ABSTRACT
Podocyte apoptosis is considered as the important element that promotes the development and progress of membranous nephropathy (MN). Unfortunately, the underlying mechanism of podocytes apoptosis in MN remains elusive. We compared the renal expressions of miR-130a-5p and M-type phospholipase A2 receptor (PLA2R) between MN patients (n = 30) and 30 controls by qRT-PCR and western blot, respectively. The podocyte damage model in vitro was established by angiotensin II (Ang II, 100 nmol/L) exposure for 24 h. Interaction between miR-130a-5p and PLA2R was determined using dual-luciferase reporter gene assay. MN mice were induced by intravenous injection of cBSA. In this study, miR-130a-5p expression was significantly decreased both in the renal biopsy specimens from MN patients and podocyte cell line AB8/13 following stimulation of Ang II. Overexpressed miR-130a-5p in AB8/13 cells significantly attenuated the Ang II induced-apoptosis in vitro. In contrast, down-regulated miR-130a-5p induced podocyte apoptosis. PLA2R was identified as the target of miR-130a-5p in AB8/13 cells. And up-regulated or down-regulated PLA2R could obviously attenuate the effect of miR-130a-5p overexpression or knockdown on the apoptosis of AB8/13 cells. Furthermore, it was also observed that overexpressed miR-130a-5p by miR-130a-5p agomir could obviously alleviate renal injury in MN mice. In conclusion, decreased miR-130a-5p was contributed to the pathological mechanism of MN through increasing PLA2R expression, which induced podocyte apoptosis.
Keywords:
Introduction
Membranous nephropathy (MN) is a common primary glomerular disease, which accounts for 22% to 33% of the adult nephrotic syndrome (NS) [Citation1]. Clinically, MN showed slow progression, prolonged course and different prognosis of patients. About 25% to 50% of MN can be spontaneous remission, and about 30% deteriorated to end stage renal disease (ERSD) within 5 to 10 years or died of the related complications [Citation2]. An abnormally small number of podocytes resulted from excessive apoptosis is a key event contributing to the development of most forms of NS, including MN [Citation3]. In progressive MN, the loss of podocytes leads to glomerular sclerosis and impaired glomerular filtration function, thereby increasing the synthesis of glomerular cytokines, mesangial matrix, and extracellular matrix (ECM) and promoting the progression of MN [Citation4,Citation5]. The membrane attack of complement (C5b-9) was considered as the crucial regulator for inducing the alterations and injuries of podocytes that causes proteinuria [Citation6–Citation8]. However, the underlying mechanism of podocytes apoptosis in MN remains elusive. Therefore, studying the molecular pathophysiology of podocyte apoptosis can provide a powerful theoretical basis for clinical application of approaches that delay the development of MN.
The fundamental role of microRNAs (miRNAs) has been gradually revealed in the developing process of MN. MiRNAs, belonging to small non-coding RNAs, have an important role in modulating gene expression. The mature miRNAs could bind on the 3′-untranslated regions (3′-UTR) of messenger RNA (mRNA) depending on a Watson-Crick base-pairing manner [Citation9]. The interaction between miRNA and mRNA’s 3′-UTR consequently elicited repression on mRNA expression [Citation10]. Interestingly, although miRNAs could not to code for functional proteins, miRNAs have been confirmed to involve in tumors, cardiovascular diseases, autoimmune diseases and a variety of human diseases through targeting one or several causative genes. Increasing studies showed a close connection between abnormal miRNAs and MN progression. For example, the relative expression of miR-217 was significantly downregulated in the tissue and plasma of MN patients and the receiver operating characteristic (ROC) curve analysis indicated that miR-217 might serve as a useful diagnostic biomarker for MN [Citation11]. Both miR-217 and miR-186 were reported to be related with podocytes apoptosis in MN [Citation11,Citation12].
Although the etiology and pathogenesis of MN are still not completely understood, auto-antibodies reacting with the target antigens of autoimmunity to form immune complexes is the main incentive. M-type phospholipase A2 receptor (PLA2R), a transmembrane receptor constitutively expressed in podocytes, has been identified as the target of autoimmunity in MN [Citation13]. In addition, PLA2R could induce human podocyte apoptosis via activating ERK1/2 and cytosolic PLA2a (cPLA2a) in vitro [Citation14]. Previous study showed that miR-130a was altered in response to ochratoxin A nephrotoxicity in rats [Citation15]. Moreover, miR-130a-3p was found to abnormally express in the urine and plasma of patients with advanced chronic kidney disease (CKD) [Citation16]. By using bioinformatics techniques, we identified that PLA2R was one of the potential targets of miR-130a-5p rather than miR-130a-3p. In this study, we investigate expression of miR-130a-5p and PLA2R in MN, and access the in vitro and in vivo interactive relationship between miR-130a-5p and PLA2R in preventing angiotensin II-induced podocyte apoptosis.
