ABSTRACT
Dynorphins act as endogenous anticonvulsants via activation of kappa opioid receptor (KOR). However, the mechanism underlying the anticonvulsant role remains elusive. This study aims to investigate whether the potential protection of KOR activation by dynorphin against epilepsy was associated with the regulation of PI3K/Akt/Nrf2/HO-1 pathway. Here, a pilocarpine-induced rat model of epilepsy and Mg2+-free-induced epileptiform hippocampal neurons were established. Decreased prodynorphin (PDYN) expression, suppressed PI3K/Akt pathway, and activated Nrf2/HO-1 pathway were observed in rat epileptiform hippocampal tissues and in vitro neurons. Furthermore, dynorphin activation of KOR alleviated in vitro seizure-like neuron injury via activation of PI3K/Akt/Nrf2/HO-1 pathway. Further in vivo investigation revealed that PDYN overexpression by intra-hippocampus injection of PDYN-overexpressing lentiviruses decreased hippocampal neuronal apoptosis and serum levels of inflammatory cytokines and malondialdehyde (MDA) content, and increased serum superoxide dismutase (SOD) level, in pilocarpine-induced epileptic rats. The protection of PDYN in vivo was associated with the activation of PI3K/Akt/Nrf2/HO-1 pathway. In conclusion, dynorphin activation of KOR protects against epilepsy and seizure-induced brain injury, which is associated with activation of the PI3K/Akt/Nrf2/HO-1 pathway.
Introduction
Epilepsy is a chronic neurologic disorder characterized by recurrent and unprovoked seizure, which is a paroxysmal alteration of neurologic function caused by the excessive, hypersynchronous discharge of neurons in the brain [Citation1]. A traumatic brain injury or a stroke may lead to a seizure by downregulation of the inhibitory neurotransmission or by over-activation of the excitatory synaptic neurotransmission [Citation2]. However, to date, the mechanisms underlying epilepsy have not been elucidated.
Dynorphins were reported to act as endogenous anticonvulsants, which could possibly protect against seizure. Dynorphins are a class of opioid peptides derived from the precursor protein prodynorphin (PDYN). When PDYN is cleaved by proprotein convertase 2 (PC2), several active peptides are released: dynorphin-A, dynorphin-B, α/β-neo-endorphin, and big dynorphin [Citation3,Citation4]. Dynorphins are produced in many different parts of the brain, including the hippocampus, the hypothalamus, and the spinal cord. As kappa (κ) opioid receptor (KOR) endogenous ligands, dynorphins are believed to act as endogenous anticonvulsants via KOR activation [Citation5–Citation8]. However, the underlying mechanism for such a role in the termination of seizures remains elusive.
Oxidative stress and neuronal apoptosis are key events in the pathogenesis of epilepsy [Citation9,Citation10]. Indeed, significant levels of free radicals and oxidative damage have been observed in the brain during seizures [Citation11,Citation12]. As one of the most important antioxidant pathways [Citation13,Citation14], the nuclear factor erythroid 2-related factor (Nrf2)/heme oxygenase-1 (HO-1) (Nrf2/HO-1) pathway has been recently shown to exert a significantly neuroprotective role in epilepsy [Citation15,Citation16]. Under normal conditions, Nrf2 is located in the cytoplasm as an inactive complex bound to the repressor protein named as Kelch-like ECH-associated protein 1 (Keap1); Under oxidative/electrophilic stress, Nrf2 dissociates from Keap1, then translocates to the nucleus where it binds to the antioxidant response element (ARE) to regulate the transcription of numerous antioxidant and cytoprotective genes, such as HO-1 and superoxide dismutase 1 (SOD1) [Citation17,Citation18]. It has been recently reported that activation of Nrf2/ARE pathway conferred neuroprotective effects against pentylenetetrazole- or pilocarpine-induced brain damage in vivo and Mg2+ free-induced seizure-like neuron injury [Citation10,Citation15,Citation16,Citation19].
The phosphatidylinositol-3-kinase (PI3K)/Akt pathway is a key regulator of cell survival and proliferation that is widely expressed in the central nervous system. Activation of the PI3K/Akt signaling pathway can alter neuronal apoptosis and attenuate the severity of seizures in experimental epilepsy-induced rats [Citation20–Citation22]. Interestingly, Tong et al. [Citation23] have demonstrated that U50448H, a KOR-selective agonist, protects against heart failure following myocardial ischemia/reperfusion via activation of HO-1 expression through the PI3K/Akt/Nrf2 pathway. However, whether the PI3K/Akt pathway is involved in the anticonvulsant role of KOR activation by dynorphin is unclear. Here, in this study, we established a rat model of epilepsy and Mg2+-free-induced epileptiform hippocampal neurons to explore the role of dynorphin activation in alleviating epilepsy. Furthermore, we investigated whether PI3K/Akt/Nrf2/HO-1 pathway was involved in the protective role of dynorphin.
