ABSTRACT
Fungal keratitis is a relatively common ocular disease requiring positive medical management combined with surgical intervention. Interleukin-17 (IL-17) was reported to promote the activation and mobilization of neutrophile granulocyte to foci of inflammation. This study investigated the effect of IL-17 production from Th17 cells on the progression of fungal keratitis. A mouse model of fungal keratitis induced by Candida albicans was successfully constructed to detect infiltration of inflammatory cells in corneal tissues by hematoxylin-eosin (HE) staining and immunohistochemistry. Fungal load capacity of mouse cornea was also detected. The regulatory role of IL-17 in fungal keratitis with the involvement of CX43 was investigated with the relevant expression of inflammatory factors detected and activation of vascular endothelial cells assessed. Furthermore, in vivo experiment was also performed to confirm the role of CX43 in keratitis. Mice with fungal keratitis showed increased level of inflammatory cytokines and infiltration of inflammatory cells. Silencing IL-17 in Th17 cells and overexpressing CX43 could inhibit the activation of vascular endothelial cells. Besides, CX43 knockdown in vivo alleviated fungal keratitis in mice. The possible mechanism of the above findings could be IL-17 inhibiting the level of CX43 through the AKT signaling pathway. Taken together, IL-17 could inhibit the occurrence and development of fungal keratitis by suppressing CX43 expression through the AKT signaling pathway. Therefore, this study provides a potential target for the treatment of fungal keratitis.
Introduction
Fungal keratitis, a common eye disease, can result in ocular morbidity and blindness. With an increased incidence in the past 30 years, this disease is featured by pain and redness in the eye, blurred vision, sensitivity to light as well as excessive tearing or discharge [1]. Fungi infection is the major reason for fungal keratitis including yeast-like fungi as Candida albicans and filamentous fungi like Aspergillus fumigatus [Citation2]. There are some major factors leading to fungal keratitis, involving widespread application of broad-spectrum antibiotics and steroids, frequent use of contact lens, trauma to the eye, seasonal variation ocular surface disease and underlying diseases that compromise the immune defense of the host [Citation3]. Fungal keratitis may lead to great morbidity, particularly in tropical climates, and it is challenging to treat this disease [Citation4]. Commonly, aggressive medical treatment combined with surgical intervention is required for fungal keratitis to obtain better outcome [Citation5]. However, when the infection is combined with bacterial or acanthamoeba co-infections, the diagnosis and management for fungal keratitis become more difficult [Citation6]. Therefore, it is very necessary to identify an effective therapeutic target for fungal keratitis and to figure out the specific mechanism.
Interleukin (IL)-17 plays a critical role in connecting the communication between immune cells and tissues, and it also promotes the activation and mobilization of neutrophils to inflammation sites [Citation7]. IL-17 mediated immunity is reported to play a significant part in mucocutaneous protection against Candida albicans in both mice and humans [Citation8]. IL-17a induced by acanthamoeba infection plays an important role in host protection against invading parasites [Citation9]. IL-17, as a member of the pro-inflammatory cytokine, is produced by diverse cell types such as neutrophils, and it can facilitate asthma, uveitis, as well as chronic autoimmune conditions like rheumatoid arthritis, inflammatory bowel disease and so on [Citation10]. Moreover, connexins are a family of proteins with expression in a cell-specific manner, of which connexin43 (CX43) has major expression in mammalian cells [Citation11]. CX43 is found to be expressed in mammalian cornea and its expression is closely related to the inflammatory responses of injured cornea [Citation12]. At the same time, in primary mixed glial cell culture, IFN-γ or IL-17 at high concentrations reduced the protein level of CX43, and Th1 cell-conditioned medium decreased the protein level of CX43 in mixed glial cell cultures, Th1 cell-derived IFN-γ activated microglia to release IL-1β that downregulates the gap junction of CX43 in astrocytes [Citation13]. Besides, AKT, also known as protein kinase B, serves as a serine threonine kinase which is significant for various biological processes including cell survival, proliferation, migration, and metabolism; it functions as a downstream of phosphatidylinositol 3-kinase (PI3K) activated by multiple growth factors and cytokines [Citation14]. On the basis of the above findings, we speculate that IL-17 and CX43 may play a pivotal role in fungal keratitis by regulating the AKT signaling pathway. Therefore, the present study was conducted to identify the role of IL-17 in fungal keratitis through the AKT signaling pathway to regulate CX43 expression and the associated regulatory network.
Materials and methods
Ethics statement
Animals were handled according to the “ARVO statement of animals in ophthalmology and vision studies” and the study protocol was approved by the animal care and use committee of the First Affiliated Hospital of Dalian Medical University.
Establishment of fungal keratitis model
Fungal strains: Candida albicans, numbered MYA-2876, were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Candida albicans were inoculated into Sabouroud medium and cultured in an incubator at 28°C for 5 days. Candida albicans were prepared into the fungal spore suspension by aseptic phosphate buffer saline (PBS).
A total of 80 adult female Balb/c mice (Beijing Institute of Pharmacology, Chinese Academy of Medical Sciences, Beijing, China) (aged 6 ~ 8 weeks) were used to establish fungal keratitis models. The mice were fed at specific pathogen-free (SPF) level animal laboratory with the humidity of 60% ~ 65% and the temperature of 22 ~ 25 °C. According to a previous study, 24 mice were randomly selected to establish a fungal keratitis mouse model [Citation15]. Mice were assigned into 2 groups (12 for each group): Candida albicans group (transfected with Candida albicans) and saline group (treated with sterile PBS). In short, when general anesthesia was performed at the Balb/c mice, their corneas were superficially scarified [Citation16]. The scarified cornea was treated with a 5 μL inoculum of each Candida albicans yeast strain with a total of 106 colony forming unit (CFU) (the Candida albicans group) or 5 μL of sterile PBS (as negative control, that is, saline group). Another 48 mice were selected to knock down CX43 expression in vivo. In the feeding process, mice were injected with shRNA-CX43 adenovirus vector (1 × 109 pfu/100 μL) or adenovirus empty vector through subconjunctival injection for 6 weeks, 2 times every week. After injection of adenovirus vector, the above methods were used to establish fungal keratitis mouse models. The mice were specifically classified into 4 groups (12 for each group): a WT group (subconjunctival injection of adenovirus empty vector and transfected with sterile saline), a CX43 KD group (subconjunctival injection of shRNA-CX43 adenovirus vector and transfected with sterile saline), a WT + Candida albicans group (subconjunctival injection of adenovirus empty vector and transfected with Candida albicans), a CX43 KD + Candida albicans group (subconjunctival injection of shRNA-CX43 adenovirus vector and transfected with Candida albicans).
