ABSTRACT
REGγ is a member of the 11S regulatory particles family of proteasome activators and has been shown to promote the degradation of intact cellular proteins in a ubiquitin- and ATP-independent manner in the progression of various diseases. Our previous studies showed that REGγ-proteasome promotes Protein kinase A catalytic subunit α (PKAcα) turnover to modulate Forkhead box protein O1 (FoxO1) cellular activity in vascular endothelial cell migration and angiogenesis. We, therefore, studied the expression and novel functional implications and pathways involving REGγ in atherogenesis. We studied the expression of REGγ in atherosclerotic plaques in the ApoE-/- mouse model. Using immunohistochemistry, we showed that REGγ was highly expressed in these plaques, and the result of RNA-seq in Human umbilical vein endothelial cells (HUVECs), led us to explore and indentify that REGγ significantly promoted cyclophilin A (CyPA) expression, which is a proinflammatory and proapoptotic molecule in atherosclerosis progression. Next, we studied the regulation of REGγ in CyPA expression, and the proapoptotic effect on Endothelial cells (ECs). REGγ promoted CyPA expression via the REGγ-PKA-FoxO1-CyPA axis, and stimulated CyPA-dependent ECs apoptosis in vitro. Our data indicated that REGγ had proapoptotic effects on ECs depends on CyPA pathway in vitro and functional implications in atherogenesis in vivo.
Introduction
ECs apoptosis, migration and permeability play significant roles in the focal pathogenesis and development of several vascular inflammatory and cardiovascular diseases, including atherosclerosis, and in vascular remodeling. Atherosclerosis is a disease of the vasculature that is characterized by chronic inflammation of the arterial wall [Citation1], and involving complex interactions with modified lipoproteins, monocyte-derived macrophages, or foam cells, T lymphocytes, ECs, and smooth muscle cells (SMCs) [Citation2,Citation3].The development of atherosclerosis is initiated by the activation of ECs, oxidized LDL (oxLDL), and other triggers that induce dysfunction of ECs, which leads to increased adhesiveness of ECs to leukocytes and production of adhesion molecules and proinflammatory cytokines, including E-selectin, vascular cell adhesion molecule (VCAM)-1 [Citation4] and monocyte chemotactic protein-1 (MCP-1), leading to recruitment of inflammatory cells [Citation5] into the intima. In addition, the activated ECs facilitate the passage of lipid components (such as LDL) in plasma. These recruited inflammatory cells uptake oxLDL to become foam cells in arterial lesions [Citation6]. The interaction between ECs and VSMCs plays an important role in regulating vascular integrity. ECs secrete several vasoactive substances, including nitric oxide (NO) and prostacyclin, which maintain vascular integrity and limit intima formation, and protect against vascular remodeling [Citation7–Citation9].
REGγ (also known as PA28γ; PSME3) is a member of the 11S family of proteasome activators. It has been shown to promote the degradation of several intact cellular proteins in a ubiquitin- and ATP-independent manner, which differs from the ubiquitin proteasome pathway, involving SRC-3, p21, PKAcα, CK1δ, SirT1, ikBε, GSK3β, SirT7, KLF2, and c-Myc, among others [Citation10–Citation13], in the regulation of a broad range of important physiological processes, including cancer progression [Citation14–Citation16], aging [Citation17], hepatic lipid and energy metabolism [Citation18,Citation19], angiogenesis [Citation20], oxidative responses [Citation21], bacterial infection [Citation22], innate immunity and inflammatory diseases [Citation23,Citation24], and may also play a role in the regulation of viral and cardiovascular diseases [Citation25].
CyPA is a ubiquitously distributed protein belonging to the immunophilin family and is recognized as the intracellular receptor for the potent immunosuppressive drug cyclosporine A [Citation26]. CyPA possesses peptidyl-prolyl isomerase (PPIase) activity that regulates cis-trans isomerization of Xaa-Pro peptide bonds [Citation27]. In recent years, CyPA (both intracellular and extracellular) has been described as having a key role in inflammation and cardiovascular disease pathologies. ROS and inflammation-related stimuli, including cardiovascular diseases, promote CyPA expression and secretion from vascular [Citation28–Citation31], CyPA stimulates proinflammatory signals in ECs, induces expression of adhesion molecules, including E-selectin and VCAM-1, and activates mitogen-activated protein kinases, including ERK1/2, JNK, and p38, stimulates IkBα phosphorylation and NF-kB activation, and induces EC apoptosis in vitro [Citation4,Citation29]. In addition to effects on vascular cells, CyPA has been shown to be a potent leukocyte chemoattractant for inflammatory cells (human monocytes, neutrophils, eosinophils, and T cells) [Citation32–Citation40]. Furthermore, vascular-derived CyPA plays a crucial role in vascular remodeling and intima formation, the development of angiotensin II-induced abdominal aortic aneurysms (AAA), and the progression of atherosclerosis in vivo [Citation5,Citation41–Citation43].
In this study, we hypothesized that REGγ had functional implications in atherogenesis, and based on the result of RNA-seq in HUVECs, we indentified that REGγ significantly promoted the important proinflammatory and proapoptotic molecule in atherosclerosis progression-CyPA expression. Here, we showed that REGγ was highly expressed in atherosclerotic plaques from ApoE-/- mice, and that REGγ stimulated the expression of CyPA via the REGγ-PKA-FoxO1-CyPA axis, and induced CyPA-dependent ECs apoptosis in vitro and had functional implications in atherogenesis in vivo.
Materials and methods
Mice
ApoE −/− mice with C57BL/6 genetic background were purchased from Jackson Laboratory. Mice were bred in the Animal Core Facility by following procedures approved by the Baylor College of Medicine Institutional Animal Care and Use Committee.