Materials and methods
Human tissue specimens
Patients with primary membranous nephropathy (MN) (n = 30) and control patients (n = 30) in this study were collected at The First Affiliated Hospital of Zhengzhou University. All MN cases were diagnosed by the described criteria [Citation17]. The control patients were testified without any kidney disease. The study was supported by the Ethics Committee of The First Affiliated Hospital of Zhengzhou University and written informed consent was obtained from all patients. All patients were underwent renal biopsy in our hospital and the collected glomerulus samples were stored at −80°C until use.
Quantitative real-time PCR
Total RNA was obtained from the frozen tissues and cells using TRIzol reagent (Invitrogen, Grand Island, NY, USA) according to the manufacturer’s instructions. For quantification of miR-130a-5p expression, total RNA was reverse transcripted into cDNA using the stem-loop-specific transcription primers provided by RiboBio Co. Ltd. (Guangzhou, China). For analysis of the mRNA level of PLA2R, total RNA was reverse transcripted into cDNA using the M-MLV Reverse Transcriptase (Takara, Dalian, China) according to the manufacturer’s instructions. RT-qPCR was performed using the SYBR Premix Ex Taq II (Takara, Dalian, China) according to the manufacturer’s instructions. U6 level served as the internal control for miR-130a-5p and GAPDH level was used as the internal control for PLA2R mRNA. The specific primers for PLA2R (NC_000002.12) and GAPDH (NC_000012.12) were as follows: PLA2R (F) 5′-GCTTCCGAGGCTGACACTTA-3′ and (R) 5′-CCGCCGAGTTAGTTCGTTAC-3′; GAPDH, (F) 5′-TGCACCACCAACTGCTTAGC-3′ and (R) 5′-GGCATGGACTGTGGTCATGAG-3′.
Western blotting
The proteins of tissue specimens and podocytes were extracted using RIPA buffer containing protease/phosphatase inhibitors (Invitrogen, Grand Island, NY, USA) according to the manufacturer’s instructions. BCA assay (Beyotime Institute of Biotechnology, Nantong, Jiangsu, China) was used to quantify the extracted proteins. After boiling for 5 min at 95°C, the protein samples (50 μg) were separated using sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and then electrotransferred to polyvinylidene difluoride membranes (PVDF; PerkinElmer, Inc., Waltham, MA, USA). The membranes were blocked with 5% skim milk for 1 h at room temperature. Subsequently, the membranes were incubated with the primary antibodies against PLA2R (1:5000, Abcam, Cambridge, MA, USA) or β-actin (1:500, Abcam, Cambridge, MA, USA) overnight at 4°C. The membranes were then incubated with the horseradish peroxidase-conjugated secondary antibodies (1:1000, Abcam, Cambridge, MA, USA) for 2 h at room temperature. The blotted proteins were visualized using the ECL Western Blotting Substrate (Thermo Scientific, Grand Island, NY, USA) on an Odyssey infrared imaging system (LI-COR Biotechnology, Lincoln, NE, USA).
Cell culture and treatment
Human conditionally immortalized podocyte cell line, AB8/13 from American Type Culture Collection (ATCC, Manassas, VA, USA) was cultured in RPMI 1640 Medium (HyClone) supplemented with 10% fetal bovine serum (FBS, Gibco, Grand Island, NY, USA), 1% penicillin/streptomycin (HyClone) and 1% Insulin-Transferrin-Selenium (ITS) (Invitrogen, Grand Island, NY, USA) at 33°C with 5% CO2 and 95% air for proliferation. For Angiotensin II (Ang II) stimulation, the cells were cultured with Ang II (100 nmol/L) (Sigma, Missouri, St. Louis, USA) for 24h [Citation18].
Cell transfection
The pcDNA 3.0 vectors were purchased from Invitrogen (Grand Island, NY, USA) and full-length PLA2R cDNA was cloned into pcDNA 3.0 vector by Ribobio Co., Ltd. (China) to generate the human PLA2R plasmid (pcDNA-PLA2R). Small interfering RNAs (siRNA) for PLA2R (si-PLA2R) were designed and synthesized by Ribobio Co., Ltd. (China). The sequence of si-PLA2R was as follows: sense: 3′-TTAACGGGACGAGGAACUGUU-5′ and antisense: 5′-UUCAGGUGCAGAACAAGGGTT-3′. MiR-130a-5p mimic, miR-130a-5p inhibitor and their negative controls were purchased from Ribobio Co., Ltd. (China). AB8/13 cells (3 × 105 cells/well) were cultured in 6-well plates overnight. Then the cells were transfected with 70 nmol miR-130a-5p mimic, miR-130a-5p inhibitor, si-PLA2R, pcDNA-PLA2R or their corresponding negative controls using the Lipofectamine2000 reagent (Invitrogen, Grand Island, NY, USA) according to the manufacturer’s instructions.