Mice were monitored for 8 days and the severity of the disease was evaluated with the help of an anatomical microscope [Citation16,Citation17]. The total scoring of each mouse was calculated on the basis of 3 criteria (opacity area, opacity density, and surface regularity) (each criterion for 0–4 points). The total score under 5 points was deemed as mild oculopathy, 5 to 9 points as moderate oculopathy, and 9 to 12 points as severe oculopathy. The results of scoring were analyzed by one-way analysis of variance (ANOVA). Results of modeling displayed that two mice died in the Candida albicans group. No death was found in the control group.
Isolation and collection of Th17 cells and corneal limbus vascular endothelial cells
The CD4 immunomagnetic beads positive selection method (Miltenyi Biltec company, Germany) was used to isolate and purify CD4+ T cells in spleen of 2 mice in the Candida albicans group. Methods referred to instructions and were improved according to specific experimental conditions. After collection of CD4+ T cells, a flow cytometer was used to identify cell purity (> 90%). CD4+ T cells were cultured in a 6-well plate with the density of 5 × 106 cells/well. Each well was added with CD3/CD 28 T Cell Expander (Invitrogen Inc., Carlsbad, CA, USA) r, 5 ng/mL transforming growth factor-β1 (TGF-β1), 10 ng/mL interleukin-6 (IL-6), 10 μg/mL FNY-γ monoclonal antibody and 10 μg/mL IL-4 monoclonal antibody to be cultured for 6 days. After that, Th17 cells were obtained [Citation18].
Mice in the Candida albicans group were used to isolate vascular endothelial cells from the surrounding tissues of infected cornea. The specific methods were similar to the isolation of rat vascular endothelial cells in “Gene expression profiles of ATP-binding cassette transporter A and C subfamilies in mouse retinal vascular endothelial cells” [Citation19].
Hematoxylin-eosin (HE) staining
The mouse corneal tissues were used to prepare paraffin blocks. The samples were continuously cut into slices with the thickness of 5 µm. And then, the slices were dewaxed and hydrated by xylene I for 10 min, xylene II for 10 min, mixture of anhydrous ethanol and xylene at the ratio of 1: 1 for 1 min, and ethanol for 10 min. Hematoxylin was used to stain cells for nuclei staining for 10 min, followed by a large quantity of running water washing. Next, the slices were rinsed with 0.25% hydrochloric acid ethanol for 3 s, followed by washing. After that, the slices were dehydrated with 95% ethanol for 1 min, stained with 0.5% eosin ethanol for cytoplasm staining for 1 min, and rinsed with 3% ethanol for 30 s. Finally, the slices were dehydrated with ethanol for 20 min, and treated with mixture of anhydrous ethanol and xylene at the ratio of 1: 1 for 1 min, xylene I and II, each for 5 min. Following that, the slices were sealed with neutral balsam. After being naturally dried, the slices were observed under a light microscope.
Immunohistochemistry
The paraffin sections of the mouse corneas were selected, and routinely dewaxed and hydrated in a 60°C oven for 2 h. And then the antigen was repaired under the high pressure. The slices were put into wet boxes and incubated with F4/80 (ab111101, 1: 100, Abcam Inc., Cambridge, MA, USA), Ly6g (ab25377, 1: 100, Abcam Inc., Cambridge, MA, USA) and RORγT (ab60134, 1: 250, Abcam Inc., Cambridge, MA, USA) overnight at 4°C. The next day, the slices were placed at room temperature for 30 min. And then the slices were added with secondary goat anti mouse antibody to IgG H&L fluorescein isothiocyanate (FITC) (ab6785, 1: 1000, Abcam Inc., MA, USA) and incubated at 37°C for 20 min, followed by adding diaminobenzidine (DAB) solution, 50 μL for each slice, under the condition void of light. Developing was conducted under the control of a microscope, followed by rinsing cells with under tap water. The slices were counter-stained with hematoxylin for 1 min, followed by returning to blur under tap water. And then the slices were dried by gradient alcohol, cleaned with xylene, and sealed with neutral balsam. PBS solution was used as negative control in replacement of the primary antibody. In all the experimental processes, PBS buffer was used for washing. Results were identified by the double blind method. Four fields of view were taken randomly at high magnification and analyzed by the Image-pro plus 5 image analysis system. The data were calculated based on the percentage of the positive cells.
Enzyme linked immunosorbent assay (ELISA)
ELISA kits for IL-17, IL-12, tumor necrosis factor α (TNF-α) and IL-6 were purchased from R&D Systems (Minneapolis, MN, USA) and operated according to the company instructions.
