ABSTRACT
Environmental stressors in early childhood can have a detrimental impact later in life, manifesting in functional gastrointestinal disorders including irritable bowel syndrome (IBS). The phenomenon is also observed in rodents, where neonatal–maternal separation, a model of early life stress, induces phenotypes similar to IBS; however, the underlying mechanisms remain unelucidated. Our recent study provided a mechanism for the pathogenesis in the gut, demonstrating that increased visceral hyperalgesia resulted from the expansion of the intestinal stem cell compartment leading to increased differentiation and proliferation of serotonin (5-hydroxytryptamine/5-HT)-producing enterochromaffin cells. Moreover, it identified nerve growth factor (NGF) as a key mediator of the pathogenesis; surprisingly, it exerts its effect via cross talk with Wnt/β-catenin signaling. This article addresses the roles of NGF in driving IBS and its potential clinical implications, outstanding questions in how psychological stimuli are transduced into physical phenotypes, as well as future directions of our findings.
Abbreviations
5-HT: 5-hydroxytryptamine/serotonin; BDNF: brain-derived neurotrophic factor; CRF: corticotrophin-releasing factor; EC: enterochromaffin; ENS: enteric nervous system; GI: gastrointestinal; GPCR: G-protein-coupled receptor; IBS (-D): irritable bowel syndrome (diarrhea predominant); LRP5/6: low-density lipoprotein receptor-related protein 5/6; MAPK: mitogen-activated protein kinase; NGF: nerve growth factor; NMS: neonatal–maternal separation; PI3K: phosphoinositode3-kinase; PLCγ: phospholipase c, gamma subtype; TrkA: tropomyosin receptor kinase A
The role of nerve growth factor (NGF)/TrkA signaling in the development of functional gastrointestinal disorders
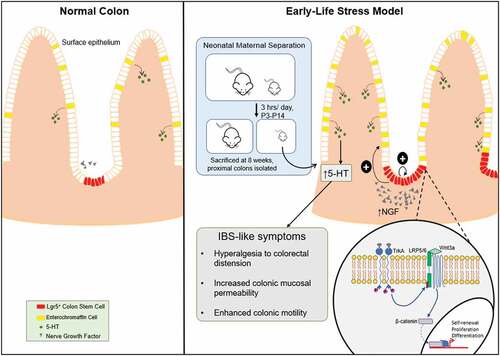
Irritable bowel syndrome (IBS) is a common, lifelong gastrointestinal (GI) motility disorder which manifests in constipation or diarrhea, abdominal pain and stomach bloating; there is no known cure [Citation1,Citation2]. The exact causes of IBS are still unknown, but several factors, ranging from immune responses and microbiota changes to abnormalities in the enteric nervous system (ENS), have been suggested, while the neurotransmitter 5-HT has also been shown to be heavily implicated in IBS pathogenesis by influencing gut motility and nociception [Citation3–Citation17]. Interestingly, early life stress has also been reported to be one of the major risk factors for IBS later in life; however, the mechanism underlying this pathogenesis process remains poorly understood [Citation18–Citation21]. For the first time, our recent findings identified the early life stress-induced upregulation of NGF as a mechanism to drive the pathogenesis of IBS-like phenotypes, via direct activation of tropomyosin receptor kinase A (TrkA) on intestinal stem cells (ISCs) in both the small intestine and colon, leading to increased self-renewal and expansion of the ISC compartment while also driving the differentiation of ISCs towards secretory lineages, including enterochromaffin (EC) and Paneth cells; the resulting increased production of 5-HT from the expanded EC cell population could promote IBS-like symptoms including hyperalgesia and enhanced colonic motility (summarized in ) [Citation18]. Importantly, specific anti-TrkA antibody MNAC13 abolished the effects on ISCs, further evidencing the key role of NGF [Citation22,Citation23]. Interestingly, instead of acting via its canonical tyrosine kinase signaling pathways – MAPK, PI3K or PLCγ, our study revealed cross talk between NGF and Wnt signaling, revealing the ability of the former to transactivate the latter Citation[24].
