eʹ)) and eye discs (-fʹ)). A previous study has shown that expression level of cyclin E and Dap are positively correlated [Citation29]. Although forced expression of e2f1 can elevate expression of cyclin E (-aʹ,b-bʹ)), our data revealed that expression of Dap is negatively regulated by forced expression of e2f1. This discrepancy raised the possibility that complex feedback regulation exists among Clbn-E2F1-cyclin E and Dap in Drosophila.
Discussion
Precise modulation of cell cycle regulators is critical for maintenance of genome integrity and normal development, whereas cell cycle disorder may cause severe diseases such as cancer, aging, neurodegeneration etc. To cope with DNA damage caused by endogenous or exogenous factors, eukaryotic cells have developed complicated DNA damage response mechanisms, which involve the activation of checkpoint pathways operating at the G1-S boundary, intra-S phase and G2-M boundary. Identification and characterization of genes involved in regulation of cell cycle progression will improve our understanding of the cell cycle control and DNA damage signaling networks, and provide us the strong tool for treating related diseases.
We previously identified Drosophila Clbn as a tumor suppressor and demonstrated that it regulates DNA damage-induced apoptosis through p53-dependent and -independent activity [Citation25,Citation26]. In this study, we made further investigation to show that Clbn is involved in G1-to-S transition and is required for DNA damage induced S phase checkpoint. Our results showed that expression of Clbn protein is elevated by ionizing radiation, and the clbn knock-out flies have defective S phase checkpoint in response to DNA damage, while the G2/M DNA damage checkpoint is functional. These data support the conclusion that clbn participates in DNA damage response and functions predominantly in regulating S phase checkpoint. Physiologically, Clbn is expressed at moderate level and displays low tissue specificity. However, in the absence of genotoxic stress, we detected normal DNA synthesis activity and normal expression level of cyclin E in the clbn knock-out flies, indicating that the G1-to-S progression is functional in the clbn knock-out mutant under physiological condition and Clbn is only required for S phase checkpoint after DNA damage. To clarify the underlying mechanisms, we performed genetic analysis by over-expressing clbn in larval brain and imaginal disc and examined the effect of clbn over-expression on G1-to-S transition. Our results showed that elevated expression of clbn in multiple tissues greatly blocks the G1-to-S transition with reduced expression of cyclin E. As cyclin E is an evolutionarily conserved nuclear cyclin and acts as the limiting factor for G1-to-S phase transition [Citation30,Citation31], and reduced cyclin E protein level can cause G1-S arrest [Citation32]. In Drosophila, Dacapo (Dap) is the only Cdk inhibitor identified thus far and was shown to be specific for the cyclin E-dependent kinases [Citation22,Citation24]. Dap expression parallels the exit of cells from the cell cycle in embryos and mutation of dap leads to an extra division during embryogenesis [Citation23,Citation24]. Consistently, we observed increased expression of Dap driven by clbn over-expression, suggesting that increased expression of clbn blocks G1-to-S transition through activating Dap and suppressing cyclin E. Since cyclin E is the target gene of E2F1, another well-identified regulator of G1-to-S transition, we further explored the genetic interaction between E2F1 and Clbn. Our results showed that clbn over-expression significantly reduced expression of E2F1. Moreover, clbn over-expression induced G1-S blocking can be rescued by over-expression of e2f1, suggesting that E2F1 works downstream of Clbn. Interestingly, expression of clbn was negatively regulated by E2F1, as over-expression of e2f1 or knocking down of rbf, the E2F1 inhibitor, greatly reduced Clbn expression level. Thus, our results revealed an antagonistic interaction between E2F1 and Clbn in regulating G1-to-S transition. Previous studies in mammals showed that ionizing radiation leads to the stabilization of E2F1 protein, induction of S phase checkpoint, and subsequent cell death [Citation33,Citation34]. On the hand, pRB has been shown to be required for an intra-S phase response to DNA damage [Citation35]. These data suggests the regulation of E2F1 activity in DNA damage response and checkpoint control is context dependent. The nature of the antagonistic interaction between E2F1 and Clbn in cell cycle control is intriguing and required further investigation. As Clbn is highly conserved, the Clbn ortholog SDCCAG1 may have similar functions in mammals to control G1-to-S transition and regulates S phase checkpoint in DNA damage response.