Cell culture, grouping and transfection
Th17 cell [Citation20] and vascular endothelial cells were added with the Roswell park memorial institute (RPMI) 1640 medium (Gibco Company, Grand Island, N.Y., USA) containing 10% fetal bovine serum (FBS), 10 μg/mL streptomycin and 100 U/mL penicillin and cultured in an incubator at 37°C with 5% CO2 (Thermo Fisher Scientific, Carlsbad, CA, USA). The cells in the logarithmic phase were treated with trypsin, and inoculated to a 6-well plate at the density of 1 × 105 per well. After conventional culture for 24 h, the cell confluence was about 75%, and the transfection was carried out according to the instructions of Lipofectamine 2000. Th17 cells were classified into a siRNA-NC group (Th17 cells transfected with siRNA-NC sequences), and a siRNA- IL-17 group (Th17 cells transfected with siRNA-IL-17 sequence). The vascular endothelial cells were assigned into the following groups: a blank group (cells without any treatment), a NC group (cells transfected with siRNA-NC sequence), a CX43 group (cells transfected with pcDNA3-CX43 plasmid), an IL-17 (1 μM) group (treated with 1 μM IL-17 recombination protein for 24 h), an IL-17 (5 μM) group (treated with 5 μM IL-17 recombination protein for 24 h), an IL-17 + CX43 (transfected with pcDNA3-CX43 plasmid and treated with 5 μM IL-17 recombination protein for 24 h), a MK-2206 2HCl group [treated with 5 μM MK-2206 2HCl (AKT inhibitors) for 24 h], and an IL-17 + MK-2206 2HCl group (simultaneously treated with 5 μm IL-17 recombination protein and 5 μm MK-2206 2HCl for 24 h). SiRNA nonsense sequence, siRNA-CX43 sequence and vector were purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China). IL-17 cytokine was purchased from PeproTech Inc. (Rocky Hill, NJ, USA). AKT inhibitors (MK-2206 2HCl) were purchased from Selleck Chemicals (Houston, TX, USA).
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
The Trizol reagent (Invitrogen Inc., Carlsbad, CA, USA) was used to extract the total RNA in each group, and then the total RNA was reversely transcribed to cDNA by the Takala Sensiscript RT Kit (Invitrogen Inc., Carlsbad, CA, USA). The specific experimental procedures were conducted according to the instructions. SYBR Green Mix fluorochrome (Hoffmann-La Roche Ltd., Basel, Switzerland) method was used for RT-qPCR. The reaction system of each gene was 20 μL, including 10 μL of 2 × SYBR Green, 0.3 μL of forward primers (20 μM), 0.3 μL of reverse primers (20 μM), 1.0 μL of cDNA and 8.4 μL of ddH2O. The reaction processes were: denaturalization at 95 °C for 5 min, naturalization at 95°C for 30 s and annealing at 60 °C for 1 min, a total of 40 cycles. All primers were synthesized by Shanghai Sangon Biotechnology Co., Ltd. (Shanghai, China), as shown in . β-actin was used as the internal reference. The 2−ΔΔCt method was applied to calculate the relative expression of CX43, Dectin-1 receptor, IL-17, IL-12, TNF-α and IL-6 in each group.
Table 1. Primer sequences.
Western blot analysis
The cultured cells in each group were treated with trypsin and lysed with enhanced radio immunoprecipitation assay (RIPA) lysate containing protease inhibitors (Boster Biological Technology, Co., Ltd., Wuhan, Hubei, China). And then bicinchoninic acid (BCA) protein quantitative Kit (Boster Biological Technology, Co., Ltd., Wuhan, Hubei, China) was used to determine the protein concentration. The protein was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto polyvinylidene fluoride (PVDF) membranes. After being mounted with 5% bovine serum albumin (BSA) for 2 h, the membranes were incubated with diluted VCAM-1 (ab181315, 1: 100), E-selectin (CTB202, 1: 1000), p-PI3K (ab133458, 1: 1000), PI3K (ab151549, 1: 1000), AKT (ab8805, 1: 500), p-AKT (ab133458, 1: 1000), COL-IV (ab19808, 1: 1000) and β-actin (ab8227, 1: 1000) at 4°C overnight. All antibodies were purchased from Abcam Inc. (Cambridge, MA, USA) except for E-selectin (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). After being washed 3 times with phosphate buffered saline with tween-20 (PBST), the membranes were incubated with horseradish peroxidase (HRP)-labeled secondary goat anti-rabbit antibody (ab205719, 1: 2000, Abcam Inc., Cambridge, MA, USA) at room temperature for 1 h. After being washed 3 times with PBST, the membranes were colored with electroluminescence (ECL) luminescent solution (EMD Millipore Corp., Belgium, MA, USA). The Image J analysis software was used to determine gray values of the bands in Western blot images with β-actin as the internal reference.
Co-culture of Th17 cells and vascular endothelial cells
Co-culture of Th17 cells/vascular endothelial cells was carried out in Transwell chambers. Th17 cells (WT) or Th17 (KO) cells were inoculated and cultured in the apical chamber of the 6-well Transwell chambers at the density of 50,000 cells/well. The vascular endothelial cells were inoculated and cultured in the basolateral chamber of the 6-well Transwell chamber with the density of 105 cells/well. After cell adherence, TGF-β was added to induce Th17 cells to express IL-17. After 48 h of culturing, vascular endothelial cells were collected for subsequent experiments.
Immunofluorescence
The vascular endothelial cells were collected and cooled down in an ice bath. Then the cells were centrifuged in a desk centrifuge at 4°C at 800 g for 5 min. The culture solution was sucked out and the cells were suspended with PBS at 4°C. PBS was centrifugally removed and the cells were suspended with 1 ~ 2 mL of 2% paraformaldehyde (PFA) fixation solution/0.1% Triton X-100, and fixed on ice for 30 min. After the fixation solution was removed through centrifugation, the cells were suspended with 15 mL of PBS at 4°C for 5 min, followed by PBS rinsing. The cells were incubated with CX43 antibody (ab11370, 1: 250, Abcam Inc., Cambridge, MA, USA) at 37°C overnight, followed by two PBS rinsing (3 min each time). And then FITC-labeled IgG (ab6717, 1: 1000, Abcam Inc., Cambridge, MA, USA) was added and incubated for 1 h, followed by two PBS washing (3 min each time). Then the slices were sealed with anti-fluorescence quenching sealing solution and observed under a microscope. The experiments were repeated 3 times.