Caspase-3 activity assay
Protein of the treated or transfected AB8/13 cells were extracted using the buffer containing Tris–HCl (25 mM), MgCl2 (20 mM), NaCl (150 mM), Triton X-100 (1%), leupeptin (25 μg/mL), and aprotinin (25 μg/mL). After centrifugation at 12,000 rpm for 15 min, the collected protein samples were quantified using BCA kit (Beyotime Institute of Biotechnology, Nantong, Jiansu, China). The protein samples (30 μg) were incubated with Ac-DEVD-pNA (20 ng) provided by caspase-3 activity assay kit (Beyotime Institute of Biotechnology, Nantong, Jiangsu, China) in 96-well plates for 2 h at 37°C. The absorbance of each well was measured at 405 nm on a Microplate Reader (Molecular Devices, USA).
Flow cytometry analysis
Apoptosis analysis was performed using Annexin V-FITC Apoptosis Detection Kit (Invitrogen, Grand Island, NY, USA). The treated or transfected AB8/13 cells were collected. After centrifugation at 1500 rpm for 5 min, the cells were resuspend in 1× Annexin V binding buffer (195 μL). Then the cells were incubated with Annexin-V/FITC (5 μL) and PI (10 μL) at room temperature in the dark for 15 min. The apoptosis of AB8/13 cells was analyzed on a flow cytometry (FACScan; BD Biosciences, San Jose, CA).
Dual-luciferase reporter gene assay
Dual-luciferase reporter gene assay was performed to determine the interaction between miR-130a-5p and the 3-UTR of PLA2R. According to the putative miR-130a-5p binding sites in the 3-UTR of PLA2R (PLA2R-WT, 5ʹ- CACAGAUGCCAUUAGAAUGUGAA-3ʹ), the 3-UTR of PLA2R with mutated miR-130a-5p binding sites (PLA2R-Mut, 5ʹ- CACAGAUGCCAUUAGCCGUGUCC-3ʹ) was generated using QuikChange Multi Site-Directed Mutagenesis kit (Stratagene). The recombinant luciferase reporter vectors containing PLA2R-WT (pGL-PLA2R-WT) or PLA2R-Mut (pGL-PLA2R-Mut) were constructed by Ribobio Co., Ltd. (China). And pGL-PLA2R-WT or pGL-PLA2R-Mut combined with miR-130a-5p mimic, pre-NC, miR-130a-5p inhibitor, or NC were co-transfected into AB8/13 cells using the Lipofectamine2000 reagent (Invitrogen, Grand Island, NY, USA). After 48 h of transfection, luciferase activity was detected using Dual Luciferase Assay System (Promega, Madison, WI, USA) according to the manufacturer’s instructions.
Animal experiments
BALB/c female mice (4–6 weeks old) were provided by The First Affiliated Hospital of Zhengzhou University. The mice were randomly divided into 4 groups (n = 7 per group): control, MN, MN+ agomir-miR-130a-5p, MN+ agomir-NC.
All mice received cationic bovine serum albumin (0.2 mg, cBSA) emulsified in complete Freund’s adjuvant (CFA) (Sigma-Aldrich, St. Louis, MO, USA) [Citation19]. Two weeks later, the mice in MN, MN+ agomir-miR-130a-5p and MN+ agomir-NC groups were intravenously injected with cBSA (100 μg) three times per week (every other day) for 6 weeks, and the control mice received pure saline on the same schedule [Citation20]. For agomir-miR-130a-5p and agomir-NC administration, MN mice received agomir-miR-130a-5p or agomir-NC (0.2 mg/mouse) on days 0, 3, 7, and 14 of MN induction. And 28 days after the first injection, urine and serum samples were collected from each mouse. The proteinuria was measured using a urine dipstick (Albustix, Bayer, Tarrytown, NY, USA). And the levels of serum albumin and serum cholesterol were detected using the commercially kits (Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s instructions. The renal tissues were used for qRT-PCR and Western blot analysis.
Statistical analysis
All experiments performed in triplicate. Data were expressed as means ± standard deviations (SD). Statistical analysis was performed on SPSS package version 19.0 (SPSS Inc., Chicago, IL, USA). And the significant difference was calculated using Bonferroni test or one-way ANOVA with P < 0.05 as considered significant.
Results
Renal expression patterns of miR-130a-5p and PLA2R in MN
We first compared the renal expressions of miR-130a-5p between MN patients (n = 30) and 30 controls, and results showed that miR-130a-5p was significantly decreased in the renal biopsy specimens from MN patients (Figure 1(a)). Moreover, according to the Western blot results, PLA2R protein was increased in the renal tissues from MN patients (n = 30) compared with the controls (). Next, the podocyte damage model in vitro was established by Ang II exposure [Citation18]. Following stimulation of Ang II, miR-130a-5p expression was significantly decreased in human podocyte cell line AB8/13 (Figure 2(a)). As expected, the protein expression of PLA2R was increased after AB8/13 cells exposed to Ang II ().