Cell culture, expression constructs, and reagents
HUVECs were purchased from ScienCell Research Laboratories (Carlsbad, CA, USA) and were cultured in endothelial cell medium ECM (ScienCell Research Laboratories). REGγ WT (REGγ+/+), REGγ Knockout (REGγ−/−) mouse embryonic fibroblast (MEF) cells and human embryonic kidney 293 (HEK293) shN (shRNA-Negative control), shR (shRNA targeting REGγ) cells were previously generated [Citation12], and were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Invitrogen). Plasmids pcDNA3.1-GFP-CyPA in this study was constructed, pcDNA3. 1-Flag- REGγ, pcDNA3. 1-Flag/GFP-PKAcα was constructed previously [Citation20] and pcDNA3.1-Flag/GFP-FoxO1 WT, pcDNA3.1-Flag/GFP-FoxO1 A3 (Two Ser and one Thr-three phosphation sites mutated to Ala, expressing constitutively active gain-of-function FoxO1 mutant), and pcDNA3.1-Flag/GFP-FoxO1 ∆DB (deletion of the DNA binding domain, expressing loss-of-function FoxO1 mutant) plasmids were kindly gifted by Dr. Weiguo Zhu, Peking University Health Science Center.Dimethyl sulfoxide (DMSO), Forskolin (FSK), H89, and cycloheximide (CHX) were obtained from Sigma, and 4’,6-diamidino-2-phenylindole (DAPI) was obtained from Invitrogen. The following antibodies were used in this study: anti-β-actin antibody (Sigma), anti-Flag antibody (Cell Signaling), anti-GFP antibody (Cell Signaling), anti-REGγ antibody (Abcam), anti-CyPA antibody (Abcam), anti-PKAcα antibody (Cell Signaling), anti-FoxO1 antibody (Cell Signaling), anti-phosphorylated FoxO1 (Ser256) antibody (Cell Signaling), anti-VCAM-1 antibody (R&D Systems), anti-Immunoglobulin G (IgG) (Cell Signaling), and chromatin immunoprecipitation (ChIP)-grade anti-FoxO1 antibody (Abcam). Real time quantitative-polymerase chain reaction (qPCR) and reverse transcription semi-quantitative polymerase chain reaction (RT-sqPCR) were performed using a real-time PCR kit (TAKARA) and Ex Taq PCR kit (TAKARA), respectively, cell viability assays were performed using CellTiter-Glo® Luminescent Cell Viability Assay Kit (Promega), and TdT-UTP nick end labeling (TUNEL) assays were performed using the one step TUNEL kit (Roche).
Immunohistochemistry
Immunohistochemistry was performed as described previously [Citation15]. Atherosclerotic plaques were obtained from ApoE-/-mice fed a Western diet for 12 weeks.
RNA-seq and data analysis
HUVECs were transfected with REGγ-specific siRNA and a negative oligonucleotide siRNA (siNeg) (each n = 3). Total RNA was isolated after 48 hours and the RNA-seq reads, gene expression profile and quantification, and differential analysis to identify genes with significant expression changes between siREGγ and siNeg groups were assessed and performed in WuXi NextCODE (Shanghai) as described [Citation44–Citation47].
Immunoblotting, real-time qPCR and RT-sqRCR
Immunoblotting, real-time qPCR, and RT-sqRCR were performed as described [Citation10,Citation12].
siRNA and plasmid transfection
REGγ, PKAcα, FoxO1 and negative control siRNA duplexes were purchased from Dharmacon (Lafayette, CO, USA). siRNA targeting REGγ (siREGγ/siR, catalog #L-012133–00-0010), PKAcα (siPKAcα, catalog #L-004649–00), FoxO1 (siFoxO1, catalog #M-003006–01-05) or negative control siRNA (siNeg/siN, catalog #D-001810–10-20) were procured. All cells in this study were transfected with siRNAs using Lipofectamine™ RNAiMAX (Invitrogen), and with the respective plasmids using Lipofectamine™ LTX reagent with PLUS™ reagent (Invitrogen), according to the manufacturer’s instructions.
Luciferase assay
The pGL3-CyPA-2000 promoter Luc reporter gene construct was generated using a plasmid pGL3-basic (Promega) containing the luciferase gene and a 2.0-kb human CyPA promoter fragment to evaluate the transcriptional activity of the CyPA promoter. HUVECs were transiently transfected with the reporter gene construct and the FoxO1 plasmid, pretreated with a FoxO1 inhibitor FSK, or pre-transfected with siFoxO1. The Luciferase assay was performed as described [Citation24,Citation48].
ChiP assay
The ChIP assay was performed as described [Citation24], by incubating the cell lysates with ChIP-grade anti-FoxO1 antibody or IgG antibody. The precipitated DNA was amplified and quantified by performing qPCR with primers corresponding to an approximately 250-bp fragment of the human CyPA promoter containing forkhead-response element (FHRE) (forward primer, 5’-AAGGTGGGACGCTTGCTCGAGCA-3’ and reverse primer, 5’-TCCGTCTCAAATAAATAAACAAA-3’) and to the GAPDH promoter (off-target site control) and with an IgG antibody (negative antibody control).
Cell viability assays
HUVECs were grown in six-well plates, transfected with the specific siRNAs or plamids for the indicated time periods, and dissociated. Next, the dissociated transfected cells were seeded at the same density into a 96-well plate, cultured for 4 h, and treated with CHX for 24 h at 37°C. Cell viability assays were performed using the CellTiter-Glo® Luminescent Cell Viability Assay Kit, according to the manufacturer’s instructions.
Apoptosis assays
Apoptotic cells were identified by performing morphological staining of nuclei or TUNEL assays. For morphological staining of nuclei, HUVECs were fixed in 3.7% formaldehyde, stained with DAPI for 10 min, and photographed using a fluorescence microscope (Lecia). Apoptotic cells were identified based on their typical morphological appearance, including chromatin condensation and nuclear fragmentation. Cell tunel staining were performed using the Tunel Assay Kit, according to the manufacturer’s instructions. The TUNEL assays were performed with the one step TUNEL kit according to the manufacturer’s instructions. The FITC-labeled TUNEL-positive cells were photographed using a fluorescence microscope (Lecia). The cells with green fluorescence were defined as apoptotic cells.
Statistical analysis
All experiments were repeated at least three independent times. Data are expressed as mean ± SD. For experiments concerning multiple groups, one-way ANOVA with post-hoc Tukey test were performed to evaluate the differences. The differences between two (control and experimental) groups were determined by two-tailed Student’s t-test. A p value of <0.05 was considered to be statistically significant (*,p < 0.05, **,p < 0.01, and ***,p < 0.001). .
Results
REGγ is expressed in ApoE-/- mouse atherosclerotic plaques and REGγ induces CyPA expression
To determine whether REGγ had functional implications in atherogenesis, immunohistochemistry of atherosclerotic plaques from ApoE-/- mice (n = 6) fed a Western diet for 12 weeks was performed. As shown in ) (arrow indicated), (b) (magnification of the boxed area in a) and (c) (statistical analysis of relative REGγ protein expression intensity of plaque area per vascular area normalized by plaque adjacent area), REGγ was highly expressed in atherosclerotic plaques, indicating that REGγ had functional implications in atherogenesis.