Cell autonomous and non-autonomous actions of NGF
NGF is a neurotrophic factor and thus is capable of inducing neuronal growth in the ENS. It has been shown to induce neurite growth upon secretion by enteric glial cells, highlighting its potential impact on an ENS-based neuroimmune response [Citation25,Citation26]. This was further evidenced by a study from Barreau et al., which observed a significant increase in enteric cholinergic neuron and colonic mucosal nerve fiber density following neonatal–maternal separation (NMS), and this synaptogenesis was NGF dependent and lost upon administration of anti-NGF-neutralizing antibodies [Citation27]. Indeed, this cell non-autonomous effect of NGF upon the stem cell population is not restricted to GI motility disorders; Hayakawa et al. have reported a similar mechanism in gastric and colorectal cancers, where cholinergic stimulation upregulated NGF expression, promoting ENS innervation. This, in turn, would trigger the malignant proliferation of intestinal epithelial cells since the expanded cholinergic stimulation would induce YAP-mediated activation of Wnt signaling in muscarinic receptor 3 (M3R)+ stem cells and, in a feed-forward fashion, promote gastric tumorigenesis [Citation28]. Surprisingly, a similar mechanism is used in embryonic pancreatic development as reported by Borden et al., in which neuronal norepinephrine secretion used to drive pancreatic β-cell migration is reciprocated by β-cell secretion of NGF to drive neuronal proliferation and innervation, forming a symbiotic, positive feedback loop to ensure proper β-cell architecture formation as well as sufficient neuronal innervation during early development [Citation29]. On the other hand, this mechanism may also be hijacked in a pathological setting as demonstrated by Renz et al., showing that adrenergic signaling upregulated NGF (and BDNF) concentrations in pancreatic ductal carcinoma to promote nerve growth and drive cancer progression [Citation30].
In contrast, our mechanistic work revealed the ability of NGF to positively regulate stem cell proliferation in a cell-autonomous manner. We observed that upregulation of NGF, acting directly on the stem cell niche, drove colonic hypersensitivity. This phenotype has been shown to occur both in vivo and ex vivo/in vitro using an intestinal organoid culture from mice and humans. We observed NGF-induced hyperplasia of ISCs, thus increasing the amount of daughter EC cells following differentiation. Moreover, we observed that NGF directed ISC differentiation towards secretory lineages, resulting in even greater density of EC cells and vastly elevated 5-HT secretion, thus driving IBS-like pathogenesis. Since the aforementioned cell-non-autonomous mechanism is (1) used in multiple organ systems and (2) present in both physiological and pathological settings, it is likely that cell-autonomous pathway may also be present in other contexts and warrants further study [Citation27–Citation30]. Both Hayakawa’s and our mechanisms converge on the role of NGF and subsequent Wnt-signaling as key mediators of cell proliferation, and given the well-described Wnt signaling as well as the ISC niche in colorectal cancer, it is worth investigating the capability of NGF to drive tumorigenesis via direct stimulation of ISC proliferation.
An NGF-centric mechanism of IBS-like pathogenesis
Our mechanism may serve to be key in answering why or how patients who have suffered through early life stress lie particularly susceptible to GI disorders including IBS. The pleiotropic effect of enteric NGF on both nerve growth as described by Barreau et al., and our observed direct effect on ISC stem cell proliferation may exert a synergistic effect to drive IBS pathogenesis in patients as follows; as per our proposed mechanism, NGF acting directly on the ISC compartment drives its expansion and differentiation into secretory cells, EC cells in particular, leading to increased 5-HT production. In the GI tract, 5-HT can act to modulate gut motility and transit, acting on 5-HT3 or 5-HT4 receptors G-protein-coupled receptor to increase peristaltic transit through the gut [Citation8,Citation10–Citation13]. 5-HT may also act on the nociceptive process, and primary afferent sensory neurons in the gut viscera also express 5-HT3 and 4 receptors which may respond to local 5-HT levels to modulate pain-sensing/perception/transmission, although the exact mechanisms and effects of 5-HT at the level of ENS nociception are still not well understood [Citation14–Citation16]. NGF acting non-cell-autonomously to drive neuronal innervation and synaptogenesis may also then result in a hyper-innervated GI tract which may thus lead to exacerbated visceral hyperalgesia and GI motility in the presence of elevated 5-HT levels or may further contribute to local 5-HT levels through neurotransmitter secretion. This highly synergistic effect of NGF acting on the two different target cell types, via two distinct mechanisms, may account for the high prevalence of IBS following early life stress.