Materials and methods
Fly genetics
All flies were maintained at 18 °C or 25 °C on standard corn meal unless specified. Fly lines used in this study were as follows: w1118; hh-Gal4; hh-Gal4, Gal80ts; elav-Gal4; clbn 1Q(clbn KO); UAS-clbn; mei-41 RT(mei-41 −/-); UAS-e2f1,UAS-dp (BL#4770); UAS-e2f1 RNAi 1#; UAS-e2f1 RNAi 2#; UAS-dp RNAi were obtained from Tsing Hua Fly Center (THFC).
Larvae irradiation
Active crawling third-instar larvae were collected,and irradiated at dosages of 10 Gy or 40 Gy by using an X-ray Biological Irradiator (X-RAD 320iX, Precision X-ray Inc) respectively.
Clonal analysis
Flp-out clones were induced 48 h after egg laying (AEL) in staged larvae by heat shock at 37 °C for 60 min. The larval genotypes were as follows: yw, hs-flp; act> y+> Gal4, UAS-mRFP/UAS-clbn; yw, hs-flp; act> y+> Gal4, UAS-mRFP/UAS-e2f1, UAS-dp and yw, hs-flp; act> y+> Gal4, UAS-mRFP/UAS-clbn; UAS-e2f1, UAS-dp. The flp-out clones were marked by mRFP. For MARCM clonal analysis, the clones were induced 48 h AEL by a 60 min, 37 °C heat shock. The genotypes used were as follows: yw, hs-flp; UAS-GFP; tubGal4, FRT82B, tubGal80/FRT82B, clbn KO.
Generation of anti-E2F1 and anti-Clbn antibodies
The cDNA fragment of E2F1-aa 460–653 was amplified with primers e2f1-F: CGCGGATCCGACATCCGTTGACCAACCAGC and e2f1-R: CCGGAATTC TCAGGGCACATCGCTGCGTCGC, and PCR product was constructed into BamHI/EcoRI sites of pRSETc plasmid (Invitrogen). Purified His-tagged E2F1 fusion protein was used to immunize rabbits, and rabbit serum was purified with Protein A beads (Abgent Biotechnology, Suzhou, China).
The cDNA fragment of Clbn-aa 657–992 was amplified with primers clbn-F: CGGGATCCGGAGGATAGCTTCATTGAGCG and clbn-R: GGAATTCTTATTTGT GATACTTCTGAAGTTGCG, and PCR product was constructed into BamHI/EcoRI sites of pRSETb plasmid (Invitrogen). Purified His-tagged Clbn fusion protein was used to immunize rabbits, and rabbit serum was purified with Protein A beads (Abgent Biotechnology, Suzhou, China).
Flow cytometry
The flow cytometry was performed as previously described [Citation36]. Third instar larvae at 94 ± 2 h AEL were collected, and brains were dissected in cold PBS. After removal of PBS, 600 µl cold Partec buffer (200 mM Tris-HCl, pH 7.5, 4 mM MgCl2, 0.1% Triton X-100) was added. The samples were treated at room temperature for 20 min, and chopped with a single-edged razor blade during digestion. Then samples were filtered with 40 µm filter membrane (BD BioSciences), and PI (50 µg/mL, ThermoFisher) was added and put on ice for 15 min in dark, then analyzed on a BD FACS Aria II (BD BioSciences). At least 15,000 events were analyzed using Modfit LT (Verity Software House) with G0/G1phase Coefficient of Variation (CV) less than 10. Three independent experiments were performed.
BrdU and EdU incorporation assay
The BrdU incorporation assay was performed as described [Citation37]. Larval brains were dissected in SFX-Insect Media (Hyclone) and incubated in the same medium with 0.5mg/ml BromoDeoxyUridine (BrdU, Sigma) for 10 min. Samples were incubated with monoclonal anti-BrdU antibody (1:500, Sigma) overnight at 4°C, and detected with anti-rabbit Alexa 488 or 555 antibody (Cell Signaling Technology, 1:400).
The EdU incorporation assay was performed according to the manufactureʹs protocol (Click-iTTM EdU Alexa Fluor 488, Click-iTTM EdU Alexa Fluor 555, Invitrogen). The imaginal wing discs and eye discs were dissected and immediately incubated in 10 mg/mL EdU for 30 min.