Fungal load capacity measurement
According to the method in “Topical Flagellin-Mediated Innate Defense against Candida albicans Keratitis” [Citation21], the fungal load capacity in mouse cornea was measured. In short, 5 mice in each group were selected and the infected corneas was removed at the first and third days. And then the infected corneas were minced and homogenized in 100 μL of PBS with a tissue grinder (Micro Dounce; Bellco Glass Corp., Vineland, NJ, USA). The homogenates were used to count fungal colonies. Serially diluted aliquots (100 μL) were plated onto Yeast Extract Peptone Dextrose (YPD) agar plates in triplicate. The plates were incubated for 3 days at 25°C, and the fungal colonies were counted.
Statistics analysis
All data were processed with the SPSS 21.0 statistical software (IBM Corp. Armonk, N.Y., USA). All data were performed the normal distribution test and homogeneity test of variance. Measurement data accorded with normal distribution were expressed as mean ± standard deviation, and those not in lines with normal distribution or homogeneity test of variance were presented as interquartile range. The comparisons between two groups were analyzed by unpaired t-test. When the data was inequality of variance, Welch’s test was performed. Comparisons among multiple groups were analyzed by one-way analysis of variance (ANOVA) and tested by Tukey’s post-hoc test. All of the non-parametric test was analyzed by using rank sum test. A p < 0.05 value was considered to be statistically significant.
Results
A mouse model of fungal keratitis is successfully established
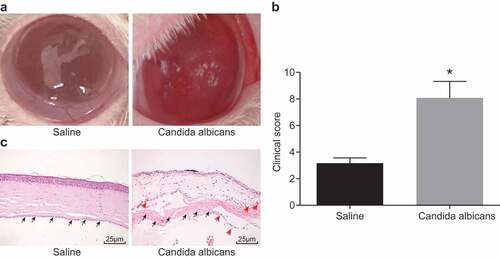
The cornea changes in mice were observed. The results showed that at the 7th day of infection of Candida albicans, the mice in Candida albicans group had conjunctival congestion, erosive necrosis of the cornea, and ulcerative focus. However, in saline group, the mice basically recovered to normal at the 7th day (). According to the clinical scoring criteria, the clinical scores at the 7th day in saline group and Candida albicans group were evaluated. Scoring results displayed that at the 7th day, Candida albicans group had significantly higher scores than saline group (p < 0.05) (). In addition, HE staining was used to detect the degree of corneal injury. The results showed that Candida albicans group appeared more serious corneal injury and more cell infiltration (as the arrow indicates) (). The above results suggested that we obtained a successful mouse model of fungal keratitis.
Figure 1. A successful mouse model of fungal keratitis is established. (a), Comparison of corneal state after the 7th day of Candida albicans infection in mice; (b), Clinical scoring in the Candida albicans group and the saline group; (c), The HE staining of the corneal tissue in mice at the 7th day after modeling with black arrows indicating corneal stromal fibers and red arrows indicating inflammatory cells (× 400), *, p < 0.05; HE, hematoxylin-eosin; n = 12 in the saline group and n = 10 in the Candida albicans group; all data were measurement data, expressed as mean ± standard deviation and analyzed by t-test; the experiment was repeated three times.
Mice with fungal keratitis present with increased inflammatory cytokine secretion and inflammatory cell infiltration
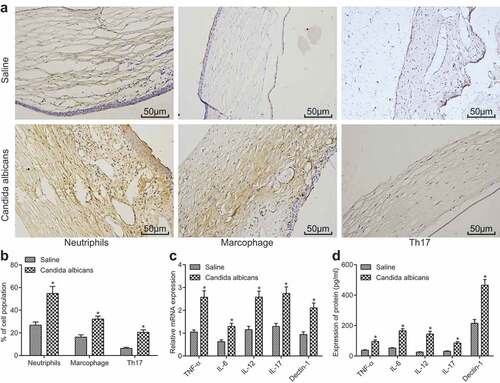
The increase of inflammatory cytokine secretion and inflammatory cell infiltration suggested that the occurrence of keratitis affected the inflammation environment of cornea. We determined the levels of the inflammatory factors in the corneal tissue of the mice with keratitis at the 7th day by RT-qPCR. In addition, the macrophages, neutrophile granulocytes and Th17 were marked by immunohistochemistry to observe the immune cell infiltration in the process of keratitis development. The results displayed that the mice in the Candida albicans group had remarkably higher infiltration of macrophages and neutrophile granulocytes than the saline group (), and obviously higher Th17 level than control group. At the same time, compared with the saline group, the levels of inflammatory factors IL-12, Il-17, TNF-α, IL-6 and Dectin-1 in corneal tissue increased significantly in the Candida albicans group (p < 0.05) (). The above results showed that elevated level of inflammatory factors and infiltration of inflammatory cells were found in mice with fungal keratitis.
Figure 2. Mice with fungal keratitis present with increased inflammatory cytokine secretion and inflammatory cell infiltration. (a), The images of macrophages (F4/80 positive), neutrophile granulocytes (Ly6g positive) and Th17 (RORγ positive) in the Candida albicans group and the saline group (× 200); (b), The histogram of the positive cell rate of three different cell types; (c), The histogram of relative mRNA levels of different cytokines in each group; (d), The histogram of protein levels of different cytokines in each group; TNF, tumor necrosis factor; IL, interleukin; *, p < 0.05 vs. saline group; n = 12 in the saline group and n = 10 in the Candida albicans group; all data were measurement data, expressed as mean ± standard deviation and analyzed by t-test; the experiment was repeated for three times.