Figure 1. REGγ is expressed in ApoE-/- mouse atherosclerotic plaques and induces CyPA expression. REGγ is expressed in ApoE-/- mouse atherosclerotic plaques (n = 6). (a) (arrow indicated), (b) (magnification of the boxed area in (a) and (c) (statistical analysis of relative REGγ protein expression intensity of plaque area per vascular area normalized by plaque adjacent area), REGγ was highly expressed in atherosclerotic plaques from ApoE-/-mice fed a Western diet for 12 weeks. REGγ induces CyPA expression. Knockdown of REGγ with specific shRNA or siRNA transfection, or knockout of REGγ in mice, significantly decreased CyPA mRNA expression by real time-qPCR (d and e) and RT-sqPCR (f and g) detection in indicated cells and mouse tissues. Knockdown or knockout of REGγ significantly decreased CyPA protein expression in the indicated cells and mouse tissues (h and i). Overexpression of REGγ with Flag- REGγ plasmid transfection dramatically increased CyPA expression by real time-qPCR (j), RT-sqPCR (k) and Immunoblotting (l) detection in indicated cells. Data were presented as mean ± SD of 3 independent experiments. ***,p < 0.001 (two-tailed Student’s t-test).
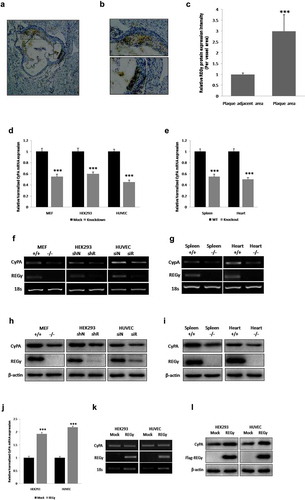
Next, to determine and indentify the result of RNA-seq in HUVECs, whether REGγ significantly promoted the improtant proinflammatory and proapoptotic molecule in atherosclerosis progression-CyPA expression, we established intracellular REGγ knockdown with specific shRNA or siRNA transfection, and REGγ knockout mouse tissue for CyPA expression by real time-qPCR, RT-sqPCR, and immunoblotting. As shown in , knockdown or knockout of REGγ significantly decreased CyPA mRNA expression, with real time-qPCR ( and ), and RT-sqPCR ( and ) detection in indicated cells and mouse tissues.
Immunoblotting was performed to confirm the mRNA results, as shown in , knockdown or knockout of REGγ significantly decreased CyPA protein expression in indicated cells and mouse tissues ( and ).
To ensure the specificity of REGγ knockdown or knockout regulation of CyPA expression, we overexpressed REGγ by transfecting indicated cells with Flag-REGγ plasmid and determined the effect of REGγ overexpression on CyPA expression. Consistent results were observed, REGγ overexpression dramatically increased CyPA expression, with real time-qPCR ()), RT-sqPCR ()),and Immunoblotting()) detection. REGγ-knockdown, -knockout and -overexpression efficiency were quantified by real time-qPCR assay (Figure S1).
FoxO1 promotes CyPA expression
Previously, we had found REGγ activated 20S proteasomes to promote PKAcα degradation and inhibited the phosphorylation at Ser256 and nuclear export of FoxO1, which increased FoxO1 nuclear accumulation and transcriptional activity in vascular ECs migration and angiogenesis [Citation20]. We hypothesized whether REGγ also acted via the PKA-FoxO1 axis to regulate CyPA expression.
First, to determine whether FoxO1 regulated CyPA expression, we examined the effect of FoxO1 knockdown, or overexpression with siFoxO1, or the indicated plasmids transfection, respectively, on CyPA expression by performing real time-qPCR, RT-sqPCR, and immunoblotting. FoxO1 knockdown by transfection with siFoxO1 significantly decreased CyPA mRNA expression according to the results of real time-qPCR ()).Consistently, CyPA mRNA expression was significantly upregulated in cells expressing WT FoxO1 and constitutively active FoxO1 A3, but not in cells expressing loss-of-function FoxO1 ∆DB, as indicated by the results of real time-qPCR ()). RT-sqPCR was performed to confirm the results of real time-qPCR ( and ), the RT-sqPCR results were consistent with the real time-qPCR shown. FoxO1-knockdown and -overexpression efficiency were quantified by real time-qPCR assay (Figure S2). FoxO1-knockdown, WT FoxO1-, FoxO1 A3- and FoxO1 ∆DB-overexpressing cells were evaluated by performing RT-sqPCR and agarose gel electrophoresis (Figure S3).
Figure 2. FoxO1 promotes CyPA expression. FoxO1 knockdown by using siFoxO1 significantly decreased CyPA mRNA expression, as determined by performing (a) real time-qPCR and CyPA mRNA expression was significantly upregulated in cells expressing WT FoxO1 and constitutively active FoxO1 A3, but not in cells expressing loss-of-function FoxO1 ∆DB, as determined by performing (b) real time-qPCR and real time-qPCR results were confirmed by performing corresponding (c and d) RT-sqPCR, which were consistent with the real time-qPCR shown. (e and f) FoxO1 knockdown with siFoxO1 significantly decreased CyPA protein expression in the indicated cells. (g and h) CyPA protein expression was significantly upregulated in cells expressing the constitutively active FoxO1 A3. Data were presented as mean ± SD of 3 independent experiments. ***,p < 0.001 (two-tailed Student’s t-test).
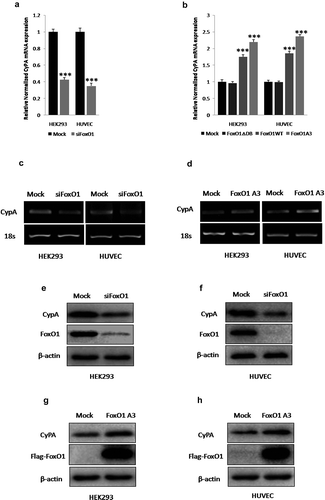
Immunoblotting was performed to confirm the results of mRNA expression (). FoxO1 knockdown by transfection with siFoxO1, significantly decreased CyPA protein expression ( and ). Consistently, CyPA protein expression was significantly upregulated in cells expressing constitutively active FoxO1 A3 ( and ).
FoxO1 stimulates the transcriptional activity of the CyPA promoter and binds to its specific region containing FHRE
To determine whether FoxO1 promoted CyPA expression by stimulating the transcriptional activity of and binding to a specific site in the CyPA promoter, we performed the luciferase and ChIP assays. FoxO1 inhibition or knockdown by treatment with FSK or transfection with siFoxO1, respectively, significantly decreased the basal transcriptional activity of the CyPA promoter, as assessed by performing the reporter gene assay by using an upstream 2,000-bp fragment of the human CyPA gene ( and ). Consistently, overexpression of WT FoxO1 and constitutively active FoxO1 (FoxO1 A3) but not of loss-of-function FoxO1 (FoxO1 ∆DB) increased the basal transcriptional activity of the CyPA promoter ()). FoxO1-knockdown, WT FoxO1-, FoxO1 A3- and FoxO1 ∆DB-overexpressing cells were evaluated by performing RT-sqPCR and agarose gel electrophoresis (Figure S3).