Histology and imaging
The brains, wing discs, and eye discs of the desired genotypes were dissected from third instar larvae in cold PBS and fixed immediately in PBS containing 4% (wt/vol) paraformaldehyde. The samples were washed with PBT (PBS containing 0.2% Triton X-100) three times, blocked in PBTB (PBT containing 5% (vol/vol) normal goat serum), and incubated with primary antibodies overnight. The following primary antibodies were used: rabbit anti-Clbn (1:400), rabbit anti-E2F1 (1:200), rabbit polyclonal anti-phospho Ser 10 histone 3 (1:400, Upstate Biotechnology/Millipore, no.06–570), mouse anti-Dap (DSHB, 1:20), mouse anti-cyclin B (DSHB, 1:20) and goat anti-cyclin E (1:200, Santa Cruz sc-15,903). After three washes with PBT, secondary anti-rabbit (1:400, Cell Signaling Technology) or anti-goat (1:400, Life Technologies) fluorescence antibodies, including Alexa 488 and 555, were used. Samples were mounted and analyzed on a Leica SP5 or Olympus FV1000 confocal laser-scanning microscope. The images were processed using Adobe Photoshop, Illustrator, and ImageJ.
Western blot
The third instar larvae were irradiated and let recover for 4 h. The wing discs of 30 larvae were dissected in PBS, and then dissociated by eight passes through a 25 gauge needle after addition of ice-cold NP40 buffer with protease inhibitors (Cell Signaling Technology, #5871). The lysate was subjected to western-blot analysis with rabbit anti-Clbn antibody (1:2,000) detection. And secondary antibody used was ECL donkey anti-rabbit HRP (1:10,000, GE Healthcare).
Statistics
Statistic significance (P value) was determined by two-way analysis of variance (ANOVA) and Student-Newman-Keuls test (SNK-q test).
Supplemental Material
Download MS Word (60 KB)Acknowledgments
We thank the Bloomington Stock Center, Dr. Zengqiang Yuan for fly stocks, and members of the Bi laboratory for advice and discussions. This work was supported by grants from National Natural Science Foundation of China (Grant No 31771437, 31501165), Natural Science Foundation of Liaoning Province (Grant No 2015020674),Liaoning Provincial Program for Top Discipline of Basic Medical Sciences, Pandeng scholarship of Liaoning province and Qizhen Gongcheng from Dalian Medical University to X.B.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplementary data can be accessed here.
Additional information
Funding
References
- Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166.
- Murray AW. Recycling the cell cycle: cyclins revisited. Cell. 2004;116:221–234.
- Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development. 2013;140:3079–3093.
- Bertoli C, Skotheim JM, de Bruin RA. Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol. 2013;14:518–528.
- DeGregori J, Kowalik T, Nevins JR. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol Cell Biol. 1995;15:4215–4224.
- Hsu JY, Reimann JD, Sorensen CS, et al. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1). Nat Cell Biol. 2002;4:358–366.
- Meyer CA, Jacobs HW, Lehner CF. Cyclin D-cdk4 is not a master regulator of cell multiplication in Drosophila embryos. Curr Biol : CB. 2002;12:661–666.
- Datar SA, Jacobs HW, de la Cruz AF, et al. The Drosophila cyclin D-Cdk4 complex promotes cellular growth. Embo J. 2000;19:4543–4554.
- Meyer CA, Jacobs HW, Datar SA, et al. Drosophila Cdk4 is required for normal growth and is dispensable for cell cycle progression. Embo J. 2000;19:4533–4542.
- Knoblich JA, Sauer K, Jones L, et al. Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell. 1994;77:107–120.
- Richardson H, O’Keefe LV, Marty T, et al. Ectopic cyclin E expression induces premature entry into S phase and disrupts pattern formation in the Drosophila eye imaginal disc. Development. 1995;121:3371–3379.
- van den Heuvel S, Dyson NJ. Conserved functions of the pRB and E2F families. Nat Rev Mol Cell Biol. 2008;9:713–724.
- Du W, Vidal M, Xie JE, et al. RBF, a novel RB-related gene that regulates E2F activity and interacts with cyclin E in Drosophila. Genes Dev. 1996;10(10):1206–1218.
- Duronio RJ, O’Farrell PH. Developmental control of a G1-S transcriptional program in Drosophila. Development. 1994;120:1503–1515.