Figure 1. Renal expression patterns of miR-130a-5p and PLA2R in MN.
(a) The expression of miR-130a-5p in renal biopsy specimens from MN patients (n = 30) or 30 controls was detected using qRT-PCR. (b) Western blot analysis of PLA2R expression in renal biopsy specimens from MN patients (n = 30) or 30 controls. **P < 0.01 compared with the control (Normal).
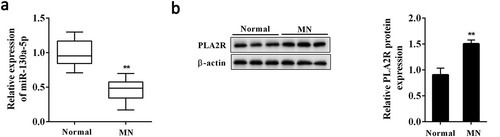
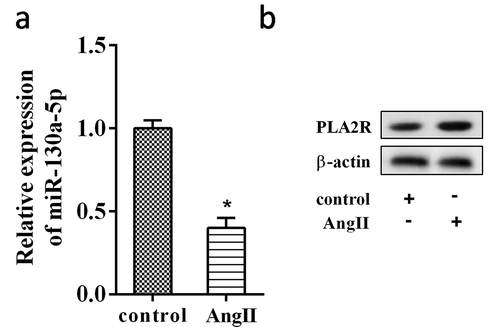
Figure 2. Ang II inhibited miR-130a-5p expression in AB8/13 cells.
Human podocyte cell line AB8/13 was treated with Ang II (100 nmol/L) for 24 h. (a) The expression of miR-130a-5p in podocytes as determined by qRT-PCR. (b) Western blot analysis of PLA2R expression in podocytes. **P < 0.01 compared with Control.
miR-130a-5p was involved in the podocyte apoptosis induced by Ang II
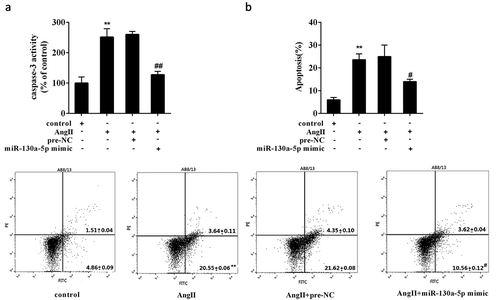
To determine the role of miR-130a-5p in MN, we explored whether miR-130a-5p was involved in regulating the Ang II induced-podocyte damage. AB8/13 cells overexpressed miR-130a-5p were treated by Ang II. It was observed that the caspase-3 activity in AB8/13 cells was significantly increased by Ang II, but overexpressed miR-130a-5p significantly attenuated the induction of Ang II on caspase-3 activity (Figure 3(a)). Moreover, apoptotic analyses showed that AB8/13 cells overexpressed miR-130a-5p had a low rate of apoptosis under Ang II exposure compared to the cells transfected with pre-NC ().
Figure 3. Effect of miR-130a-5p on podocyte apoptosis induced by Ang II.
AB8/13 cells were transfected with miR-130a-5p mimic or its negative control, pre-NC. After 24 h of transfection, the cells were treated with Ang II (100 nmol/L) for another 24h. (a) The caspase-3 activity was detected using a Caspase-3 Activity Assay Kit. (b) Cell apoptosis was analyzed by flow cytometry. **P < 0.01 compared with Control; #P < 0.01 compared with Ang II+ pre-NC.
Interaction between miR-130a-5p and PLA2R
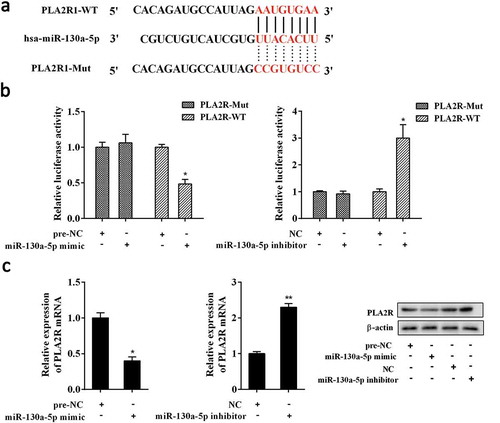
Bioinformatics analysis revealed the putative miR-130a-5p binding sites in the 3-UTR of PLA2R (PLA2R1-WT) (Figure 4(a)). And the 3-UTR of PLA2R with mutated miR-130a-5p binding sites (PLA2R1-Mut) was also generated (). By using dual-luciferase reporter gene assay, we found that miR-130a-5p mimic and miR-130a-5p inhibitor significantly decreased and increased the relative luciferase activity of luciferase reporter gene vector containing PLA2R-WT, but neither had marked impact on the luciferase activity of luciferase reporter gene vector containing PLA2R-Mut in AB8/13 cells (). Also, overexpression of miR-130a-5p evidently decreased the expression of PLA2R; whereas miR-130a-5p knockdown significantly elevated PLA2R expression in AB8/13 cells ().