Materials and methods
Pilocarpine-induced epilepsy in rats
Adult male Wistar rats (weight, 200–220 g; years, 8 wk old) were purchased from Charles River Laboratories (Beijing, China). All animals were fed a healthy diet with controlled condition (temperature, 25°C ± 1°C; humidity, 50%). All animals were used in strict accordance with national animal experiment requirements that were approved by the animal ethics association of the Third Xiangya Hospital (Hunan, China).
Rats were randomly assigned into four groups (n = 8/group): Control group, epileptic model group, LV-NC group, and LV-PDYN group. The epilepsy was induced by pilocarpine injection as previously described [Citation24,Citation25], with some alterations. The rats in the model group were injected intraperitoneally (i.p.) with lithium chloride (LiCl, 127 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) 18 h prior to the first administration of pilocarpine (30 mg/kg, i.p., Sigma-Aldrich). Pilocarpine (10 mg/kg, i.p.) was repeatedly injected every 30 min until the rats developed seizures. Rats in the control group were injected with normal saline instead of pilocarpine. One hour after the onset of the status epileptics, rats were injected with diazepam (10 mg/kg, i.p.) to terminate seizures.
The behavioral changes of the rats were observed. Animals were sacrificed by decapitation 24 h after status epilepticus. The hippocampus was removed and stored in liquid nitrogen for TUNEL staining and extraction of RNA and protein.
Lentivirus production and stereotactic injection
A lentiviral vector that stably overexpressing PDYN (LV-PDYN) and a negative control lentiviral vector (LV-NC) were purchased from GenePharma (Shanghai, China). Stereotaxic intra-hippocampus injection was done as previously described [Citation24]. After the pilocarpine-induced seizure, rats were deeply anesthetized and the head of rats was fixed in a stereotaxic frame. A volume of 5 mL LV-PDYN and LV-NC were infused through a glass pipette bilaterally in the dorsal hippocampus of epileptic rats.
RT-qPCR
RT-qPCR was performed to examine the mRNA expression of PDYN and HO-1 in rat hippocampus and cultured hippocampal neurons. Total RNA was extracted from rat hippocampus using TRIzol reagent (Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA, USA), and reverse-transcribed to cDNA using First-strand cDNA synthesis kit (Tiangen Biotech, Beijing, China) according to the manufacturer’s protocol. Relative mRNA expression of PDYN and HO-1 were detected using SYBR® Premix Dimer Eraser kit (Takara Shiga, Japan) performing on an ABI 7500 Real-Time PCR system (Applied Biosystems, Carlsbad, CA, USA). The relative quantification was calculated using the 2−ΔΔct method. β-actin was used as the internal control.
Western blot
Western blot was performed to examine the expression of the PI3K/Akt/Nrf2/HO-1 pathway-related proteins in rat hippocampus and cultured hippocampal neurons. Briefly, hippocampus tissues were extracted in RIPA lysis buffer (Beyotime, Shanghai, China). Nuclear and cytosolic proteins were extracted using the Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime) according to the manufacturer’s instructions. Then equal protein from cell lysates was separated by 10% SDS-PAGE gels and transferred onto PVDF membrane (Millipore, Billerica, MA, USA). After being blocked with 5% non-fat milk, the membrane was incubated with the following primary antibodies: anti-PDYN (OriGene, Rockville, MD, USA), anti-PI3K (Cell Signaling Technology Inc., Danvers, MA, USA), anti-p-Akt (Santa Cruz Biotechnology, Dallas, TX, USA), anti-Akt (Santa Cruz Biotechnology), and anti-Nrf2 (Santa Cruz Biotechnology), anti-HO-1 (Santa Cruz Biotechnology), followed by horseradish peroxidase (HRP)-labeled secondary antibodies (Beyotime). The protein analysis was visualized by Quantity One software (Bio-Rad, Hercules, CA, USA). β-actin and lamin B1 were used as the internal controls for total and nuclear proteins, respectively.
TUNEL assay
TUNEL assay was performed to analyze the apoptosis in rat hippocampus. Serial sections (5 μm thick) sections were deparaffinized with xylene, rehydrated through descending concentrations of ethanol and rinsed in phosphate buffered saline (PBS) twice for 10 min at room temperature. Proteinase-K (20 μg/ml) was applied for 20 min at room temperature to chromatin protein digestion. After washing in PBS, TUNEL reaction was performed using the in situ cell death detection kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions. Apoptotic nuclei were stained in dark brown.