Silencing IL-17 in Th17 cells activates vascular endothelial cells
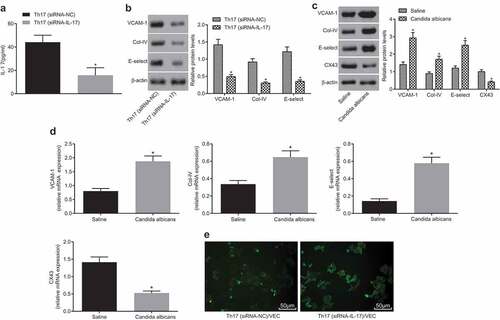
IL-17 is a well-known pro-inflammatory factor, mainly secreted by TH17 cells. Studies have shown that the activation of vascular endothelium can lead to a large number of inflammatory cell exudates and participates in inflammatory response. In order to investigate whether the expression of IL-17 in Th17 cells was involved in the activation of vascular endothelial cells, we interfered the expression of IL-17 in Th17 cells through the use of siRNA-IL-17, and co-cultured Th17 cells with vascular endothelial cells to demonstrate the relationship between IL-17 and vascular endothelial cells. The results displayed that IL-17 small interference RNA could effectively interfere the expression of IL-17 in Th17 cells (); the normal Th17 cells and Th17 cells with IL-17 gene silencing were co cultured with vascular endothelial cells respectively. It was observed that the vascular endothelial cells in the IL-17 gene silencing group were not activated. On the contrary, in the normal Th17 cell group, the vascular endothelial cells were significantly activated, and the endothelial activation related genes such as E-selectin, VCAM-1, and Col-IV were all highly expressed (). The above data showed that inflammation factor IL-17 secreted by Th17 cells could significantly activate vascular endothelial cells. As shown, the mRNA and protein levels of E-selectin, VCAM-1, and COL-IV were higher and CX43 mRNA and protein level was lower in vascular endothelial cells of the Candida albicans group than the saline group. Cell co-culture experiment showed that after co-culture of normal Th17 cells and vascular endothelial cells, which was observed by a fluorescence microscope, the level of CX43 in vascular endothelial cells was significantly lower than that in co-culture of IL-17 gene silencing Th17 cells and vascular endothelial cells (). The above results showed that vascular endothelial cells could activate vascular endothelial cells under the stimulation of IL-17, while inhibited CX43 level.
Figure 3. IL-17 gene silencing in Th17 cells inhibits activation of vascular endothelial cells. (a), The histogram of IL-17 level in Th17 (siRNA-NC) and Th17 (siRNA-IL-17) groups; (b), Relative mRNA levels of VCAM-1, E-selectin and Col-IV in Th17 (siRNA-NC) and Th17 (siRNA-IL-17) groups; (c), Relative mRNA levels of VCAM-1, E-selectin, Col-IV and CX43 in the Candida albicans and saline groups; (d), The histogram of mRNA levels of CX43 in different conditions; (e), The images of CX43 expression detected by immunofluorescence (× 200); IL, interleukin; Col-IV, Collagen 4; VCAM-1, vascular cell adhesion molecule 1; CX43, connexin43; *, p < 0.05; n = 12 in the saline group and n = 10 in the Candida albicans group; all data were measurement data, expressed as mean ± standard deviation and analyzed by t-test; the experiment was repeated for three times.
Overexpression of CX43 inhibits the activation of vascular endothelial cells
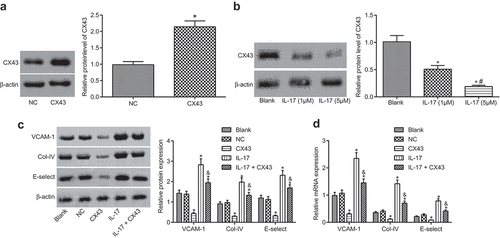
In the previous experiment, we found that IL-17 could inhibit the expression of CX43. In order to further investigate the role of IL-17 in fungal keratitis through CX43, we conducted a related study. IL-17 was applied as an in vitro stimulator to facilitate vascular endothelial cells. The adhesion factors and collagen expression were measured to determine whether endothelial cells were activated. The results showed that the level of CX43 in CX43 group was significantly higher than that in the control group (). IL-17 group could significantly reduce the level of CX43, and the level of CX43 gradually decreased in sycn with the increase of IL-17 concentration (). The IL-17 group could significantly activate vascular endothelial cells to induce high levels of E-selectin, VCAM-1, and COL-IV. After overexpressing CX43 in vascular endothelial cells, the activation of vascular endothelial cells through IL-17 remarkably reduced (). These results suggested that IL-17 regulated the activation of vascular endothelial cells through the level of CX43.
Figure 4. The activation of vascular endothelial cells is inhibited by overexpressing CX43. (a), Relative protein level of CX43 in NC and CX43 groups; *, p < 0.05 vs. the NC group; (b), Relative protein level of CX43 in saline group, IL-17 (1 μM) and IL-17 (5 μM); *, p < 0.05 vs. the blank group; #, p < 0.05 vs. IL-17 (1 μM); (c), Relative protein levels of VCAM-1, E-selectin and Col-IV in each group; (d), Relative mRNA levels of VCAM-1, E-selectin and Col-IV in each group; (c) and (d), * p < 0.05 vs. the blank group; &, p < 0.05 vs. the IL-17 group; NC, negative control; IL, interleukin; CX43, connexin43; all data were measurement data, expressed as mean ± standard deviation; data in panel A were analyzed by t-test, and the other data were tested by one-way analysis of variance; the experiment was repeated for three times.