Figure 4. Interaction between miR-130a-5p and PLA2R.
(a) Putative miR-130a-5p binding sites in the 3-UTR of PLA2R (PLA2R-WT). And the 3-UTR of PLA2R with mutated miR-130a-5p binding sites (PLA2R-Mut) was also generated. (b) Dual-luciferase reporter gene assay. Luciferase reporter gene vector containing PLA2R-WT or PLA2R-Mut combined with miR-130a-5p mimic, pre-NC, miR-130a-5p inhibitor, or NC were co-transfected into AB8/13 cells. After 48h, luciferase activity was detected using Dual Luciferase Assay System. (c) Effect of miR-130a-5p on PLA2R expression. AB8/13 cells were transfected with miR-130a-5p mimic, pre-NC, miR-130a-5p inhibitor, or NC. The expression of PLA2R was analyzed by qRT-PCR and Western blot analysis. NC, the negative control for miR-130a-5p inhibitor. *P < 0.05 compared with PLA2R-WT+ pre-NC, PLA2R-WT+ NC, or pre-NC; **P < 0.01 compared with NC.
Down-regulated miR-130a-5p induced podocyte apoptosis via modulating PLA2R
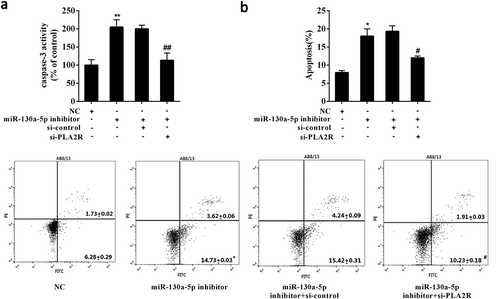
To reveal the functional interaction between miR-130a-5p and PLA2R in podocyte apoptosis, AB8/13 cells were transfected with miR-130a-5p inhibitor alone or combined with si-PLA2R. As compared with control, knockdown of miR-130a-5p resulted in a obvious increase in caspase-3 activity and apoptosis of AB8/13 cells (Figure 5(a,b)). Notably, additional silencing of PLA2R significantly reduced the caspase-3 activity and apoptosis of AB8/13 cells induced by miR-130a-5p knockdown (). Collectively, the data suggested that down-regulated miR-130a-5p induced podocyte apoptosis via elevating PLA2R.
Figure 5. Down-regulated miR-130a-5p induced podocyte apoptosis via modulating PLA2R.
AB8/13 cells were transfected with miR-130a-5p inhibitor alone or combined with siRNA targeting PLA2R (si-PLA2R). Analysis of (a) the caspase-3 activity and (b) cell apoptosis after 48 h of transfection. si-control, the negative control for si-PLA2R. *P < 0.05, **P < 0.01 compared with NC; #P < 0.05, ##P < 0.01 compared with miR-130a-5p inhibitor+ si-control.
Overexpressed miR-130a-5p attenuated the Ang II induced-podocyte apoptosis by modulating PLA2R
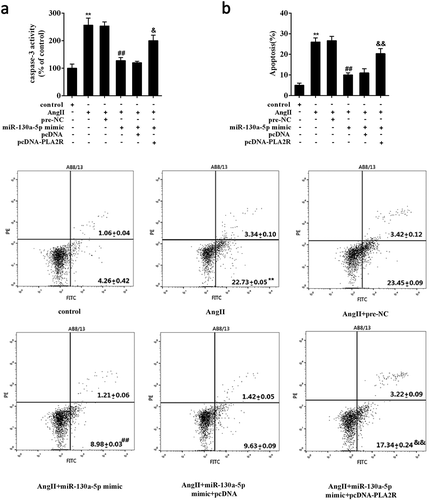
To further confirm the implication of miR-130a-5p/PLA2R axis in podocyte damage, we explored the effect of miR-130a-5p and PLA2R on podocyte apoptosis under Ang II exposure. We observed that overexpression of miR-130a-5p significantly reduced the Ang II-increased caspase-3 activity in AB8/13 cells, and overexpression of PLA2R partly impaired the inhibition of miR-130a-5p on caspase-3 activity (Figure 6(a)). Similarly, under Ang II exposure, overexpression of miR-130a-5p significantly relieved the Ang II-induced apoptosis of AB8/13 cells, but overexpression of PLA2R could substantially attenuate this effect ().
Figure 6. Overexpressed miR-130a-5p attenuated the Ang II induced-podocyte apoptosis by modulating PLA2R.
AB8/13 cells were transfected with miR-130a-5p mimic alone or combined with expression plasmids of PLA2R (pcDNA-PLA2R) followed by treatment of Ang II. (a) The caspase-3 activity and (b) cell apoptosis were determined. pcDNA, the negative control for pcDNA-PLA2R. **P < 0.01 compared with Control; ##P < 0.01 compared with Ang II+ pre-NC; &P < 0.05, &&P < 0.01 compared with Ang II+ miR-130a-5p mimic+ pcDNA.