Figure 3. FoxO1 stimulates the transcriptional activity of and binds to a specific region containing the FHRE in the CyPA promoter. FoxO1 promotes the transcriptional activity of the CyPA promoter. (a and b) FoxO1 inhibition or knockdown by using FSK or siFoxO1, respectively, significantly decreased the basal transcriptional activity of the CyPA promoter, as assessed by performing the reporter gene assay by using the upstream 2,000-bp fragment of the human CyPA gene. (c) Overexpression of WT FoxO1 and constitutively active FoxO1 A3, but not of loss-of-function FoxO1 ∆DB increased the basal transcriptional activity of the CyPA promoter.FoxO1 binds to the specific region in the CyPA promoter. (d) The results of the ChIP assay showed that FoxO1 associated with the CyPA promoter at a region containing the FHRE that was 2,000-bp upstream of the transcription start site. (d) However, no binding was observed when the GAPDH promoter off-site control primers or negative antibody control IgG antibody was used. Data were presented as mean ± SD of 3 independent experiments. ***,p < 0.001 (two-tailed Student’s t-test).
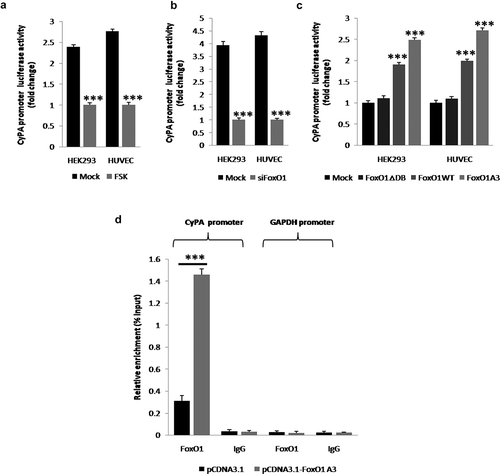
Results of promoter analysis indicated that the CyPA promoter contained a conserved optimal FHRE at position −2,000 relative to the transcription start site (TTGTTTAT). Results of the ChIP assay showed that the overexpression of constitutively active FoxO1 (FoxO1 A3) increased CyPA promoter enrichment relative to the basal level ()), indicating that FoxO1 directly interacted with the CyPA promoter region containing the FHRE that was present 2,000 bp upstream of the transcription start site ()). However, no binding was detected when off-target control primers corresponding to the GAPDH promoter or negative antibody control IgG antibody was used ()), thus confirming the specificity of the interaction between FoxO1 and the CyPA promoter.
PKA inhibites CyPA expression
As we had demonstrated that FoxO1 regulated CyPA expression by binding to a specific region containing the forkhead-responsive element in its promoter.Then, to determine whether PKA regulated CyPA expression, we examined the effects of PKA inhibition, knockdown, activation, or overexpression with H89 treatment, siPKAcα transfection, FSK treatment or the PKAcα plasmid transfection, respectively, on CyPA expression by performing real time-qPCR, RT-sqPCR, and immunoblotting, with VCAM-1, which is regulated by PKA, as the positive control. PKA inhibition or knockdown by treatment with H89, or transfection with siPKAcα, respectively, significantly increased CyPA mRNA expression, according to real time-qPCR results ( and ). Consistently, CyPA mRNA expression was significantly downregulated in cells treated with PKA activator FSK in a time-dependent manner or expressing PKAcα, as indicated by the results of real time-qPCR () and ). RT-sqPCR () was performed to confirm the results of real time-qPCR. The RT-sqPCR results were consistent with those obtained by real time-qPCR.
Figure 4. PKA inhibites CyPA expression. PKA inhibition or knockdown by treatment with H89, or transfection with siPKAcα, respectively, significantly increased CyPA mRNA expression according to the results of real time-qPCR (a and b). CyPA mRNA expression was significantly downregulated in cells treated with PKA activator FSK in a time-dependent manner or expressing PKAcα, as indicated by the results of real time-qPCR (c and d). RT-sqPCR was performed to confirm the results of real time-qPCR (E, F, G and H), with VCAM-1, which is regulated by PKA, as the positive control. Immunoblotting was performed to confirm the results of mRNA expression measurements, with VCAM-1, which is regulated by PKA, as the positive control. PKA inhibition or knockdown by treatment with H89 or transfection with siPKAcα, respectively, significantly increased CyPA protein expression (i and j). CyPA protein expression was significantly downregulated in cells treated with PKA activator FSK in a concentration-dependent manner or expressing PKAcα (K and L). Data were presented as mean ± SD of 3 independent experiments. **,p < 0.01, and ***,p < 0.001 (two-tailed Student’s t-test).
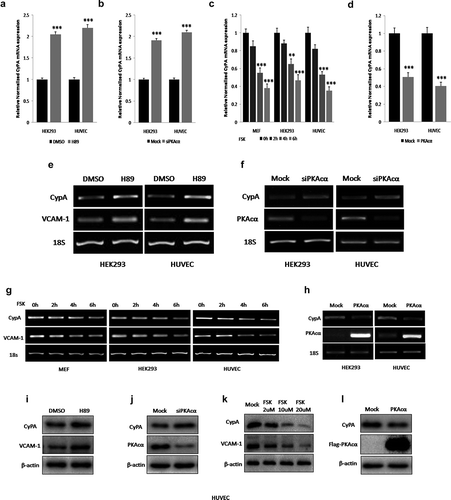
Immunoblotting was performed to confirm the results of mRNA expression detection (). PKA inhibition or knockdown by treatment with H89, or transfection with siPKAcα, respectively, significantly increased CyPA protein expression ( and ). Consistently, CyPA protein expression was significantly downregulated in cells treated with PKA activator FSK in a concentration-dependent manner or expressing PKAcα ( and ).
PKA inhibites CyPA expression via FoxO1 pathway
To further determine whether PKA caused decreased CyPA expression via FoxO1, we used intracellular PKAcα inhibition, knockdown, activation or overexpression with H89 treatment, siPKAcα transfection, FSK treatment or the PKAcα plasmid transfection, and FoxO1 knockdown or overexpression with siFoxO1 or the indicated plasmids transfection for CyPA expression detected by real time-qPCR and immunoblotting.
As shown in , inhibition or knockdown of PKAcα by H89 treatment or siPKAcα transfection significantly increased CyPA mRNA expression, but this PKAcα-derived increase was diminished when cells were knocked down FoxO1 by siFoxO1 transfection with real time-qPCR ( and ) detection in the indicated cells, and activation or overexpression of PKAcα by FSK treatment or PKAcα plasmid transfection significantly decreased CyPA mRNA expression, but this PKAcα-derived decrease was rescued in cells overexpressed WT FoxO1 and constitutively active FoxO1 A3, and the FoxO1 A3 group rescued more compared to the WT group, but not in cells overexpressed loss-of-function FoxO1 ∆DB by the indicated plasmid transfection, as indicated by the results of real time-qPCR ( and ), indicating that PKAcα mediated CyPA mRNA expression via regulated FoxO1 phosphorylation and transcriptional activity. Primers used in real time-qPCR are listed in Table S1. PKAcα and FoxO1-knockdown, FoxO1 ∆DB, FoxO1 WT, FoxO1 A3 and PKAcα-overexpressing cells, were evaluated by performing RT-sqPCR and agarose gel electrophoresis (Figure S4).