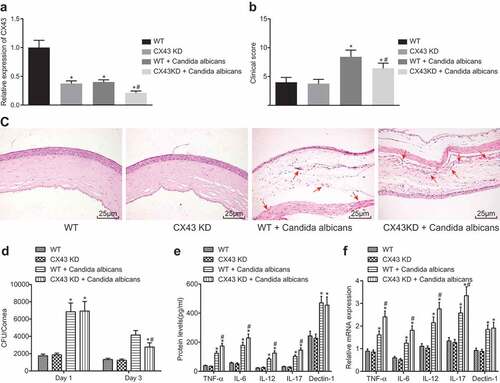
CX43 knockdown in vivo alleviates fungal keratitis in mice
The above results demonstrated that IL-17 inhibited the development of fungal keratitis by inhibiting CX43 expression in vascular endothelial cells. Therefore, the mechanism of CX43 in vivo was explored by figuring out whether the fungal keratitis in mice was controlled after knockdown of CX43 gene in mice. The results showed that after subconjunctival injection of sh-CX43 adenovirus, the expression of CX43 in corneal vascular endothelial cells decreased significantly (). At the 7th day after model establishment, the clinical score of mice with CX43 knockdown was lower, indicating that the severity of fungal keratitis reduced after CX43 knocking down (). Similarly, we observed the corneal tissue morphology and inflammatory cell infiltration by HE staining. It was found that after CX43 knockdown, the corneal morphology of mice was more complete and inflammatory cell infiltration (as the arrow indicates) increased compared with the model group (). The Fungal load of mice with infected cornea in each group was detected. It was found that compared with the normal mice, Fungal load in the CX43 knockdown mice decreased significantly (). RT-qPCR was used to detect the mRNA levels of IL-12, IL-17, TNF-α, IL-6 and Dectin-1 in CX43 knockdown mice, fungal keratitis mice and normal mice. Similarly, ELISA detected the protein levels of these corresponding indicators. Immunohistochemistry was used to detect the infiltration of inflammatory cells in fungal keratitis tissues and corneal tissues of CX43 knockdown mice. The above results showed that the mRNA levels of IL-12, IL-17, TNF-α, IL-6 and Dectin-1 in the corneal tissue of model mice increased significantly. There was no significant difference in the levels of these factors between CX43 knockdown mice and model mice (). The above results showed that CX43 knockdown could lead to activation of vascular endothelial cells, increase of inflammatory cells and inflammatory factors in the corneal tissues, decrease of fungi in corneal tissues, and reduction of the corneal injury.
Figure 5. The symptoms of fungal keratitis are alleviated after CX43 knockdown. (a), The expression of CX43 in mouse vascular endothelial cells determined by RT-qPCR; (b), Comparison of clinical scoring in each group; (c), Mice morphological characteristics of corneal tissues in three groups (× 400) with black arrows indicating corneal stromal fibers and red arrows indicating inflammatory cells; (d), Fungal load capacity in infected cornea of each group; (e), Relative mRNA level of each gene in corneal tissues of each group; (f), Protein levels of each gene in corneal tissues of each group; *, p < 0.05 vs. the WT group; #, p < 0.05 vs. the WT + Candida albicans group; HE, hematoxylin-eosin; TNF, tumor necrosis factor; CX43, connexin43; IL, interleukin; n = 12; all data were measurement data, expressed as mean ± standard deviation and analyzed by one-way analysis of variance; the experiment was repeated for three times.
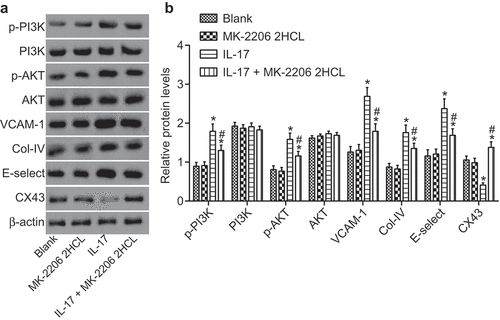
IL-17 inhibits the level of CX43 through the AKT signaling pathway
In this study, we assessed the expression of CX43 and the use of inhibitors of related signaling pathways to find out the underlying mechanism in the process of IL-17 regulating CX43. The results showed that the extent of AKT and PI3K phosphorylation in IL-17 treatment group increased significantly, and the level of CX43 reduced significantly. After the application of MK-22062HCL (AKT inhibitor), IL-17 did not reduce the level of CX43 in vascular endothelial cells. At the same time, compared with the IL-17 group, the levels of vascular endothelial activation related factors E-selectin, VCAM-1, and COL-IV in the IL-17 + MK-22062HCL group declined significantly (). These results indicated that IL-17 inhibited the expression of CX43 through the AKT signaling pathway, thereby regulating the activation of vascular endothelial cells.
Figure 6. IL-17 regulates endothelial cell activation through the AKT signaling pathway. (a), The gray values of PI3K, p-PI3K, p-AKT, AKT, E-selectin, VCAM-1, COL-IV and CX43 protein bands; (b): The histogram of relative protein levels of PI3K, AKT, E-selectin, VCAM-1, COL-IV and CX43 as well as the extent of AKT and PI3K phosphorylation; *, p < 0.05 vs. the blank group; #, p < 0.05 vs. the IL-17 group; IL, interleukin; Col-IV, Collagen 4; VCAM-1, vascular cell adhesion molecule 1; AKT, serine/threonine kinase; all data were measurement data, expressed as mean ± standard deviation and analyzed by one-way analysis of variance; the experiment was repeated for three times.
Discussion
This study investigated how IL-17 in Th17 cell inhibited the development of fungal keratitis by suppressing CX43 expression in corneal endothelial cells. It was found that IL-17 and CX43 knockdown could promote the activation of vascular endothelial cells and alleviate the symptoms of fungal keratitis in mice. In short, IL-17 inhibited the level of CX43 through the AKT signaling pathway to suppress the occurrence and progression of fungal keratitis.
Initially, one of our major findings was that vascular endothelial cells could be activated and endothelial activation related molecules including E-selectin, VCAM-1 and Col-IV were highly expressed through IL-17. A recent study found that E-selectin promotes adhesion and migration of endothelial-colony forming cells after endotoxic endothelial injury and it can also repair endothelial injury [Citation22]. VCAM-1, also known as an important adhesion molecule, induced leukocytes to adhere to endothelial cells and its expression could be suppressed by miR-126 to inhibit endothelial cell inflammatory response [Citation23]. A recent evidence showed that cell adhesion molecules such as E-selectin and VCAM1 increased on the surface of endothelial cells in such inflammatory diseases as atherosclerosis and cerebral aneurysms [Citation24]. Col-IV, a major component of basement membrane, was a crucial factor for structural support to form a fibrillary network of basal lamina, as well as for regulating adhesion, proliferation and migration of endothelial cells [Citation25]. Interestingly, endothelial cell density was found to downregulate in herpes simplex keratitis and was closely associated with corneal nerve density, indicating an underlying correlation between corneal innervation and endothelial cell homeostasis [Citation26]. Another study reported that the inflammatory response in diffuse lamellar keratitis may be related to endothelial cell loss [Citation27]. IL-17 was reported to upregulate levels of adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1), VCAM-1 and E-selectin in endothelial cells [Citation28]. IL-17 was produced by peripheral blood neutrophils and markedly increased in corneal ulcers in fungal keratitis where neutrophils make up the majority of infiltrating cells [Citation29]. Besides, our study also found that CX43 knockdown could abate symptoms of fungal keratitis by activating vascular endothelial cells, increasing inflammatory factors in corneal tissues, reducing fungi in corneal tissues and corneal injury. A recent study indicated that the alpha-carboxy terminus 1 (αCT1) peptide, as a 25 amino acid peptide from the C-terminus of CX43, could promote wound healing, and the downregulation of CX43 and ZO-1 were related to the healing process of corneal injury, which was consistent with the role of CX43 in fungal keratitis in our study [30].