Overexpressed miR-130a-5p alleviated renal injury in MN mice
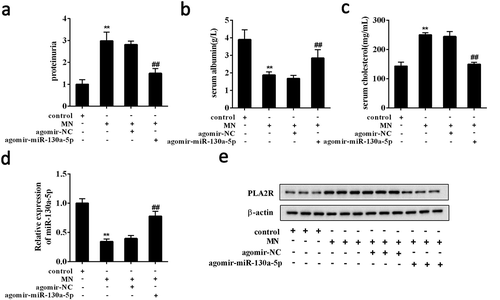
To verify the involvement of miR-130a-5p in MN, we investigated the in vivo activity of miR-130a-5p in MN mice. MN mice receiving agomir-miR-130a-5p (n = 7) had significant reduction in the levels of proteinuria and serum cholesterol accompanied by an increase in serum albumin (). We also found that the renal expression of miR-130a-5p was significantly increased in the MN mice receiving agomir-miR-130a-5p (n = 7) (). In contrast, Western blot analysis showed that PLA2R protein was decreased in the MN mice receiving agomir-miR-130a-5p (n = 7) ().
Figure 7. Overexpressed miR-130a-5p alleviated renal injury in MN mice.
BALB/c female mice were randomly divided into 4 groups (n = 7 per group): control, MN, MN+ agomir-miR-130a-5p, MN+ agomir-NC. MN mice induced by intravenous injection of cBSA received agomir-miR-130a-5p or agomir-NC. The levels of (a) proteinuria, (b) serum albumin, and (c) serum cholesterol were detected in each mouse. (d) The expression of miR-130a-5p in renal tissues was detected using qRT-PCR. (e) The protein expression of PLA2R was determined using Western blot analysis. **P < 0.01 compared with control; ##P < 0.01 compared with MN+ agomir-NC.
Discussion
The pathogenesis of MN is complicated, involving the dysregulation of multiple genes and signaling pathways. Podocyte apoptosis is considered as the important element that promotes the development and progress of MN. MiRNAs have recently been identified as a major modulator involved in MN. In this study, it was found that the renal expression of miR-130a-5p was decreased in MN patients and confirmed that overexpressed miR-130a-5p was sufficient to attenuate the Ang II-induced podocyte apoptosis in vitro. Furthermore, our data showed that knockdown of miR-130a-5p resulted in an obvious increase in the apoptosis of podocytes in vitro. These data suggested that down-regulated miR-130a-5p might be an important regulatory event in the injury and apoptosis of podocytes.
MiR-130a-5p has been proved to be ubiquitously expressed in various types of tissues and cells and responded to many pathologic and physiological processes. However, the functional role and mechanism by which miR-130a-5p regulates biological activities remains largely unknown. MiR-130a-5p was reported to significantly upregulate in the lungs infected with a mouse-adapted, highly virulent avian H5N2 virus in mice [Citation21]. MiR-130a-5p was also differently expressed in ectopic cells in response to the peritoneal fluid from endometriosis patients and was suggested to implicate in angiogenesis [Citation22]. In pulmonary hypertension, dysregulated miR-130a-5p appeared to have an effect on at least one intermediate of the following signaling pathways: nitric oxide, endothelin, prostacyclin, calcium channels, PDGF, protein kinase, MAP kinase, and oncogenes [Citation23]. Most recently, up-regulated miR-130a-5p was documented in human retinal pigment epithelium cells ARPE-19 against the oxidative stress induced by hydrogen peroxide [Citation24]. In our preliminary experiment, the screening of microRNA showed miR-130a-5p was abnormally expressed, whereas other microRNAs had no remarkable changes (Supplemental Figure 1). Besides these known abnormalities, our data indicated that the renal expression of miR-130a-5p was significantly decreased in MN patients. As a major active effector of the renin angiotensin system (RAS), Ang II was able to damage podocytes through a variety of mechanisms, and subsequent inducing cell apoptosis both in vivo and in vitro [Citation25]. A key finding of the current study revealed that down-regulated miR-130a-5p induced podocyte apoptosis, highlighting an essential role of miR-130a-5p in Ang II induced-podocyte apoptosis.
PLA2R, a member of the mannose receptor family, deposits along glomerular capillary loops in 69% of MN patients [Citation26]. PLA2R was able to undergo endocytosis, indicating that PLA2R might involve in the internalisation of extracellular ligands [Citation27]. Accumulating evidence suggested that PLA2R, as the target of autoimmunity in MN, mediated the event of podocyte apoptosis [Citation14]. In the present study, PLA2R was identified as the target of miR-130a-5p in podocytes. Indeed, enforced overexpression of PLA2R substantially attenuated the effect of miR-130a-5p on podocyte apoptosis, indicating miR-130a-5p preventing the Ang II induced-podocyte apoptosis by inhibiting PLA2R. These data illustrated a new mechanism for the Ang II induced-podocyte apoptosis in the progression of MN.