Figure 5. PKA inhibites CyPA expression via FoxO1 pathway. PKA inhibition or knockdown by treatment with H89, or transfection with siPKAcα, respectively, significantly increased CyPA mRNA expression, but this PKAcα-derived increase was diminished when cells were knocked down FoxO1 by specific siRNA transfection according to the results of real time-qPCR (a and b). CyPA mRNA expression was significantly downregulated in cells treated with PKA activator FSK or expressing PKAcα, but this PKAcα-derived decrease was rescued when cells were overexpressed WT FoxO1 and constitutively active FoxO1 A3, and the FoxO1 A3 group rescued more compared to the WT group, but not in cells overexpressed loss-of-function FoxO1 ∆DB, as indicated by the results of real time-qPCR (c and d). Immunoblotting was performed to confirm the results of mRNA expression measurements, with VCAM-1, which is regulated by PKA, as the positive control. PKA inhibition or knockdown by treatment with H89, or transfection with siPKAcα, respectively, significantly decreased FoxO1 phosphorylation at Ser256 as previously we had indentified, and associated with significant increase of CyPA protein expression, but this PKAcα-derived CyPA increase was diminished when cells were knocked down FoxO1 by specific siRNA transfection according to the results of Immunoblotting (E and F). FoxO1 phosphorylation at Ser256 was significantly upregulated as previously we had demonstrated, and associated with significant downregulation of CyPA protein expression in cells treated with PKA activator FSK or expressing PKAcα, but this PKAcα-derived CyPA decrease was rescued when cells were overexpressed constitutively active FoxO1 A3 by plasmid transfection as indicated by the results of Immunoblotting (g and h), indicating that PKA mediated CyPA protein expression partly via regulated FoxO1 phosphorylation and transcriptional activity. Data were presented as mean ± SD of 3 independent experiments. **, p < 0.01,***,p < 0.001 (one-way ANOVA with post hoc Tukey test).
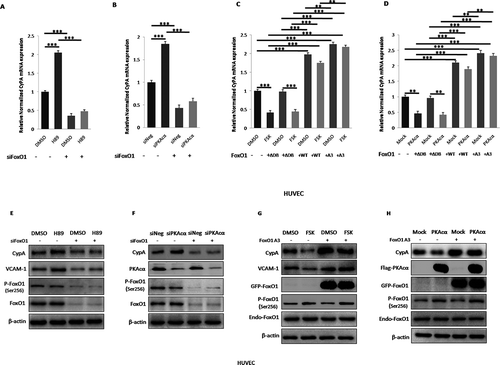
Immunoblotting was performed to confirm the mRNA results. As shown in , with VCAM-1, which is regulated by PKA, as the positive control. Inhibition or knockdown of PKAcα by H89 treatment or siPKAcα transfection significantly decreased FoxO1 phosphorylation (P-FoxO1) at Ser256 as previously had indentified [Citation20], and associated with significant increase of CyPA protein expression, but this PKAcα-derived CyPA increase was diminished when cells were knocked down FoxO1 by siFoxO1 transfection with immunoblotting ( and ) detection, and activation or overexpression of PKAcα by FSK treatment or PKAcα plasmid transfection significantly increased FoxO1 phosphorylation at Ser256 as previously had demonstrated [Citation20], and associated with significant decrease of CyPA protein expression decreased CyPA protein expression, but this PKAcα-derived CyPA decrease was rescued when cells were overexpressed constitutively active FoxO1 A3 by plasmid transfection with immunoblotting ( and ) detection in the indicated cells, indicating that PKAcα mediated CyPA protein expression partly via regulated FoxO1 phosphorylation and transcriptional activity. Non-overexpressed protein indicated as Endogenous (Endo) to differentiate from the Exogenous overexpression of plasmid transfection.
REGγ induces CyPA expression via the PKA-FoxO1 axis
As we had established that the PKA-FoxO1 axis regulates CyPA expression, to determine whether REGγ promoted CyPA expression via the same pathway, we used intracellular REGγ knockdown with specific shRNA or siRNA transfection, and PKA-FoxO1 axis activation or inhibition by treatment with chemical compounds, siRNA or plasmid transfection for CyPA expression detected by real time-qPCR, RT-sqPCR and immunoblotting.
As shown in , treatment with the PKA activator FSK significantly decreased CyPA mRNA expression in REGγ WT cells (MEF+/+ and HUVEC siN), but this decrease was not observed in REGγ knockout or knockdown cells (MEF-/- and HUVEC siR) by real time-qPCR ( and ) detection in the indicated cells, suggesting that REGγ mediated CyPA expression partly via the PKA pathway. Primers used in real time-qPCR and RT-sqPCR are listed in Table S1. REGγ-knockout or knockdown cells were evaluated by performing RT-sqPCR and agarose gel electrophoresis (Figure S5).
Figure 6. REGγ induces CyPA expression via the PKA-FoxO1 axis. Treatment with the PKA activator FSK significantly decreased CyPA mRNA expression in REGγ WT cells (MEF+/+ and HUVEC siN), but this decrease was not observed in REGγ knockout or knockdown cells (MEF-/- and HUVEC siR) by real time-qPCR (a and b) detection in the indicated cells, suggesting that REGγ mediated CyPA expression partly via the PKA pathway. Knockdown of REGγ significantly decreased CyPA mRNA expression, but this REGγ-derived decrease was diminished or rescued in cells after treated with the PKA activator FSK, knocked down or overexpressed PKA, knocked down FoxO1 or overexpressed FoxO1 WT and constitutively active FoxO1 A3 (the FoxO1 A3 group rescued more compared to the WT group), but not in cells overexpressed loss-of-function FoxO1 ∆DB, with real time-qPCR (c and d) detection in the indicated cells, indicating that REGγ mediated CyPA mRNA expression indeed partly via the PKA-FoxO1 pathway. Knockdown of REGγ significantly increased PKAcα protein level and FoxO1 phosphorylation at Ser256 as previously we had identified, and associated with significant decrease of CyPA protein expression, but the REGγ-derived CyPA decrease was diminished or rescued after treated with the PKA activator FSK, overexpressed PKAcα, knocked down FoxO1 or overexpressed constitutively active FoxO1 A3 by specific siRNA, or indicated plasmids transfection as indicated by the results of Immunoblotting (e–h), indicating that REGγ mediated CyPA protein expression partly via regulated PKAcα protein stability and PKAcα-derived FoxO1 phosphorylation and transcriptional activity, depended on REGγ-PKA-FoxO1 axis. Data were presented as mean ± SD of 3 independent experiments. **, p < 0.01, ***,p < 0.001 (one-way ANOVA with post hoc Tukey test).