- Duronio RJ, O’Farrell PH. Developmental control of the G1 to S transition in Drosophila: cyclin Eis a limiting downstream target of E2F. Genes Dev. 1995;9:1456–1468.
- Duronio RJ, O’Farrell PH, Xie JE, et al. The transcription factor E2F is required for S phase during Drosophila embryogenesis. Genes Dev. 1995;9:1445–1455.
- Foley E, O’Farrell PH, Sprenger F. Rux is a cyclin-dependent kinase inhibitor (CKI) specific for mitotic cyclin-Cdk complexes. Curr Biol : CB. 1999;9:1392–1402.
- Sprenger F, Yakubovich N, O’Farrell PH. S-phase function of Drosophila cyclin A and its downregulation in G1 phase. Curr Biol : CB. 1997;7:488–499.
- Thomas BJ, Zavitz KH, Dong X, et al. roughex down-regulates G2 cyclins in G1. Genes Dev. 1997;11:1289–1298.
- Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell. 2008;14:159–169.
- Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol. 2004;5:739–751.
- Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nature Reviews Cancer. 2006;6:369–381.
- de Nooij JC, Letendre MA, Hariharan IK. A cyclin-dependent kinase inhibitor, Dacapo, is necessary for timely exit from the cell cycle during Drosophila embryogenesis. Cell. 1996;87:1237–1247.
- Lane ME, Sauer K, Wallace K, et al. Dacapo, a cyclin-dependent kinase inhibitor, stops cell proliferation during Drosophila development. Cell. 1996;87:1225–1235.
- Bi X, Jones T, Abbasi F, et al. Drosophila caliban, a nuclear export mediator, can function as a tumor suppressor in human lung cancer cells. Oncogene. 2005;24:8229–8239.
- Wang Y, Wang Z, Joshi BH, et al. The tumor suppressor Caliban regulates DNA damage-induced apoptosis through p53-dependent and -independent activity. Oncogene. 2013;32:3857–3866.
- Carbonnelle D, Jacquot C, Lanco X, et al. Up-regulation of a novel mRNA (NY-CO-1) involved in the methyl 4-methoxy-3-(3-methyl-2-butenoyl) benzoate (VT1)-induced proliferation arrest of a non-small-cell lung carcinoma cell line (NSCLC-N6). Int J of Cancer. 2001;92:388–397.
- Scanlan MJ, Chen YT, Williamson B, et al. Characterization of human colon cancer antigens recognized by autologous antibodies. Int J of Cancer. 1998;76:652–658.
- de Nooij JC, Graber KH, Hariharan IK. Expression of the cyclin-dependent kinase inhibitor Dacapo is regulated by cyclin E. Mech Dev. 2000;97:73–83.
- Neufeld TP, de la Cruz AF, Johnston LA, et al. Coordination of growth and cell division in the Drosophila wing. Cell. 1998;93:1183–1193.
- Lane ME, Elend M, Heidmann D, et al. A screen for modifiers of cyclin E function in Drosophila melanogaster identifies Cdk2 mutations, revealing the insignificance of putative phosphorylation sites in Cdk2. Genetics. 2000;155:233–244.
- Mandal S, Guptan P, Owusu-Ansah E, et al. Mitochondrial regulation of cell cycle progression during development as revealed by the tenured mutation in Drosophila. Dev Cell. 2005;9:843–854.
- Huang Y, Ishiko T, Nakada S, et al. Role for E2F in DNA damage-induced entry of cells into S phase. Cancer Res. 1997;57:3640–3643.
- Lin WC, Lin FT, Nevins JR. Selective induction of E2F1 in response to DNA damage, mediated by ATM-dependent phosphorylation. Genes Dev. 2001;15:1833–1844.
- Knudsen KE, Booth D, Naderi S, et al. RB-dependent S-phase response to DNA damage. Mol Cell Biol. 2000;20:7751–7763.
- Callan MA, Cabernard C, Heck J, et al. Fragile X protein controls neural stem cell proliferation in the Drosophila brain. Hum Mol Genet. 2010;19:3068–3079.
- Jaklevic B, Purdy A, Su TT. Control of mitotic entry after DNA damage in Drosophila. Methods Mol Biol. 2004;280:245–256.