Quantification of cytokine levels
The levels of tumor necrosis factor-α (TNF-α), interleukin-2 (IL-2), and IL-6 were measured using their commercial ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Determination of MDA content and SOD activity
The malondialdehyde (MDA) content and superoxide dismutase (SOD) activity were analyzed with their commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
Isolation, culture, and epileptiform activity induction of hippocampal neurons
Isolation, culture, and epileptiform activity induction of hippocampal neurons were performed as previously described [Citation10]. Briefly, the rat hippocampal tissues were dissected, triturated, and plated onto poly-D-lysine-treated cell plates. Cells were cultured in the Neurobasal™ medium supplemented with 2% B27 (Neurobasal/B27; Gibco, Thermo Fisher Scientific, Inc.) and 10% fetal bovine serum (FBS; Gibco) in a humidified atmosphere containing 95% air and 5% CO2 at 37°C. After plating for 3 d, the hippocampal neurons were treated with cytosine arabinoside (5 μM; Sigma-Aldrich) to inhibit astrocyte proliferation. Culture medium was refreshed every 3 d. The hippocampal neurons at days in vitro 7–18 were used for subsequent experiments.
For epileptiform activity induction, the culture medium of hippocampal neurons was replaced with magnesium-free (Mg2+-free) physiological solution (pH 7.3) supplemented with 2.5 mM KCl, 145 mM NaCl, 2 mM CaCl2, 10 mM HEPES, 10 mM glucose and 0.002 mM glycine at day in vitro 6, followed which neurons in the epilepsy group were kept in Mg2+-free solution for 3 h. After treatment, neurons were transferred to the conventional Neurobasal/B27 medium and returned to the original incubator as previously described [Citation26]. Cells in the control group were cultured in Neurobasal/B27 medium under the same incubation condition. After that, the hippocampal neurons were used in these experiments including determination of MDA content and SOD activity, cell apoptosis assay, RT-qPCR, and western blot.
Cell treatment
To investigate the role of KOR activation in epilepsy, the Mg2+-free solution-exposed hippocampal neurons were treated with dynorphin-A (a KOR agonist; Sigma-Aldrich) and GNTI (a KOR antagonist; R&D Systems), both alone and in combination ().
Figure 1. Decreased PDYN expression and suppressed PI3K/Akt/Nrf2/HO-1 pathway in a rat model of epilepsy Serum levels of TNF-α, IL-2, and IL-6 (a) using ELISA, MDA content and SOD activity (b) using commercial kits in control and epileptic model rats. In situ cell apoptosis (c) using TUNEL staining (Scale bar: 25.0 μm in overview; 2 μm in CA1 and CA3), mRNA expression of PDYN and HO-1 (d) using RT-qPCR, and protein expression of PDYN, total Nrf2, nuclear Nrf2, HO-1, PI3K, as well as phosphorylation level of Akt (p-Akt) (e) using western blot, in the hippocampus of control and epileptic model rats. n = 8/group. *p < 0.05, **p < 0.01, vs. Control group.
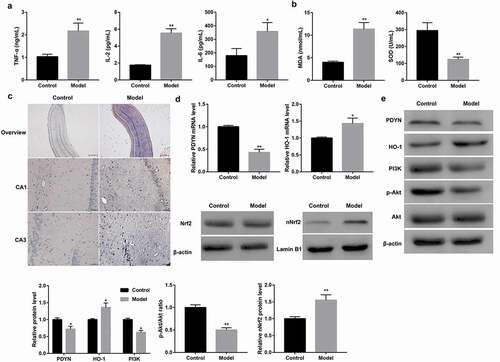
Figure 2. Decreased PDYN expression and suppressed PI3K/Akt/Nrf2/HO-1 pathway in epileptiform hippocampal neurons MDA content and SOD activity (a) using commercial kits, cell apoptosis (b) using flow cytometry, mRNA expression of DYN and HO-1 (c) using RT-qPCR, and protein expression of PDYN, total Nrf2, nuclear Nrf2, HO-1, PI3K, as well as phosphorylation level of Akt (p-Akt) (d-e) using western blot, in cultured hippocampal neurons exposed to Mg2+-free solution or not. *p < 0.05, **p < 0.01, vs. Control group.
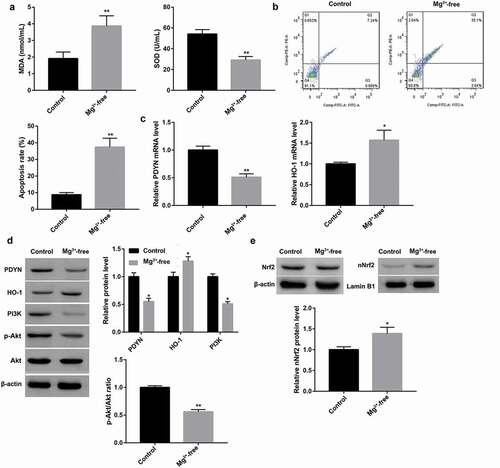
Figure 3. Dynorphin activation of KOR activated PI3K/Akt/Nrf2/HO-1 pathway and alleviated seizure-like neuron injury MDA content and SOD activity (a) using commercial kits, cell apoptosis (b) using flow cytometry, mRNA expression of HO-1 (c) using RT-qPCR, and protein expression of total Nrf2, nuclear Nrf2, HO-1, PI3K, as well as phosphorylation level of Akt (p-Akt) (d) using western blot, in cultured hippocampal neurons exposed to Mg2+-free solution, followed by treatment with the KOR agonist dynorphin-A and the KOR antagonist GNTI, both alone or in combination. Cultured hippocampal neurons without any treatment served as the control group. Dyn-A, dynorphin-A. *p < 0.05, **p < 0.01, vs. Control group; #p < 0.05, ##p < 0.01, vs. Mg2+-free group; &p < 0.05, &&p < 0.01, vs. Mg2+-free + Dyn-A group.