NGF-Wnt cross talk in non-neuronal cells
Interestingly, it was found that NGF induced ISC expansion via cross talk between MAPK and Wnt-signaling pathways to elicit transcriptional changes driving self-renewal and differentiation [Citation24]. While we cannot exclude the possibility that additional effects may be driven downstream by canonical MAPK activity, our study observed the ability of NGF to transactivate and augment Wnt signaling, demonstrating that NGF alone could elicit increased β-catenin transcriptional activity similar to Wnt3a alone [Citation31]. This was further evidenced in an in vitro organoid culture where NGF could partially rescue intestinal organoid growth and morphology both in the absence of Wnt-signaling activator R-spondin-1 or in the presence of Wnt antagonist IWP-2, demonstrating that the ability of NGF to augment Wnt signaling was independent of Wnt ligands [Citation32,Citation33]. Along this line, we have observed Wnt-independent phosphorylation of LRP5/6, a critical co-receptor for Frizzled [Citation34], suggestive of ligand-independent activation of the receptor complex by NGF. Furthermore, the addition of NGF biased cell fate of these ISCs towards secretory lineages such as EC cells, Paneth cells and enteroendocrine cells, highlighting the positive effect of NGF on both ISC proliferation and differentiation [Citation35].
Whereas the effect of NGF on growth and differentiation of neural stem cells has been well described, its effects on other stem cell types have not been well characterized [Citation36–Citation38]. Although these effects were only tested on ISCs in our study, it is possible that similar cross talk events occur in other contexts. Indeed, the recent findings of Di Donato et al., demonstrating reciprocal cross talk between NGF and the androgen receptor (AR) in prostate cancer via AR and TrkA, serves to evidence the importance of considering NGF activity in its full capacity and context [Citation39,Citation40]. In a broader view, these findings highlight the need to consider the possibility of NGF exerting effects on non-neuronal stem cell types as well as the ability of NGF to interact with other, non-canonical signaling pathways in both physiological and pathological contexts.
Source of the NGF and the role of the brain
While we have uncovered a mechanism by which NGF drives IBS-like pathogenesis, the events upstream of NGF synthesis, and indeed the source of the NGF itself, remain unclear, and further investigation across multiple disciplines is required to fully delineate the sequence of events driving IBS development. We observed a positive correlation between serum NGF and 5-HT levels in diarrhea-dominant IBS (IBS-D) patients, suggesting the possibility of systemic elevations of both NGF and 5-HT in disease. While 5-HT has been reported to exert multifarious effects on the immune and vascular systems and even in tissue regeneration, it is unclear if the elevated serum 5-HT levels are physiologically significant enough to modulate their functions [Citation41–Citation48]. Future work will aim to uncover if these elevations are also observed in other organs, and whether they are physiologically relevant outside of the GI tract, since these patients suffer predominantly from GI symptoms.
A current working model proposed by multiple groups suggests that stress may activate the hypothalamic–pituitary–adrenal axis, leading to release of corticotrophin-releasing factor (CRF) in the central nervous system and the ENS [Citation49,Citation50]. In the latter case, the released CRF may act on CRF1 and CRF2 receptors along the GI tract to drive NGF synthesis and secretion from intestinal mast cells [Citation51,Citation52]. Their subsequent findings on elevated neuron density, as well as a model proposed by Xu et al., follow the aforementioned cell-non-autonomous, neuron-dependent mechanism to drive IBS symptoms [Citation50]. Likewise, our mechanism may also rely on this mast-cell-derived pool of NGF; however, future work will be necessary to determine if this is true.
Clinical implications
Our findings suggest that inhibition of NGF/TrkA signaling may be a potential treatment against IBS, even in adulthood. Recombinant NGF drives not only proliferation and growth of mouse but also human intestinal organoids. This is strengthened by our clinical data showing the correlation between serum NGF and 5-HT levels, and both NGF and 5-HT are significantly upregulated in IBS-D relatives to healthy patients, which lies in accordance with existing published findings [Citation53]. Together this suggests that the effect of NGF seen in mice models is, at least in some capacity, observed and therefore translatable in humans as well. In addition, while NMS was carried out on newborn mice from P3-P14, intraperitoneal administration of MNAC13, a well-characterized neutralizing antibody against TrkA, in 10-wk-old adult NMS-challenged mice significantly ablated EC cell hyperplasia in the colonic mucosa [Citation22,Citation23]. Thus, despite our observed, long-lasting effect of early life trauma on colonic 5-HT levels in NMS versus control mice, intervention in the adult stage was still able to revert the physiological changes induced by early life trauma, and behavioral/visceral sensitivity tests are in the pipeline to determine if the reversion of EC cell hyperplasia in adulthood will yield functional alleviation of IBS symptoms [Citation18,Citation54]. Altogether our findings implicate NGF as a potential therapeutic target and suggest that inhibition of NGF/TrkA signaling may be a novel avenue towards the treatment of IBS.
Stress is induced in newborn mice pups via 3 h/d separation from their mother, for 12 d. NGF levels in the intestinal mucosa are elevated in response to stress, which then act on TrkA receptors on ISCs, in which activated NGF/TrkA signaling transactivates Wnt signaling. This drives the proliferation of ISCs and their subsequent differentiation to 5-HT-secreting EC cells, leading to elevated 5-HT production and promoting IBS-like pathogenesis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Drossman DA, Hasler WL. Rome IV – functional GI disorders: disorders of gut-brain interaction. Gastroenterology. 2016;150:1257–1261.