Additionally, our results also demonstrated that IL-17 inhibited CX43 expression through the AKT signaling pathway, thereby inhibiting fungal keratitis which was featured by elevated level of inflammatory cytokines including IL-12, Il-17, TNF-α, IL-6 and Dectin-1 as well as increased infiltration of inflammatory cells. Data showed that inflammatory cells like neutrophils, monocytes, macrophages, as well as lymphocytes played significant roles in resisting fungal pathogens in fungal keratitis [Citation31]. IL-1β, a pro-inflammatory cytokine, was important for resisting fungal infection in inflammatory response and Dectin-1, as a pattern recognition receptor, also played a pivotal role in this process [Citation32]. IL-6 was related to the same pro-inflammatory pathway just like IL-1β and it was expressed by corneal epithelial cells as well as keratocytes; IL-12, produced by macrophages and dendritic cells, was also correlated with the early stage of inflammation and it could induce Th1 response to increase inflammation [Citation33]. TNF-α was another pro-inflammatory cytokine to enhance corneal inflammation and it could inhibit CX43 expression and gap-junctional intercellular communication in corneal fibroblasts through the c-Jun NH2-terminal kinase (JNK) signaling pathway, indicating its important role in corneal inflammation [Citation11]. In addition, it has been found that the inhibition of CX43 can promote the healing of superficial epithelial injury and may promote the migration and accumulation of inflammatory cells in the tissues for deep trauma without inhibiting the subsequent angiogenesis [30,Citation34]. Furthermore, evidence showed that Th17 cells were crucial to regulate immune responses, promote chronic inflammation and autoimmunity, as well as resist fungal infections which mostly relied on the pro-inflammatory cytokine IL-17 [Citation35]. IL-17 has inhibitory effect on Candida albicans keratitis, then activate T cell immunity, produce inflammatory infiltration, and promote the healing of keratitis [Citation36]. In addition, Akt could be activated by PI3Ks which were a family of lipid kinases and the activation of the PI3K/Akt signaling pathway could inhibit the pro-inflammatory response by negatively regulating toll-like receptor (TLR) and also played a part in regulating the activities of macrophages [Citation37].
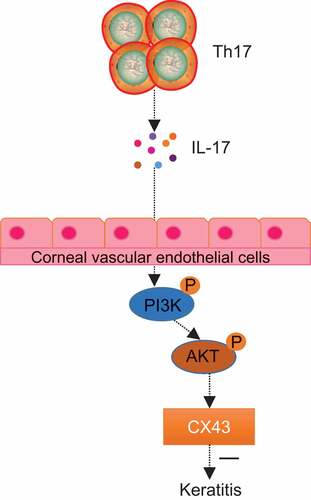
In summary, our results showed that the development of fungal keratitis could be inhibited by IL-17, which suppressed the level of CX43 by regulating the AKT signaling pathway. Therefore, IL-17 could serve as a potential target for the treatment of fungal keratitis (). However, further studies are needed to fully understand the specific mechanisms of IL-17 by regulating CX43 in the management of fungal keratitis.
Figure 7. IL-17 inhibited fungal keratitis by reducing the level of CX43 through the AKT signaling pathway. In fungal keratitis, IL-17 secreted by Th17 cells could inhibit the expression of CX43 in the limbal vascular endothelial cells through the AKT signaling pathway, thus inhibiting the development of fungal keratitis.
Competing Interests
The authors declare that they have no competing interests.
Acknowledgments
We would like show sincere appreciation to the reviewers for critical comments on this article
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Wu H, Ong ZY, Liu S, et al. Synthetic beta-sheet forming peptide amphiphiles for treatment of fungal keratitis. Biomaterials. 2015;43:44–49.
- Liu L, Wu H, Riduan SN, et al. Short imidazolium chains effectively clear fungal biofilm in keratitis treatment. Biomaterials. 2013;34:1018–1023.
- Kibret T, Bitew A. Fungal keratitis in patients with corneal ulcer attending Minilik II memorial hospital, Addis Ababa, Ethiopia. BMC Ophthalmol. 2016;16:148.
- Shapiro BL, Lalitha P, Loh AR, et al. Susceptibility testing and clinical outcome in fungal keratitis. Br J Ophthalmol. 2010;94:384–385.
- Ledbetter EC, Montgomery KW, Landry MP, et al. Characterization of fungal keratitis in alpacas: 11 cases (2003–2012). J Am Vet Med Assoc. 2013;243:1616–1622.
- Said DG, Otri M, Miri A, et al. The challenge of fungal keratitis. Br J Ophthalmol. 2011;95:1623–1624.
- Waisman A, Hauptmann J, Regen T. The role of IL-17 in CNS diseases. Acta Neuropathol. 2015;129:625–637.
- Ling Y, Puel A. IL-17 and infections. Actas Dermosifiliogr. 2014;105(Suppl 1):34–40.
- Suryawanshi A, Cao Z, Sampson JF, et al. IL-17A-mediated protection against Acanthamoeba keratitis. J Immunol. 2015;194:650–663.