MiRNA has emerged as a key regulator in kidney diseases via modulating the expression of protein-coding genes. Differentially expressed miRNAs has been found to be commonly presented in human diseases, including MN [Citation28]. The circulating miRNAs associated with a specific pathophysiological state have been demonstrated as the useful diagnostic biomarkers for MN, such as miR-217 [Citation11]. In addition, recent studies reported that miR-186 was significantly down-regulated in renal tissue from MN patients that contributed to podocytes apoptosis [Citation12]. Because of the important functional role of miRNAs, the control of miRNAs has been thought of as therapeutic strategies for addressing MN progression [Citation29]. Our data showed that overexpressed miR-130a-5p by miR-130a-5p agomir could obviously alleviate renal injury in MN mice, suggesting that miR-130a-5p was expected to be the effective biological target for MN.
According to the bioinformatics software, there are also other microRNAs that targeting PLA2R, such as miR-149, miR-218, miR-135b, etc. These microRNAs are related with renal carcinoma or kidney disease, suggesting other miRNA/PLA2R pathways in the involvement of MN.
In summary, the present study suggested that following Ang II stimulation, miR-130a-5p was down-regulated and further increased PLA2R expression, which accelerated podocyte apoptosis. These findings illustrated the role of miR-130a-5p in podocyte injury and also provided mechanistic explanation of the involvement of miR-130a-5p in MN.
Supplemental Material
Download Zip (45 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- McCloskey O, Maxwell AP. Diagnosis and management of nephrotic syndrome. Practitioner. 2017;261(1801):11–15. Epub 2017/ 10/12.PubMed PMID: 29020719.
- Rozenberg I, Kotliroff A, Zahavi T, et al. Outcome of Idiopathic Membranous Nephropathy: A Retrospective Study. Isr Med Assoc J. 2018;20(3):186–189. Epub 2018/ 03/13.PubMed PMID: 29527859.
- Nangaku M, Shankland SJ, Couser WG. Cellular response to injury in membranous nephropathy. J Am Soc Nephrol. 2005;16(5):1195–1204. . Epub 2005/ 04/01. PubMed PMID: 15800119.
- Burlaka I, Nilsson LM, Scott L, et al. Prevention of apoptosis averts glomerular tubular disconnection and podocyte loss in proteinuric kidney disease. Kidney Int. 2016;90(1):135–148. . Epub 2016/05/25. PubMed PMID: 27217195.
- Zhang C, Hu JJ, Xia M, et al. Redox signaling via lipid raft clustering in homocysteine-induced injury of podocytes. Biochim Biophys Acta. 2010;1803(4):482–491. . Epub 2009/ 12/29. PubMed PMID: 20036696; PubMed Central PMCID: PMCPmc2840204.
- Perkinson DT, Baker PJ, Couser WG, et al. Membrane attack complex deposition in experimental glomerular injury. Am J Pathol. 1985;120(1):121–128. Epub 1985/07/01. PubMed PMID: 3160245; PubMed Central PMCID: PMCPmc1887956.
- Baker PJ, Ochi RF, Schulze M, et al. Depletion of C6 prevents development of proteinuria in experimental membranous nephropathy in rats. Am J Pathol. 1989;135(1):185–194. Epub 1989/07/01.PubMed PMID: 2672823; PubMed Central PMCID: PMCPmc1880216.
- Savin VJ, Johnson RJ, Couser WG. C5b-9 increases albumin permeability of isolated glomeruli in vitro. Kidney Int. 1994;46(2):382–387. Epub 1994/08/01.PubMed PMID: 7526024.
- Reinhart BJ, Slack FJ, Basson M, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403(6772):901–906. . Epub 2000/ 03/08. PubMed PMID: 10706289.
- Wu L, Fan J, Belasco JG. MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci U S A. 2006;103(11):4034–4039. . Epub 2006/02/24. PubMed PMID: 16495412; PubMed Central PMCID: PMCPmc1449641.
- Li J, Liu B, Xue H, et al. miR-217 Is a Useful Diagnostic Biomarker and Regulates Human Podocyte Cells Apoptosis via Targeting TNFSF11 in Membranous Nephropathy. Biomed Res Int. 2017;2017:2168767. . PubMed PMID: 29214160.
- Sha WG, Shen L, Zhou L, et al. Down-regulation of miR-186 contributes to podocytes apoptosis in membranous nephropathy. Biomed Pharmacothe. 2015;75:179–184. . Epub 2015/ 09/19. PubMed PMID: 26382839.
- Beck LH Jr., Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11–21. . Epub 2009/07/03. PubMed PMID: 19571279; PubMed Central PMCID: PMCPmc2762083.