- Mokha JS, Hyams JS. Irritable bowel syndrome. In: Faure C, Thapar N, Di Lorenzo C, editors. Pediatric neurogastroenterology. Springer International Publishing; 2017. p. 399–410. DOI:10.1007/978-3-319-43268-7_37.
- Barbara G, Cremon C, Carini G, et al. The immune system in irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:349–359.
- Lazaridis N, Germanidis G. Current insights into the innate immune system dysfunction in irritable bowel syndrome. Ann Gastroenterol. 2018;31:171–187.
- Rodiño-Janeiro BK, Vicario M, Alonso-Cotoner C, et al. A review of microbiota and irritable bowel syndrome: future in therapies. Adv Ther. 2018;35:289–310.
- Menees S, Chey W. The gut microbiome and irritable bowel syndrome. F1000Res. 2018;7:1029.
- Distrutti E, Monaldi L, Ricci P, et al. Gut microbiota role in irritable bowel syndrome: new therapeutic strategies. World J Gastroenterol. 2016;22:2219.
- Crowell MD. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br J Pharmacol. 2004;141:1285–1293.
- Wood JD. Enteric nervous system, serotonin, and the irritable bowel syndrome. Curr Opin Gastroenterol. 2001;17:91–97.
- TALLEY NJ. Review article: 5-hydroxytryptamine agonists and antagonists in the modulation of gastrointestinal motility and sensation: clinical implications. Aliment Pharmacol Ther. 2007;6:273–289.
- GORE S, Gilmore IT, Haigh CG, et al. Colonic transit in man is slowed by ondansetron (GR38032F), a selective 5-hydroxytryptamine receptor (type 3) antagonist. Aliment Pharmacol Ther. 2007;4:139–144.
- Houghton LA, Foster JM, Whorwell PJ. Alosetron, a 5-HT3 receptor antagonist, delays colonic transit in patients with irritable bowel syndrome and healthy volunteers. Aliment Pharmacol Ther. 2000;14:775–782.
- Humphrey PP, Bountra C, Clayton N, et al. Review article: the therapeutic potential of 5-HT3 receptor antagonists in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 1999;13:31–38.
- Muller-Lissner SA, Fumagalli I, Bardhan KD, et al. Tegaserod, a 5-HT4 receptor partial agonist, relieves symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation. Aliment Pharmacol Ther. 2001;15:1655–1666.
- PRIOR A, READ NW. Reduction of rectal sensitivity and post-prandial motility by granisetron, a 5 HT3-receptor antagonist, in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2007;7:175–180.
- Grundy D. Towards a reduction of rectal pain? Neurogastroenterol Motil. 2002;14:217–219.
- Mavrangelos C, Campaniello MA, Andrews JM, et al. Longitudinal analysis indicates symptom severity influences immune profile in irritable bowel syndrome. Gut. 2018;67:398–399.
- Wong HLX, Qin H-Y, Tsang SW, et al. Early life stress disrupts intestinal homeostasis via NGF-TrkA signaling. Nat Commun. 2019;10:1745.
- Bradford K, Shih W, Videlock EJ, et al. Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol Hepatol. 2012;10:385–390.e3.
- Talley NJ, Fett SL, Zinsmeister AR, et al. Gastrointestinal tract symptoms and self-reported abuse: a population-based study. Gastroenterology. 1994;107:1040–1049.
- Drossman DA. Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Ann Intern Med. 1990;113:828.
- Covaceuszach S, Cattaneo A, Lamba D. Neutralization of NGF-TrkA receptor interaction by the novel antagonistic anti-TrkA monoclonal antibody MNAC13: a structural insight. Proteins Struct Funct Bioinforma. 2004;58:717–727.
- Ugolini G, Marinelli S, Covaceuszach S, et al. The function neutralizing anti-TrkA antibody MNAC13 reduces inflammatory and neuropathic pain. Proc Natl Acad Sci. 2007;104:2985–2990.
- Bothwell M. Recent advances in understanding neurotrophin signaling. F1000Res. 2016;5:1885.
- Hansebout, C. R., Su C, Reddy K, et al. Enteric glia mediate neuronal outgrowth through release of neurotrophic factors. Neural Regen Res. 2012;7:2165–2175.
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc B Biol Sci. 2006;361:1545–1564.