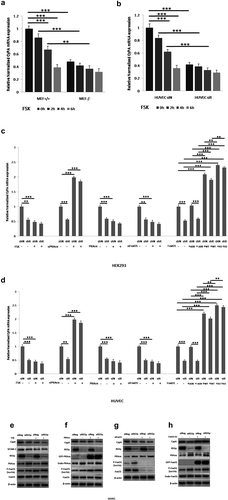
Furthermore, knockdown of REGγ significantly decreased CyPA mRNA expression, but this REGγ-derived decrease was diminished or rescued when cells were treated with the PKA activator FSK, knocked down or overexpressed PKA, knocked down FoxO1 or overexpressed FoxO1 WT and constitutively active FoxO1 A3 (the FoxO1 A3 group rescued more compared to the WT group), but not in cells overexpressed loss-of-function FoxO1 ∆DB,by specific siRNA, or indicated plasmids transfection with real time-qPCR ( and ) detection in the indicated cells, indicating that REGγ indeed mediated CyPA mRNA expression, partly via the PKA-FoxO1 pathway. Primers used in real time-qPCR and RT-sqPCR are listed in Table S1. REGγ, PKAcα and FoxO1-knockdown, FoxO1 ∆DB, FoxO1 WT, FoxO1 A3 and PKAcα-overexpressing cells, were evaluated by performing RT-sqPCR and agarose gel electrophoresis (Figure S6).
Immunoblotting was performed to confirm the mRNA results. As shown in , knockdown of REGγ significantly increased PKAcα protein level and FoxO1 phosphorylation at Ser256 as previously we had identified [Citation20], and associated with significant decrease of CyPA protein expression, whereas the REGγ-derived CyPA decrease was diminished or rescued after treated with the PKA activator FSK, overexpressed PKAcα, knocked down FoxO1 or overexpressed constitutively active FoxO1 A3 by specific plasmids, or by siRNA transfection, indicating that REGγ mediated CyPA protein expression partly via regulated PKAcα protein stability and PKAcα-derived FoxO1 phosphorylation, depended on REGγ-PKA-FoxO1 axis.
REGγ regulates HUVEC viability and triggers ECs apoptosis partly via CyPA activity
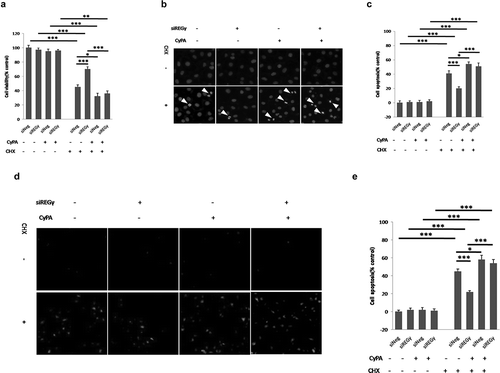
Based on the observation that REGγ induced the expression of CyPA, which is known to play key roles in EC viability and apoptosis [Citation4], we carried out HUVEC viability assays to assess REGγ’s capability in regulating ECs death in the presence of the protein synthesis inhibitor CHX ()). No significant reduction was observed in the viability of HUVECs in the absence of CHX. However, CHX treatment and siREGγ transfection significantly increased the viability of HUVECs compared with siNeg. However, the enhanced effect of REGγ knockdown on cell viability diminished in CyPA-overexpressing HUVECs. To determine whether HUVEC death was caused by apoptosis, we analyzed cell nuclei by performing fluorescence microscopy ()), and statistical analysis of apoptotic cell percentage of control was shown ()). Consistent results were observed in the apoptosis of HUVECs. No significant apoptotic induction was observed in the absence of CHX. However, CHX treatment and siNeg transfection significantly induced the apoptosis of HUVECs (indicated by an arrowhead) compared with siREGγ transfection. However, overexpression of CyPA diminished the REGγ-derived apoptosis change of HUVECs. To strengthen the HUVECs death was caused by apoptosis, we performed TUNEL assays, to verifty the results of nuclei morphological staining. Similar results ( and ) were observed, indicating that REGγ regulated ECs viability, and that apoptosis partly requires CyPA activity. REGγ and CyPA overexpression were evaluated by performing immunoblotting (Figure S7).
Figure 7. REGγ regulates HUVEC viability and triggers ECs apoptosis partly via CyPA activity. Cell viability and apoptosis assays were performed using HUVECs transiently pre-transfected with vector or CyPA plasmid for 24 h, followed by transfection with siNeg or siREGγ for 24 h, and treatment with CHX for 24 h. REGγ-stimulated CyPA-dependent ECs death. (a) Cell death decreased robustly in cells transfected with siREGγ and treated with CHX. However, this decrease in cell death was not observed in cells overexpressed CyPA. (b) ECs death was indentified to be caused by apoptosis with nuclei morphological staining (arrowhead), based on the analysis of nuclear morphology, by performing fluorescence microscopy, and (c) statistical analysis of apoptotic cell percentage was shown. For this, HUVECs were fixed, and apoptotic cells were identified based on the morphological staining of nuclei, as described in Materials and Methods. (d) TUNEL assays were performed to verifty the results of nuclei morphological staining, the FITC-labeled TUNEL-positive cells with green fluorescence were defined as apoptotic cells, and corresponding (e) statistical analysis of apoptotic cell percentage was shown. Data were presented as mean ± SD of 3 independent experiments. *, p < 0.05, **, p < 0.01, ***,p < 0.001 (one-way ANOVA with post hoc Tukey test).
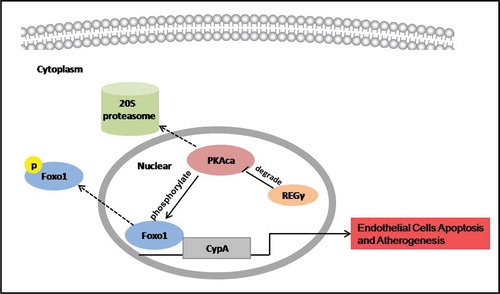
Possible mechanism of how REGγ stimulates ECs apoptosis and its functional implications in atherogenesis
REGγ activated the 20S proteasome to promote PKAcα degradation and inhibit the phosphorylation at Ser256 and nuclear export of FoxO1, thereby increasing FoxO1 nuclear accumulation and transcriptional activity. FoxO1 stimulated CyPA expression by binding to a specific region containing the forkhead-responsive element in the CyPA promoter. REGγ, via the PKA-FoxO1 axis, stimulated CyPA expression and CyPA-dependent ECs apoptosis in vitro and had functional implications in atherogenesis in vivo.