- Hou A, Tong L. Expression, regulation, and effects of interleukin-17f in the human ocular surface. Ocul Immunol Inflamm. 2018;26(7):1069–1077.
- Kimura K, Orita T, Morishige N, et al. Role of the JNK signaling pathway in downregulation of connexin43 by TNF-alpha in human corneal fibroblasts. Curr Eye Res. 2013;38:926–932.
- Li X, Zhou H, Tang W, et al. Transient downregulation of microRNA-206 protects alkali burn injury in mouse cornea by regulating connexin 43. Int J Clin Exp Pathol. 2015;8:2719–2727.
- Watanabe M, Masaki K, Yamasaki R, et al. Th1 cells downregulate connexin 43 gap junctions in astrocytes via microglial activation. Sci Rep. 2016;6:38387.
- Couture C, Desjardins P, Zaniolo K, et al. Enhanced wound healing of tissue-engineered human corneas through altered phosphorylation of the CREB and AKT signal transduction pathways. Acta Biomater. 2018;73:312–325.
- Jackson BE, Wilhelmus KR, Hube B. The role of secreted aspartyl proteinases in Candida albicans keratitis. Invest Ophthalmol Vis Sci. 2007;48:3559–3565.
- Wu TG, Wilhelmus KR, Mitchell BM. Experimental keratomycosis in a mouse model. Invest Ophthalmol Vis Sci. 2003;44:210–216.
- O’Day DM, Head WS, Csank C, et al. Differences in virulence between two Candida albicans strains in experimental keratitis. Invest Ophthalmol Vis Sci. 2000;41:1116–1121.
- Kimura A, Naka T, Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl Acad Sci U S A. 2007;104:12099–12104.
- Tachikawa M, Toki H, Tomi M, et al. Gene expression profiles of ATP-binding cassette transporter A and C subfamilies in mouse retinal vascular endothelial cells. Microvasc Res. 2008;75:68–72.
- Tamaoka M, Hassan M, McGovern T, et al. The epidermal growth factor receptor mediates allergic airway remodelling in the rat. Eur Respir J. 2008;32:1213–1223.
- Gao N, Kumar A, Guo H, et al. Topical flagellin-mediated innate defense against Candida albicans keratitis. Invest Ophthalmol Vis Sci. 2011;52:3074–3082.
- Sun J, Li Y, Graziani GM, et al. E-selectin mediated adhesion and migration of endothelial colony forming cells is enhanced by SDF-1alpha/CXCR4. PLoS One. 2013;8:e60890.
- Xu Q, Luan T, Fu S, et al. Effects of pitavastatin on the expression of VCAM-1 and its target gene miR-126 in cultured human umbilical vein endothelial cells. Cardiovasc Ther. 2014;32:193–197.
- Rafat M, Rotenstein LS, Hu JL, et al. Engineered endothelial cell adhesion via VCAM1 and E-selectin antibody-presenting alginate hydrogels. Acta Biomater. 2012;8:2697–2703.
- Heo Y, Shin YM, Lee YB, et al. Effect of immobilized collagen type IV on biological properties of endothelial cells for the enhanced endothelialization of synthetic vascular graft materials. Colloids Surf B Biointerfaces. 2015;134:196–203.
- Muller RT, Pourmirzaie R, Pavan-Langston D, et al. In vivo confocal microscopy demonstrates bilateral loss of endothelial cells in unilateral herpes simplex keratitis. Invest Ophthalmol Vis Sci. 2015;56:4899–4906.
- Chaudhry P, Prakash G, Agarwal A, et al. Endothelial cell loss associated with diffuse lamellar keratitis because of laser-assisted in situ keratomileusis. Eye Contact Lens. 2012;38:263–265.
- Chen C, Zhang Q, Liu S, et al. IL-17 and insulin/IGF1 enhance adhesion of prostate cancer cells to vascular endothelial cells through CD44-VCAM-1 interaction. Prostate. 2015;75:883–895.
- Karthikeyan RS, Vareechon C, Prajna NV, et al. Interleukin 17 expression in peripheral blood neutrophils from fungal keratitis patients and healthy cohorts in southern India. J Infect Dis. 2015;211:130–134.
- Moore K, Bryant ZJ, Ghatnekar G, et al. A synthetic connexin 43 mimetic peptide augments corneal wound healing. Exp Eye Res. 2013;115:178–188.
- Zhong J, Peng L, Wang B, et al. Tacrolimus interacts with voriconazole to reduce the severity of fungal keratitis by suppressing IFN-related inflammatory responses and concomitant FK506 and voriconazole treatment suppresses fungal keratitis. Mol Vis. 2018;24:187–200.
- Yuan K, Zhao G, Che C, et al. Dectin-1 is essential for IL-1beta production through JNK activation and apoptosis in Aspergillus fumigatus keratitis. Int Immunopharmacol. 2017;52:168–175.
- Carnt NA, Willcox MD, Hau S, et al. Association of single nucleotide polymorphisms of interleukins-1beta, −6, and −12B with contact lens keratitis susceptibility and severity. Ophthalmology. 2012;119:1320–1327.
- Elbadawy HM, Mirabelli P, Xeroudaki M, et al. Effect of connexin 43 inhibition by the mimetic peptide Gap27 on corneal wound healing, inflammation and neovascularization. Br J Pharmacol. 2016;173:2880–2893.
- Xia L, Zhang S, Cao Z, et al. Interleukin-17 enhanced immunoinflammatory lesions in a mouse model of recurrent herpetic keratitis. Microbes Infect. 2013;15:126–139.
- Zhang H, Li H, Li Y, et al. IL-17 plays a central role in initiating experimental Candida albicans infection in mouse corneas. Eur J Immunol. 2013;43:2671–2682.
- Sun M, Zhu M, Chen K, et al. TREM-2 promotes host resistance against Pseudomonas aeruginosa infection by suppressing corneal inflammation via a PI3K/Akt signaling pathway. Invest Ophthalmol Vis Sci. 2013;54:3451–3462.