- Pan Y, Wan J, Liu Y, et al. sPLA2 IB induces human podocyte apoptosis via the M-type phospholipase A2 receptor. Sci Rep. 2014;4:6660. . Epub 2014/10/23. PubMed PMID: 25335547; PubMed Central PMCID: PMCPmc4205892.
- Dai Q, Zhao J, Qi X, et al. MicroRNA profiling of rats with ochratoxin A nephrotoxicity. BMC Genomics. 2014;15:333. . Epub 2014/06/03. PubMed PMID: 24885635; PubMed Central PMCID: PMCPmc4035064.
- Muralidharan J, Ramezani A, Hubal MJ, et al. Extracellular microRNA signature in chronic kidney disease. Am J Physiol Renal Physiol. 2017;312(6):F982–F991.
- Qin W, Beck LH Jr., Zeng C, et al. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol. 2011;22(6):1137–1143. . Epub 2011/ 05/14. PubMed PMID: 21566055; PubMed Central PMCID: PMCPmc3103733.
- Yu L, Lin Q, Feng J, et al. Inhibition of nephrin activation by c-mip through Csk-Cbp-Fyn axis plays a critical role in Angiotensin II-induced podocyte damage. Cell Signal. 2013;25(3):581–588. . Epub 2012/ 12/04. PubMed PMID: 23200848.
- Wu CC, Huang YS, Chen JS, et al. Resveratrol ameliorates renal damage, increases expression of heme oxygenase-1, and has anti-complement, anti-oxidative, and anti-apoptotic effects in a murine model of membranous nephropathy. PloS one. 2015;10(5):e0125726. . Epub 2015/05/09. PubMed PMID: 25954969; PubMed Central PMCID: PMCPmc4425525.
- Huang YS, Lu KC, Chao TK, et al. Role of melatonin receptor 1A and pituitary homeobox-1 coexpression in protecting tubular epithelial cells in membranous nephropathy. J Pineal Res. 2018;e12482. DOI:10.1111/jpi.12482. PubMed PMID: 29480949.
- Choi EJ, Kim HB, Baek YH, et al. Differential microRNA expression following infection with a mouse-adapted, highly virulent avian H5N2 virus. BMC Microbiol. 2014;14:252. . Epub 2014/10/01. PubMed PMID: 25266911; PubMed Central PMCID: PMCPmc4189662.
- Braza-Boils A, Salloum-Asfar S, Mari-Alexandre J, et al. Peritoneal fluid modifies the microRNA expression profile in endometrial and endometriotic cells from women with endometriosis. Hum Reprod. 2015;30(10):2292–2302. . Epub 2015/ 08/27. PubMed PMID: 26307093.
- Rothman A, Restrepo H, Sarukhanov V, et al. Assessment of microRNA and gene dysregulation in pulmonary hypertension by endoarterial biopsy. Pulm Circ. 2017;7(2):455–464. . Epub 2017/ 06/10. PubMed PMID: 28597755; PubMed Central PMCID: PMCPmc5467936.
- Ayaz L, Dinc E. Evaluation of microRNA responses in ARPE-19 cells against the oxidative stress. Cutan Ocul Toxicol. 2018;37(2):121–126. . Epub 2017/07/15. PubMed PMID: 28707489.
- Schenk LK, Moller-Kerutt A, Klosowski R, et al. Angiotensin II regulates phosphorylation of actin-associated proteins in human podocytes. FASEB J. 2017;31(11):5019–5035. . Epub 2017/08/05. PubMed PMID: 28768720.
- Svobodova B, Honsova E, Ronco P, et al. Kidney biopsy is a sensitive tool for retrospective diagnosis of PLA2R-related membranous nephropathy. Nephrol Dial Transplant. 2013;28(7):1839–1844. . Epub 2012/12/12. PubMed PMID: 23223223.
- Jurgensen HJ, Johansson K, Madsen DH, et al. Complex determinants in specific members of the mannose receptor family govern collagen endocytosis. J Biol Chem. 2014;289(11):7935–7947. . Epub 2014/02/07. PubMed PMID: 24500714; PubMed Central PMCID: PMCPmc3953304.
- Chen W, Lin X, Huang J, et al. Integrated profiling of microRNA expression in membranous nephropathy using high-throughput sequencing technology. Int J Mol Med. 2014;33(1):25–34. . Epub 2013/ 11/14. PubMed PMID: 24220188; PubMed Central PMCID: PMCPmc3868500.
- Muller-Deile J, Dannenberg J, Schroder P, et al. Podocytes regulate the glomerular basement membrane protein nephronectin by means of miR-378a-3p in glomerular diseases. Kidney Int. 2017;92(4):836–849. . Epub 2017/05/10. PubMed PMID: 28476557; PubMed Central PMCID: PMCPmc5658661.