To explore the role of PI3K/Akt/Nrf2/HO-1 pathway in the regulation mediated by dynorphin-A-mediated effects in epilepsy, the Mg2+-free solution-exposed hippocampal neurons were treated with dynorphin-A and LY294002 (an inhibitor of PI3K/Akt pathway; Sigma-Aldrich) and ZnPPIX (an HO-1 inhibitor; Sigma-Aldrich), both alone and in combination ().
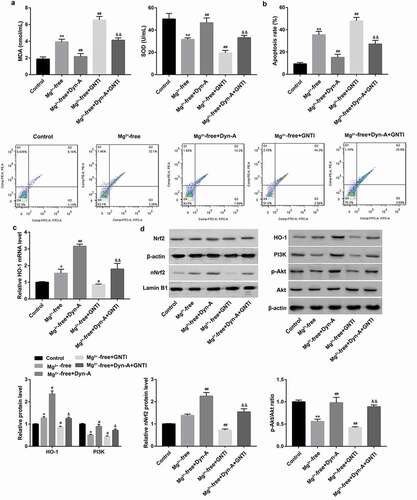
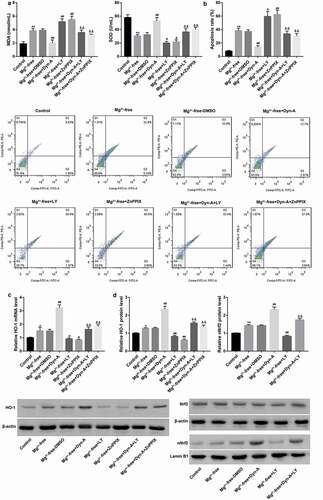
Figure 4. Dynorphin activation of KOR alleviated seizure-like neuron injury via activation of PI3K/Akt/Nrf2/HO-1 pathway MDA content and SOD activity (a) using commercial kits, cell apoptosis (b) using flow cytometry, mRNA expression of HO-1 (c) using RT-qPCR, and protein expression of total Nrf2, nuclear Nrf2 and HO-1 (d) using western blot, in cultured hippocampal neurons exposed to Mg2+-free solution, followed by treatment with dynorphin-A and LY294002 (an inhibitor of PI3K/Akt pathway)/ZnPPIX (an HO-1 inhibitor), both alone or in combination. Cultured hippocampal neurons without any treatment served as the control group. DMSO served as the vehicle of LY294002 and ZnPPIX. Dyn-A, dynorphin-A. LY, LY294002. *p < 0.05, **p < 0.01, vs. Control group; #p < 0.05, ##p < 0.01, vs. Mg2+-free or Mg2+-free + DMSO group; &&p < 0.01, vs. Mg2+-free + Dyn-A group.
Cell apoptosis assay
Cell apoptosis analysis was performed by flow cytometry using the FITC-conjugated Annexin V apoptosis detection kit (BD Pharmingen, San Jose, CA, USA) according to the manufacturer’s instructions. Flow cytometric analysis was conducted using a FACScalibur (Beckman Coulter, Fullerton, CA, USA), and the data were analyzed using FlowJo software (Tree Star, San Carlos, CA, USA).
Statistical analysis
All statistical analyses were performed using SPSS statistical software package standard version 16.0 (SPSS, Inc., Chicago, IL, USA). Group statistical significance was assessed using Student’s t‑test for comparison of two groups, one‑way analysis of variance (ANOVA) followed by LSD test for three or more groups. The data are presented as the mean ± standard deviation (SD) from three independent experiments. p < 0.05 was considered statistically significant.
Results
PDYN expression and activity of PI3K/AkT and Nrf2/HO-1 pathway in a rat model of epilepsy
Compared with the control rats, epileptic model rats showed significantly increased serum levels of pro-inflammatory cytokines (TNF-α, IL-2, and IL-6) and MDA content, accompanied with significantly decreased serum SOD level (,). MDA is a marker for oxidative stress while SOD is an antioxidant enzyme. Therefore, our results confirmed the inflammation elevation and oxidative stress enhancement in epileptic model rats. Furthermore, epileptic model rats displayed markedly increased hippocampal neuronal apoptosis as indicated by TUNEL staining ()). Moreover, PDYN was notably lower at both mRNA ()) and protein ()) levels in the model group than that in the control group. In addition, protein level of PI3K and phosphorylation level of Akt were significantly lower in the model group than that in the control group. In contrast, the epileptic model rats showed a significant increase in nuclear translocation of Nrf2 as well as expression of its target anti-oxidant gene HO-1, at both mRNA and protein levels (,). These results indicated the potential involvement of the PI3K/Akt pathway and Nrf2/HO-1 pathway in epilepsy.