Discussion
The results of the present study highlight that REGγ was highly expressed in atherosclerotic plaques and activates ECs in a proapoptotic manner via the REGγ-PKA-FoxO1-CyPA pathway. First, we found that REGγ was highly expressed in atherosclerotic plaques from high-fat diet induecd ApoE-/- mice. Next, based on the RNA-seq result and experimental validation, we indentified that in HUVECs, REGγ increased the expression of an important proinflammatory and proapoptotic molecule in atherosclerosis progression-CyPA dramatically, via the REGγ-PKA-FoxO1 axis, and stimulated CyPA-dependent ECs apoptosis in vitro and had functional implications in atherogenesis in vivo, as indicated in .
Figure 8. REGγ, via the PKA-FoxO1 axis, stimulated CyPA expression and CyPA-dependent ECs apoptosis and had functional implications in atherogenesis. REGγ activated the 20S proteasome to promote PKAcα degradation and inhibite the phosphorylation at Ser256 and nuclear export of FoxO1, thereby increasing FoxO1 nuclear accumulation and transcriptional activity. FoxO1 stimulated CyPA expression by binding to a specific region containing the forkhead-responsive element in the CyPA promoter. REGγ, via the PKA-FoxO1 axis, stimulated CyPA expression and CyPA -dependent endothelial cells apoptosis in vitro and had functional implications in atherogenesis in vivo.
The various functions identified, and previous studies of REGγ on angiogenesis, and lipid metabolism [Citation18,Citation20], suggested its potential role on vascular remodeling and pathogenesis of atherosclerosis. Previously, we demonstrated that REGγ, via the PKA-FoxO1 axis, stimulated FoxO1 activation, and VCAM-1 and E-selectin expression on ECs migration and angiogenesis. In this study, we strenghened and broadened the expression and functional implications of REGγ in vascular biology, and found a novel atherosclerosis related downstream target of REGγ and FoxO1, via CyPA in a proapoptotic manner on ECs activation. And as REGγ involved in oxidative stress response [Citation21], in the future, it also could be explored whether REGγ regulates the oxidative stress-related enzymes, such as the glyoxalase I (GLO1) etc., which involved both in oxidative stress and apoptotic signallig pathway [Citation49,Citation50], to link the oxidative stress and apoptotic regulation in this process. They may had crosstalk to form a REGγ-signalling and regulating network, play each key role in different events including inflammatory cell recruitment, ECs migration and apoptosis, oxidative stress, and lipid metabolism and depositing etc. for complex atherosclerosis development.
Increasing evidence suggests that atherosclerosis is an inflammatory disease of the blood vessel walls, characterized by monocyte infiltration in response to proatherogenic factors [Citation51]. The proinflammatory effects of CyPA on ECs play an important role in monocyte recruitment to atherosclerotic lesions. In addition, CyPA has chemotactic activity toward neutrophils [Citation33,Citation34] and monocytes [Citation33]. However, so far we do not have direct evidence in REGγ-/ – ApoE-/- double knockout mice to identify the role of REGγ in atherosclerosis in vivo. Our data indicated that REGγ can regulate CyPA expression on ECs, and as CyPA can be expressed and secreted in atherosclerotic lesions, may contribute to recurrent inflammatory events leading to atherosclerosis progression, suggesting that REGγ may also play roles in atherosclerosis via inflammatory and immune processes. In the future, the REGγ-/- ApoE-/- or REGγ-/- CyPA-/- ApoE-/- murine disease model, or more human atherosclerosis samples should be used, to strengthen and develop the presented results in inflammatory and immune events in atherogenesis, as well.
In conclusion, this study demonstrated that REGγ activates ECs in a manner that would promote atherogenesis. We propose that REGγ, via the PKA-FoxO1 axis, promotes expression of adhesion molecules and CyPA, possibly inducing ECs to release CyPA. Secreted CyPA can bind to ECs and stimulate inflammation and apoptosis, which may form a network resulting in recruitment of additional inflammatory cells, and progression of atherosclerosis via a positive feedback mechanism.
Supplemental Material
Download PDF (2.2 MB)Acknowledgments
This work is funded by the National Natural Science Foundation of China [81521001]. Y.X. designed, performed, and discussed the study experiments and wrote the manuscript. X.L. conceived the project, supervised the experiments, and wrote the manuscript. J.G. conceived the project, wrote the manuscript, and provided funding. We thank the great support from Jia Xu and Youxin Xie for the research work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519.
- Rader DJ, Daugherty A. Translating moleculardiscoveries into new therapies for atherosclerosis. Nature. 2008;451(7181):904–913.
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874.
- Jin Z-G. Cyclophilin A is a proinflammatory cytokine that activates endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:1186–1191.
- Satoh K. Cyclophilin A mediates vascular remodeling by promoting inflammation and vascular smooth muscle cell proliferation. Circulation. 2008;117:3088–3098.
- Wang F. Sphingosine-1-phosphate receptor-2 deficiency leads to inhibition of macrophage proinflammatory activities and atherosclerosis in apoE-deficient mice. J Clin Invest. 2010;120(11):3979–3995.
- Bryant SR, Bjercke RJ, Erichsen DA, et al. Vascular remodeling in response to altered blood flow ismediated by fibroblast growth factor-2. Circ Res. 1999;84:323–328.
- Chiang HY, Korshunov VA, Serour A, et al. Fibronectin is an important regulator of flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol. 2009;29:1074–1079.
- Acevedo L, Yu J, Erdjument-Bromage H, et al. A new role for Nogo as a regulator of vascular remodeling. Nat Med. 2004;10:382–388.
- Li X, Lonard DM, Jung SY, et al. The SRC-3/AIB1 coactivator is degraded in a ubiquitin- and ATP-independent manner by the REGγ proteasome. Cell. 2006;124(2):381–392.
- Chen X, Barton LF, Chi Y, et al. Ubiquitin-independent degradation of cell-cycle inhibitors by the REGγ proteasome. Mol Cell. 2007;26:843–852.
- Li X, Amazit L, Long W, et al. Ubiquitin- and ATP-independent proteolytic turnover of p21 by the REGγamma-proteasome pathway. Mol Cell. 2007;26:831–842.
- Mao I, Liu J, Li X, et al. REGγ, a proteasome activator and beyond? Cell Mol Life Sci. 2008;65:3971–3980.
- Ali A, Wang Z, Fu J, et al. Differential regulation of the REGγ–proteasome pathway by p53/TGF-b signalling and mutant p53 in cancer cells. Nat Commun. 2013;4:2667.
- Liu J, Yu G, Zhao Y, et al. REGγ modulates p53 activity by regulating its cellularlocalization. J Cell Sci. 2010;123(23):4076–4084.