- Barreau F, Salvador-Cartier C, Houdeau E, et al. Long-term alterations of colonic nerve-mast cell interactions induced by neonatal maternal deprivation in rats. Gut. 2008;57:582–590.
- Hayakawa Y, Sakitani K, Konishi M, et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell. 2017;31:21–34.
- Borden P, Houtz J, Leach SD, et al. Sympathetic innervation during development is necessary for pancreatic islet architecture and functional maturation. Cell Rep. 2013;4:287–301.
- Renz BW, Takahashi R, Tanaka T, et al. β2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell. 2018;33:75–90.e7.
- Xing J, Kornhauser JM, Xia Z, et al. Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol Cell Biol. 1998;18:1946.
- de Lau WB, Snel B, Clevers HC. The R-spondin protein family. Genome Biol. 2012;13:242.
- Chen B, Dodge ME, Tang W, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107.
- MacDonald BT, He X. Frizzled and LRP5/6 receptors for Wnt/β-catenin signaling. Cold Spring Harb Perspect Biol. 2012;4:a007880-a007880.
- Yan KS, Gevaert O, Zheng GXY, et al. Intestinal enteroendocrine lineage cells possess homeostatic and injury-inducible stem cell activity. Cell Stem Cell. 2017;21:78–90.e6.
- Heese K, Low JW, Inoue N. Nerve growth factor, neural stem cells and Alzheimer’s disease. Neurosignals. 2006;15:1–12.
- Antonov SA, Manuilova ES, Dolotov OV, et al. Effect of nerve growth factor on neural differentiation of mouse embryonic stem cells. Bull Exp Biol Med. 2017;162:679–683.
- Xiong -L-L, Chen Z-W, Wang T-H. Nerve growth factor promotes in vitro proliferation of neural stem cells from tree shrews. Neural Regen Res. 2016;11:591–596.
- Di Donato M, Cernera G, Auricchio F, et al. Cross-talk between androgen receptor and nerve growth factor receptor in prostate cancer cells: implications for a new therapeutic approach. Cell Death Discov. 2018;4:5.
- Di Donato M, Bilancio A, D’Amato L, et al. Cross-talk between androgen receptor/filamin A and TrkA regulates neurite outgrowth in PC12 cells. Mol Biol Cell. 2015;26:2858–2872.
- Michalopoulos GK, Regeneration L. Science. 1997;276:60–66.
- Lesurtel M. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104–107.
- KAUMANN A, LEVY F. 5-Hydroxytryptamine receptors in the human cardiovascular system. Pharmacol Ther. 2006;111:674–706.
- Dale GL, Friese P, Batar P, et al. Stimulated platelets use serotonin to enhance their retention of procoagulant proteins on the cell surface. Nature. 2002;415:175–179.
- Jenne CN, Kubes P. Platelets in inflammation and infection. Platelets. 2015;26:286–292.
- Amireault P, Bayard E, Launay J-M, et al. Serotonin is a key factor for mouse red blood cell survival. PLoS One. 2013;8:e83010.
- Lv J, Liu F. The role of serotonin beyond the central nervous system during embryogenesis. Front Cell Neurosci. 2017;11:74.
- Watts SW, Morrison SF, Davis RP, et al. Serotonin and blood pressure regulation. Pharmacol Rev. 2012;64:359–388.
- Barreau F, Cartier C, Leveque M, et al. Pathways involved in gut mucosal barrier dysfunction induced in adult rats by maternal deprivation: corticotrophin-releasing factor and nerve growth factor interplay. J Physiol. 2007;580:347–356.
- Xu X, Liu L, Yao S. Nerve growth factor and diarrhea-predominant irritable bowel syndrome (IBS-D): a potential therapeutic target? J Zhejiang Univ B. 2016;17:1–9.
- Taché Y, Million M. Role of corticotropin-releasing factor signaling in stress-related alterations of colonic motility and hyperalgesia. J Neurogastroenterol Motil. 2015;21:008–024.
- Yuan P-Q, Wu SV, Pothoulakis C, et al. Urocortins and CRF receptor type 2 variants in the male rat colon: gene expression and regulation by endotoxin and anti-inflammatory effect. Am J Physiol Liver Physiol. 2016;310:G387–G398.
- Dothel G, Barbaro MR, Boudin H, et al. Nerve fiber outgrowth is increased in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2015;148:1002–1011.e4.
- Chung EKY, Zhang X-J, Xu H-X, et al. Visceral hyperalgesia induced by neonatal maternal separation is associated with nerve growth factor–mediated central neuronal plasticity in rat spinal cord. Neuroscience. 2007;149:685–695.