PDYN expression and activity of PI3K/Akt and Nrf2/HO-1 pathway in epileptiform hippocampal neurons
Cultured hippocampal neurons were exposed to Mg2+-free solution to induce epilepsy. Compared with the control neurons, Mg2+-free-exposed hippocampal neurons showed significantly increased MDA content but decreased SOD level ()). Furthermore, Mg2+-free-exposed hippocampal neurons displayed markedly increased cell apoptosis ()). Moreover, PDYN was notably lower at both mRNA ()) and protein ()) levels in the Mg2+-free group than that in the control group. In addition, protein level of PI3K and phosphorylation level of Akt were significantly lower in the Mg2+-free group than that in the control group. In contrast, Mg2+-free-exposed hippocampal neurons showed increased nuclear translocation of Nrf2 as well as expression of its target anti-oxidant gene HO-1 at both mRNA and protein levels (,). These data further suggested the potential involvement of the PI3K/Akt pathway and Nrf2/HO-1 pathway in epilepsy.
Dynorphin activation of KOR activated PI3K/Akt and Nrf2/HO-1 pathway and alleviated seizure-like neuron injury
MDA content which was significantly increased by Mg2+-free solution exposure was subsequently decreased by the endogenous KOR agonist dynorphin-A ()). Furthermore, SOD level which was significantly decreased by Mg2+-free solution exposure was subsequently increased by dynorphin-A ()). These results indicated the antioxidant characteristics of KOR activation. Moreover, dynorphin-A treatment notably inhibited the Mg2+-free-induced cell apoptosis ()). These results indicated that dynorphin-A alleviated Mg2+-free-induced seizure-like neuron injury.
In addition, dynorphin-A treatment led to significantly increased PI3K protein expression and Akt phosphorylation, accompanied with induced Nrf2 nuclear translocation and HO-1 upregulation in Mg2+-free solution-exposed hippocampal neurons (,). These data indicated that dynorphin-A activated the PI3K/Akt pathway and the Nrf2/HO-1 pathway. In contrast with the endogenous KOR agonist dynorphin-A, the KOR-specific antagonist GNTI exerted the opposite effects. Of note, GNTI significantly abrogated the dynorphin-A-mediated changes in hippocampal neurons exposed to Mg2+-free solution (,).
Dynorphin activation of KOR alleviated seizure-like neuron injury via activation of PI3K/Akt/Nrf2/HO-1 pathway
To further verify whether the PI3K/Akt pathway and the Nrf2/HO-1 pathway were involved in the dynorphin-A-mediated alleviation of seizure-like neuron injury, the LY294002 (an inhibitor of PI3K/Akt pathway) and ZnPPIX (an HO-1 inhibitor) were used. Data revealed that inhibition of PI3K/Akt pathway by LY294002 significantly abolished the dynorphin-A-mediated activation of the Nrf2/HO-1 pathway, which indicated that the Nrf2/HO-1 pathway was downstream of the PI3K/Akt pathway under this condition (,). Consistent with what was indicated in , dynorphin-A decreased MDA content, increased SOD activity, and inhibited cell apoptosis in hippocampal neurons exposed to Mg2+-free solution (,). Importantly, in contrast with the dynorphin-A, both the LY294002 and ZnPPIX exerted the opposite effects. Moreover, both LY294002 and ZnPPIX significantly abrogated the dynorphin-A-mediated changes in hippocampal neurons exposed to Mg2+-free solution (,). These results indicated that dynorphin-A alleviated seizure-like neuron injury through activation of the PI3K/Akt/Nrf2/HO-1 pathway.