- Li L, Dang Y, Zhang J, et al. REGγ is critical for skin carcinogenesis by modulating the Wnt/β-catenin pathway. Nat Com. 2015;2:6875.
- Li L, Dengpan Zhao, Haibin Wei, et al. REGγ deficiency promotes premature aging via the casein kinase 1 pathway. Proc Natl Acad Sci USA. 2013;110:11005–11010.
- Dong S, Jia C, Zhang S, et al. The REGγamma proteasome regulates hepatic lipid metabolism through inhibition of autophagy. Cell Metab. 2013;18:380–391.
- Sun L, Fan G, Shan P, et al. Regulation of energy homeostasis by the ubiquitin-independent REGγ proteasome. Nat Com. 2016;7:12497.
- Liu S, Lai L, Zuo Q, et al. PKA turnover by the REGγamma-proteasome modulates FoxO1 cellular activity and VEGF-induced angiogenesis. J Mol Cell Cardiol. 2014;72:28–38.
- Zhang YY, Liu S, Zuo Q, et al. Oxidative challenge enhances REGγ-proteasome-dependent protein degradation. Free Radic Biol Med. 2015;82:42–49.
- Sun J, Luan Y, Xiang D, et al. The 11S proteasome subunit PSME3 is a positive feedforward regulator of NF-κB and important for host defense against bacterial pathogens. Cell Rep. 2016;14(4):737–749.
- Barton LF, Runnels HA, Schell TD, et al. Immune defects in 28-kDa proteasome activator gamma-deficient mice. J Immunol. 2004;172:3948–3954.
- Xu J, Zhou L, Ji L, et al. The REGγ-proteasome forms a regulatory circuit with IkBe and NF-kB in experimental colitis. Nat Com. 2016;7:10761.
- Gao G, Wong J, Zhang J, et al. Proteasome activator REGγ enhances coxsackieviral infection by facilitating p53 degradation. J Virol. 2010;84:11056–11066.
- Handschumacher. Cyclophilin: a specific cytosolic binding protein for cyclosporine A. Science. 1984;226:544–547.
- Marks AR. Cellular functions of immunophilins. Physiol Rev. 1996;76:631–649.
- Jin ZG, Melaragno MG, Liao DF, et al. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res. 2000;87:789–796.
- Suzuki J, Jin Z-G, Meoli DF, et al. Cyclophilin A is secreted by a vesicular pathway in vascular smooth muscle cells. Circ Res. 2006;98:811–817.
- Satoh K, Nigro P, Matoba T, et al. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nat Med. 2009;15:649–656.
- Liao DF, Jin ZG, Baas AS, et al. Purification and identification of secreted oxidative stress-induced factors from vascular smooth muscle cells. J Biol Chem. 2000;275:189–196.
- Pan H, Luo C, Li R, et al. Cyclophilin A is required for CXCR4-mediated nuclear export of heterogeneous nuclear ribonucleoprotein A2,activation and nuclear translocation of ERK1/2, and chemotactic cell migration. J Biol Chem. 2008;283:623–637.
- Sherry B, Yarlett N, Strupp A, et al. Identification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophages. Proc Natl Acad Sci U S A. 1992;89:3511–3515.
- Xu Q, Leiva MC, Fischkoff SA, et al. Leukocyte chemotactic activity of cyclophilin. J Biol Chem. 1992;267:11968–11971.
- Allain F, Vanpouille C, Carpentier M, et al. Interaction with glycosaminoglycans is required for cyclophilin B to trigger integrin-mediated adhesion of peripheral blood T lymphocytes to extracellular matrix. Proc Natl Acad Sci USA. 2002;99:2714–2719.
- Yurchenko V, Zybarth G, O’Connor M, et al. Active site residues of cyclophilin A are crucial for its signaling activity via CD147. J Biol Chem. 2002;277:22959–22965.
- Arora K, Gwinn WM, Bower MA, et al. Extracellular cyclophilins contribute to the regulation of inflammatory responses. J Immunol. 2005;175:517–522.
- Damsker JM, Bukrinsky MI, Constant SL. Preferential chemotaxis of activated human CD4+T cells by extracellular cyclophilin A. J Leukoc Biol. 2007;82:613–618.
- Kim H, Kim WJ, Jeon ST, et al. Cyclophilin A may contribute to the inflammatory processes in rheumatoid arthritis through induction of matrix degrading enzymes and inflammatory cytokines from macrophages. Clin Immunol. 2005;116:217–224.
- Zhu P, Ding J, Zhou J, et al. Expression of CD147 on monocytes/macrophages in rheumatoid arthritis: its potential role in monocyte accumulation and matrix metalloproteinase production. Arthritis Res Ther. 2005;7:R1023–R1033.
- Nigro P. Cyclophilin A is an inflammatory mediator that promotes atherosclerosis in apolipoprotein E-deficient mice. J Exp Med. 2010;208(1):53–66.
- Satoh K, Nigro P, Matoba T, et al. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nat Med. 2009;15:649–656.
- Weintraub NL. Understanding abdominal aortic aneurysm. N Engl J Med. 2009;361:1114–1116.
- Yu P, Wilhelm K, Dubrac A, et al. FGF-dependent metabolic control of vascular development. Nature. 2017;545(7653):224–228.
- Donato L, Bramanti P, Scimone C, et al. miRNAexpression profile of retinal pigment epithelial cells under oxidative stress conditions. FEBS Open Bio. 2018;8(2):219–233.
- Donato L, Scimone C, Rinaldi C, et al. Non-coding RNAome of RPE cells under oxidative stress suggests unknown regulative aspects of Retinitis pigmentosa etiopathogenesis. Sci Rep. 2018;8(1):16638.
- Donato L, Scimone C, Nicocia G, et al. Role of oxidative stress in Retinitis pigmentosa: new involved pathways by an RNA-Seq analysis. Cell Cycle. 2019;18(1):84–104.
- Donato L, Scimone C, Rinaldi C, et al. Stargardt phenotype associated with two ELOVL4 promoter variants and ELOVL4 downregulation: new possible perspective to etiopathogenesis? Invest Ophthalmol Vis Sci. 2018;59(2):843–857.
- Marinucci L, Balloni S, Fettucciari K, et al. Nicotine induces apoptosis in human osteoblasts via a novel mechanism driven by H2O2 and entailing Glyoxalase 1-dependent MG-H1 accumulation leading to TG2-mediated NF-kB desensitization: implication for smokers-related osteoporosis. Free Radic Biol Med. 2018;117:6–17.
- Donato L, Scimone C, Nicocia G, et al. GLO1 gene polymorphisms and their association with retinitis pigmentosa: a case-control study in a Sicilian population. Mol Biol Rep. 2018;45(5):1349–1355.
- Libby P, Ridker PM. Novel inflammatory markers of coronary risk: theoryversus practice. Circulation. 1999;100:1148–1150.