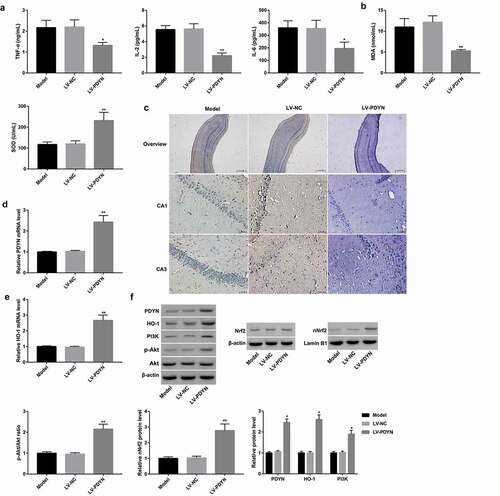
PDYN overexpression alleviated pilocarpine-induced epilepsy and neuronal apoptosis in rats
Finally, we explored the in vivo role of PDYN in rat epilepsy and investigated whether the underlying mechanism was associated with the PI3K/Akt/Nrf2/HO-1 pathway. To address this, LV-NC and LV-PDYN were respectively delivered into epilepsy model rats via intra-hippocampus injection. As expected, PDYN was successfully overexpressed by LV-PDYN injection, as evidenced by a significant increase in both mRNA ()) and protein ()) levels of hippocampal PDYN in the LV-PDYN group compared with the LV-NC group. Importantly, PDYN overexpression significantly decreased serum levels of pro-inflammatory cytokines (TNF-α, IL-2, and IL-6) and MDA content, and increased serum SOD level (,). Furthermore, PDYN overexpression markedly decreased hippocampal neuronal apoptosis as indicated by TUNEL staining ()). Moreover, PDYN overexpression significantly increased protein level of PI3K and phosphorylation level of Akt, as well as Nrf2 nuclear translocation and HO-1 expression at both mRNA and protein levels (,). These results indicated PDYN overexpression alleviated pilocarpine-induced epilepsy and neuronal apoptosis in epileptic rats, the mechanism of which was associated with activation of the PI3K/Akt/Nrf2/HO-1 pathway.
Figure 5. PDYN overexpression alleviated pilocarpine-induced epilepsy and neuronal apoptosis in rats Serum levels of TNF-α, IL-2, and IL-6 (a) using ELISA, MDA content and SOD activity (b) using commercial kits, in the groups of model, LV-NC, and LV-Dyn. In situ cell apoptosis (c) using TUNEL staining (scale bar: 25.0 μm in overview; 2 μm in CA1 and CA3), mRNA expression of PDYN (d) and HO-1 (e) using RT-qPCR, and protein expression of PDYN, total Nrf2, nuclear Nrf2, HO-1, PI3K, as well as phosphorylation level of Akt (p-Akt) (f) using western blot, in the rat hippocampus in the groups of model, LV-NC, and LV-PDYN. n = 8/group. *p < 0.05, **p < 0.01, vs. LV-NC group.
Discussion
Epilepsy is a common chronic brain disorder characterized by recurring seizures. The hippocampus is especially vulnerable to seizure-induced damage. Oxidative stress and neuronal apoptosis are key events in the pathogenesis of epilepsy [Citation9,Citation10]. Besides, elevation in cytokine levels, particularly, TNF-α, IL-2, and IL-6, has been implicated in the pathogenesis of epilepsy [Citation27]. Pilocarpine-induced epilepsy in vivo exhibits most of the clinical and neuropathological characteristics of epilepsy and has been widely used in seizure research [Citation10,Citation24]. On the other hand, Mg2+-free physiological solution was generally used to induce epileptiform hippocampal neurons in vitro [Citation10,Citation28]. Using these models, this study revealed decreased PDYN expression in both rat epileptiform hippocampal tissues and in vitro neurons. Furthermore, dynorphin A, which was derived from PDYN, alleviated in vitro seizure-like neuron injury. Moreover, PDYN overexpression attenuated key events including hippocampal neuronal apoptosis, oxidative stress, and inflammation in pilocarpine-induced epileptic rats. Together, the protective effects of dynorphin against epilepsy have been confirmed in the present study.
Dynorphins are produced in many different parts of the brain and exert many different physiological actions depending on its site of production. Dynorphins preferentially bind to KOR; however, they can also activate the other classical opioid receptors mu (μ) and delta (δ), as well as NMDA receptors [Citation6]. As indicated in in this study, the endogenous KOR agonist dynorphin-A alleviated neuronal apoptosis and oxidative stress in Mg2+-free-induced epileptiform hippocampal neuron injury, whereas the KOR-specific antagonist GNTI exerted the opposite effects. These results indicated dynorphin alleviated in vitro seizure-like neuron injury via KOR activation.
This study also explored the proteins involved in the PI3K/Akt/Nrf2/HO-1 pathway in the dynorphin-associated protection in epilepsy. Activation of the PI3K/Akt signaling pathway can alter neuronal apoptosis and attenuates the severity of seizures in experimental epilepsy-induced rats [Citation20–Citation22]. On the other hand, activation of the antioxidant Nrf2/ARE pathway confers neuroprotective effects against pentylenetetrazole- or pilocarpine-induced brain damage in vivo and Mg2+ free-induced seizure-like neuron injury [Citation10,Citation15,Citation16,Citation19]. In this study, the suppressed activity of the PI3K/Akt pathway and activated Nrf2/HO-1 pathway were observed, as indicated by decreased PI3K expression and Akt phosphorylation, as well as increased Nrf-2 nuclear translocation and HO-1 expression. Furthermore, our findings revealed that inhibition of the PI3K/Akt pathway by LY294002 treatment significantly abolished the dynorphin-A-induced Nrf2 nuclear translocation and HO-1 upregulation, which linked the PI3K/Akt and Nrf2/HO-1 pathway. Moreover, inhibition of PI3K/Akt by LY294002 and inhibition of HO-1 activity by ZnPP-IX treatment significantly abrogated the dynorphin-A-mediated protection in Mg2+-free-hippocampal neuron injury. Further in vivo investigation revealed that PDYN exerted protective effects against epilepsy in conjunction with the activation of the PI3K/Akt/Nrf2/HO-1 pathway. Collectively, these results indicated that the alleviation of seizure-like neuron injury may be due, in part, to the direct action of dynorphin-A on the activation of the PI3K/Akt/Nrf2/HO-1 pathway.
Oxidative stress is a key event in the pathogenesis of epilepsy. Intriguingly, glyoxalase 1 is a ubiquitous cellular enzyme involved in detoxification of methylglyoxal, a cytotoxic byproduct of glycolysis, whose excess can cause oxidative stress [Citation29,Citation30]. The impaired activity of glyoxalase 1 in hippocampal neurons inhibits Nrf2, and this is associated to mitochondrial dysfunction, oxidative stress and neurodegeneration [Citation31]. Thus, is there a link between dynorphin and glyoxalase system in epilepsy? Furthermore, transcriptome experiments may be also needed in future to evaluate which other pathways and which aberrantly expressed non-coding RNAs in epilepsy are involved in dynorphin-mediated epilepsy protection [Citation32,Citation33].
Conclusion
In conclusion, dynorphin activates KOR, and protects against epilepsy and seizure-induced brain injury, which is associated with the PI3K/Akt pathway activation and ensuing Nrf2 nuclear translocation and HO-1 upregulation. Our findings provide new insight into the underlying mechanism of dynorphin-mediated protection in epilepsy.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Stafstrom CE, Carmant L. Seizures and epilepsy: an overview for neuroscientists. Cold Spring Harb Perspect Med. 2015Jun 03;5:a022426-a022426.
- Goldberg EM, Coulter DA. Mechanisms of epileptogenesis: a convergence on neural circuit dysfunction. Nat Rev Neurosci. 2013 Apr 19;14:337–349.
- Day R, Lazure C, Basak A, et al. Prodynorphin processing by proprotein convertase 2. Cleavage at single basic residues and enhanced processing in the presence of carboxypeptidase activity. J Biol Chem. 1998 Feb 14;273:829–836.
- Tan‐No K, Takahashi H, Nakagawasai O, et al. Chapter 15 nociceptive behavior induced by the endogenous opioid peptides dynorphins in uninjured mice: evidence with intrathecal N‐ethylmaleimide inhibiting dynorphin degradation. Int Rev Neurobiol. 2009;85:191–205. Academic Press.
- Pirker S, Gasser E, Czech T, et al. Dynamic up-regulation of prodynorphin transcription in temporal lobe epilepsy. Hippocampus. 2009May 14;19:1051–1054.
- Schwarzer C. 30 years of dynorphins–new insights on their functions in neuropsychiatric diseases. Pharmacol Ther. 2009 Jun 02;123:353–370.
- Loacker S, Sayyah M, Wittmann W, et al. Endogenous dynorphin in epileptogenesis and epilepsy: anticonvulsant net effect via kappa opioid receptors. Brain. 2007 Mar 10;130:1017–1028.
- Schunk E, Aigner C, Stefanova N, et al. Kappa opioid receptor activation blocks progressive neurodegeneration after kainic acid injection. Hippocampus. 2011 Mar 11;21:1010–1020.
- Ashrafi MR, Shams S, Nouri M, et al. A probable causative factor for an old problem: selenium and glutathione peroxidase appear to play important roles in epilepsy pathogenesis. Epilepsia. 2007 Jun 09;48:1750–1755.
- Geng JF, Liu X, Zhao HB, et al. LncRNA UCA1 inhibits epilepsy and seizure-induced brain injury by regulating miR-495/Nrf2-ARE signal pathway. Int J Biochem Cell Biol. 2018Apr 03;99:133–139.
- Qi Z, Yu X, Xu P, et al. l-Homocarnosine, l-carnosine, and anserine attenuate brain oxidative damage in a pentylenetetrazole-induced epilepsy model of ovariectomized rats. 3 Biotech. 2018 Aug 15;8:363.
- Rodrigues AD, Scheffel TB, Scola G, et al. Neuroprotective and anticonvulsant effects of organic and conventional purple grape juices on seizures in Wistar rats induced by pentylenetetrazole. Neurochem Int. 2012 Apr 03;60:799–805.
- Ali T, Kim T, Rehman SU, et al. Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of alzheimer’s disease. Mol Neurobiol. 2017;55:6076–6093.
- Liang F, Fang Y, Cao W, et al. Tangeretin attenuates tert-Butyl Hydroperoxide (t-BHP)-induced oxidative damage in HepG2 cells: relevance of Nrf2/ARE and MAPKs signaling pathways. J Agric Food Chem. 2018;66:6317–6325.
- Singh N, Vijayanti S, Saha L, et al. Neuroprotective effect of Nrf2 activator dimethyl fumarate, on the hippocampal neurons in chemical kindling model in rat. Epilepsy Res. 2018 Apr 25;143:98–104.
- Shi Y, Miao W, Teng J, et al. Ginsenoside Rb1 protects the brain from damage induced by epileptic seizure via Nrf2/ARE signaling. Cell Physiol Biochem. 2018 Jan 23;45:212–225.
- Zhang L, Chen Z, Gong W, et al. Paeonol ameliorates diabetic renal fibrosis through promoting the activation of the Nrf2/ARE pathway via up-regulating sirt1. Front Pharmacol. 2018;9:512.
- Seo JY, Pyo E, An JP, et al. Andrographolide activates keap1/Nrf2/ARE/HO-1 Pathway in HT22 cells and suppresses microglial activation by Aβ42 through Nrf2-related inflammatory response. Mediators Inflamm. 2017;2017:5906189. 2017,(2017-3-8).
- Shekh-Ahmad T, Eckel R, Dayalan Naidu S, et al. KEAP1 inhibition is neuroprotective and suppresses the development of epilepsy. Brain. 2018 Mar 15;141:1390–1403.
- Guo XQ, Cao YL, Hao F, et al. Tangeretin alters neuronal apoptosis and ameliorates the severity of seizures in experimental epilepsy-induced rats by modulating apoptotic protein expressions, regulating matrix metalloproteinases, and activating the PI3K/Akt cell survival pathway. Adv Med Sci. 2017 May 16;62:246–253.
- Wei H, Duan G, He J, et al. Geniposide attenuates epilepsy symptoms in a mouse model through the PI3K/Akt/GSK-3beta signaling pathway. Exp Ther Med. 2018 Feb 06;15:1136–1142.
- Wu Q, Yi X. Down-regulation of long noncoding RNA MALAT1 protects hippocampal neurons against excessive autophagy and apoptosis via the PI3K/Akt Signaling Pathway in Rats with epilepsy. J Mol Neurosci. 2018 Jun 03;65:234–245.
- Tong G, Zhang B, Zhou X, et al. Kappa-opioid agonist U50,488H-mediated protection against heart failure following myocardial ischemia/reperfusion: dual roles of heme oxygenase-1. Cell Physiol Biochem. 2016 Nov 02;39:2158–2172.
- Wang D, Ren M, Guo J, et al. The inhibitory effects of Npas4 on seizures in pilocarpine-induced epileptic rats. PloS one. 2014 Dec 24;9:e115801.
- Lee SH, Choi BY, Kho AR, et al. Protective effects of protocatechuic acid on seizure-induced neuronal death. Int J Mol Sci. 2018 Jan 11;19:E187.
- Jiang Q, Wu Y, Wang J, et al. Characterization of developing rat cortical neurons after epileptiform discharges. Int J Dev Neurosci. 2010 Jul 06;28:455–463.
- Ey A-R, LA D, Bustanji Y, et al. Effect of lamotrigine on in vivo and in vitro cytokine secretion in murine model of inflammation. J Neuroimmunol. 2018 Jun 26;322:36–45.
- Lv X, Guo F, Xu X, et al. Abnormal alterations in the Ca(2)(+)/CaV1.2/calmodulin/caMKII signaling pathway in a tremor rat model and in cultured hippocampal neurons exposed to Mg(2)(+)-free solution. Mol Med Rep. 2015 Aug 25;12:6663–6671.
- Frandsen J, Narayanasamy P. Flavonoid enhances the glyoxalase pathway in cerebellar neurons to retain cellular functions. Sci Rep. 2017;7:5126.
- Donato L, Scimone C, Nicocia G, et al. GLO1 gene polymorphisms and their association with retinitis pigmentosa: a case–control study in a Sicilian population. Mol Biol Rep. 2018;45:1349–1355.
- Mastrocola R. AGEs and neurodegeneration: the Nrf2/glyoxalase-1 interaction. Oncotarget. 2016;8:5645–5646.
- Donato L, Bramanti P, Scimone C, et al. Corrigendum to: miRNA expression profile of retinal pigment epithelial cells under oxidative stress conditions. FEBS Open Bio. 2018 Nov 10;8:1884.
- Donato L, Scimone C, Rinaldi C, et al. Non-coding RNAome of RPE cells under oxidative stress suggests unknown regulative aspects of Retinitis pigmentosa etiopathogenesis. Sci Rep. 2018;45:1349–1355.
